Influence of Dietary Supplementation with an Amino Acid Mixture on Inflammatory Markers, Immune Status and Serum Proteome in LPS-Challenged Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Welfare Disclaimer
2.2. Animals, Diets and Sampling
2.3. Gut Morphology
2.4. Serum Immune and Hormonal Status
2.5. Statistical Analysis for Non-Proteomics Data
2.6. Proteomics Analysis
2.7. Data Availability
3. Results
3.1. Growth Performance and Faecal Score
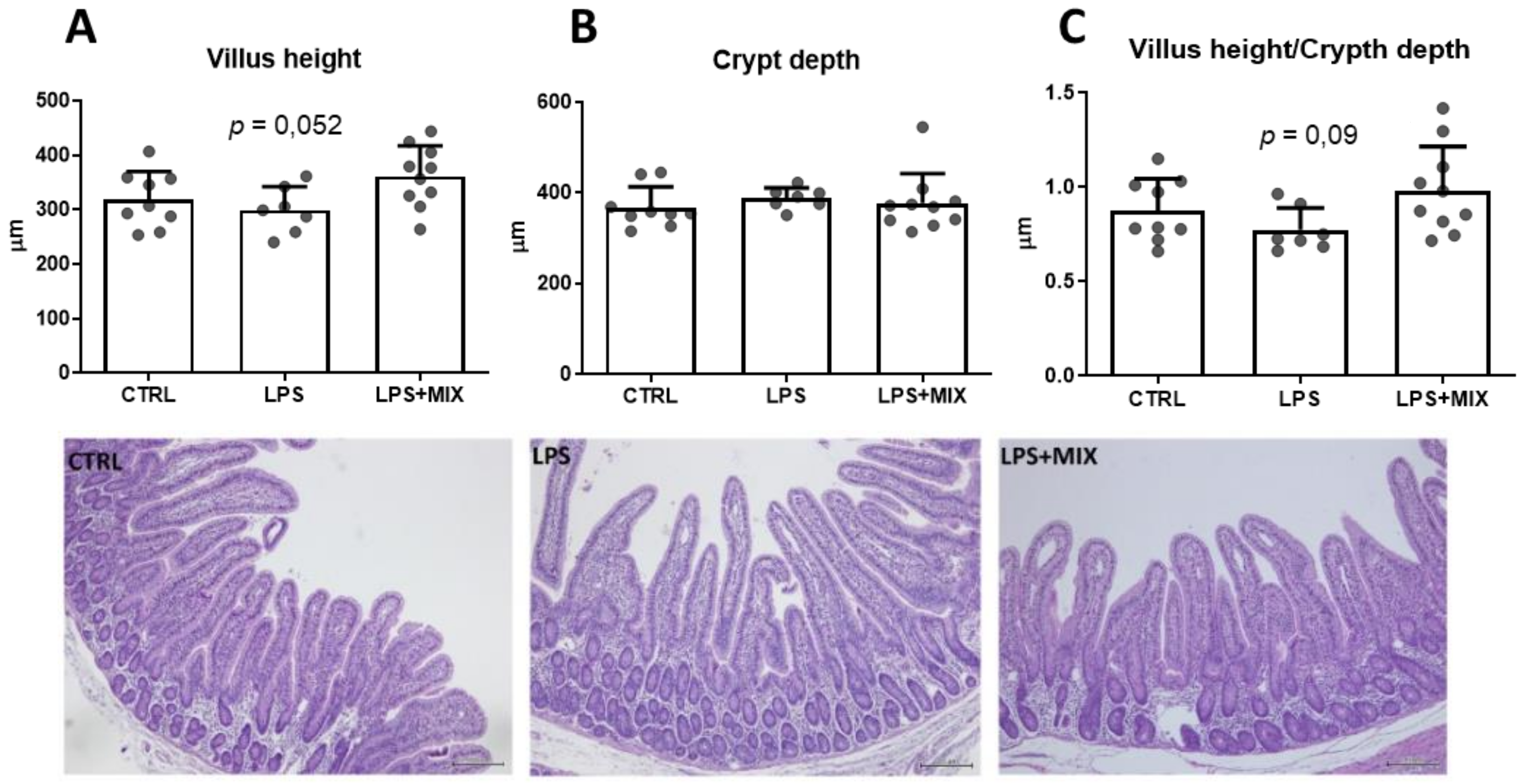
3.2. Gut Morphology
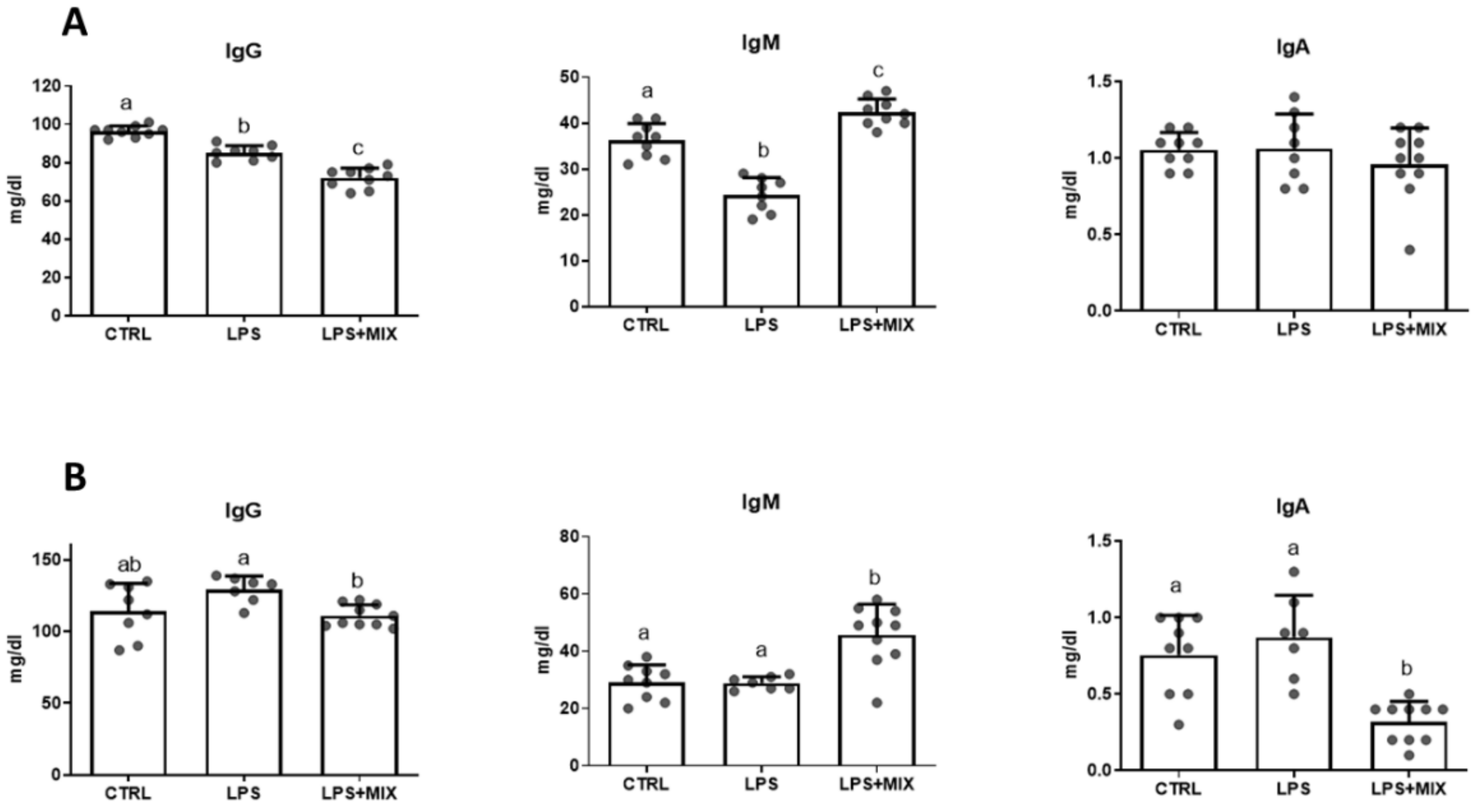
3.3. Serum Inflammatory and Hormonal Status
3.4. Serum Proteomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors Influencing the Structure and Function of the Small Intestine in the Weaned Pig: A Review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional Management of Gut Health in Pigs around Weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Turpin, D.L.; Kim, J.-C. Gastrointestinal Tract (Gut) Health in the Young Pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.; Moran, C.; Beal, J.; Demeckova, V.; Campbell, A. Liquid Feeding for the Young Piglet. Weaner Pig Nutr. Manag. 2001, 153–178. [Google Scholar]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glutamate Alleviates Intestinal Injury, Maintains MTOR and Suppresses TLR4 and NOD Signaling Pathways in Weanling Pigs Challenged with Lipopolysaccharide. Sci. Rep. 2018, 8, 15124. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of L-Arginine on Intestinal Mucosal Immune Barrier Function in Weaned Pigs after Escherichia coli LPS Challenge. Innate. Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef]
- Bergeron, N.; Robert, C.; Guay, F. Feed Supplementation with Arginine and Zinc on Antioxidant Status and Inflammatory Response in Challenged Weanling Piglets. Anim. Nutr. 2017, 3, 236–246. [Google Scholar] [CrossRef]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C. hong L-Cysteine Protects Intestinal Integrity, Attenuates Intestinal Inflammation and Oxidant Stress, and Modulates NF-ΚB and Nrf2 Pathways in Weaned Piglets after LPS Challenge. Innate. Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with Branched-Chain Amino Acids to a Low-Protein Diet Regulates Intestinal Expression of Amino Acid and Peptide Transporters in Weanling Pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef]
- Mao, X.; Liu, M.; Tang, J.; Chen, H.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P. Dietary Leucine Supplementation Improves the Mucin Production in the Jejunal Mucosa of the Weaned Pigs Challenged by Porcine Rotavirus. PLoS ONE 2015, 10, e0137380. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Gu, Y.; Song, Y.; Yin, H.; Lin, S.; Zhang, X.; Che, L.; Lin, Y.; Xu, S.; Feng, B.; Wu, D.; et al. Dietary Supplementation with Tributyrin Prevented Weaned Pigs from Growth Retardation and Lethal Infection via Modulation of Inflammatory Cytokines Production, Ileal Expression, and Intestinal Acetate Fermentation. J. Anim. Sci. 2017, 95, 226–238. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Jin, L.Z.; Kim, J.W.; Fang, L.; Frohlich, A.A.; Baidoo, S.K. Passive Protective Effect of Egg-Yolk Antibodies against Enterotoxigenic Escherichia coli K88+ Infection in Neonatal and Early-Weaned Piglets. FEMS Immunol. Med. Microbiol. 1999, 23, 283–288. [Google Scholar] [CrossRef]
- Tonel, I.; Pinho, M.; Lordelo, M.M.; Cunha, L.F.; Garres, P.; Freire, J.P.B. Effect of Butyrate on Gut Development and Intestinal Mucosa Morphology of Piglets. Livest. Sci. 2010, 133, 222–224. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-Pot, Solid-Phase-Enhanced Sample Preparation for Proteomics Experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Matos, A.; Domínguez-Pérez, D.; Almeida, D.; Agüero-Chapin, G.; Campos, A.; Osório, H.; Vasconcelos, V.; Antunes, A. Shotgun Proteomics of Ascidians Tunic Gives New Insights on Host–Microbe Interactions by Revealing Diverse Antimicrobial Peptides. Mar. Drugs 2020, 18, 362. [Google Scholar] [CrossRef]
- Almeida, A.M.; Nanni, P.; Ferreira, A.M.; Fortes, C.; Grossmann, J.; Bessa, R.J.B.; Costa, P. The Longissimus Thoracis Muscle Proteome in Alentejana Bulls as Affected by Growth Path. J. Proteom. 2017, 152, 206–215. [Google Scholar] [CrossRef]
- Moya, S.L.; Boyle, L.; Lynch, P.B.; Arkins, S. Pro-Inflammatory Cytokine and Acute Phase Protein Responses to Low-Dose Lipopolysaccharide (LPS) Challenge in Pigs. Anim. Sci. 2006, 82, 527–534. [Google Scholar] [CrossRef]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time Course of Increased Plasma Cytokines, Cortisol, and Urea Nitrogen in Pigs Following Intraperitoneal Injection of Lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef]
- Yao, K.; Guan, S.; Li, T.; Huang, R.; Wu, G.; Ruan, Z.; Yin, Y. Dietary L-Arginine Supplementation Enhances Intestinal Development and Expression of Vascular Endothelial Growth Factor in Weanling Piglets. Br. J. Nutr. 2011, 105, 703–709. [Google Scholar] [CrossRef]
- Zhu, L.; Cai, X.; Guo, Q.; Chen, X.; Zhu, S.; Xu, J. Effect of N-Acetyl Cysteine on Enterocyte Apoptosis and Intracellular Signalling Pathways’ Response to Oxidative Stress in Weaned Piglets. Br. J. Nutr. 2013, 110, 1938–1947. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Yi, D.; Ding, B.; Yang, Z.; Li, J.; Chen, X.; Qiu, Y.; Wu, G. N-Acetylcysteine Reduces Inflammation in the Small Intestine by Regulating Redox, EGF and TLR4 Signaling. Amino Acids 2013, 45, 513–522. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and Function of Immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Kim, M.; Yun, C.; Kim, G.; Ko, J.; Lee, J.-J.; Ha, J.-K. Changes of Immunoglobulins and Lymphocyte Subpopulations in Peripheral Blood from Holstein Calves Challenged with Escherichia coli Lipopolysaccharide. Asian Australas. J. Anim. Sci. 2011, 24, 696–706. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, H.; Shao, F.; Tan, B.; Hu, S. Arginine Accelerates Intestinal Health through Cytokines and Intestinal Microbiota. Int. Immunopharmacol. 2020, 81, 106029. [Google Scholar] [CrossRef]
- Han, B.; Tong, J.; Zhu, M.J.; Ma, C.; Du, M. Insulin-like Growth Factor-1 (IGF-1) and Leucine Activate Pig Myogenic Satellite Cells through Mammalian Target of Rapamycin (MTOR) Pathway. Mol. Reprod. Dev. 2008, 75, 810–817. [Google Scholar] [CrossRef]
- Wang, J.P.; Yoo, J.S.; Jang, H.D.; Lee, J.H.; Cho, J.H.; Kim, I.H. Effect of Dietary Fermented Garlic by Weissella Koreensis Powder on Growth Performance, Blood Characteristics, and Immune Response of Growing Pigs Challenged with Escherichia coli Lipopolysaccharide. J. Anim. Sci. 2011, 89, 2123–2131. [Google Scholar] [CrossRef]
- Priego, T.; Granado, M.; Ibáñez de Cáceres, I.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Endotoxin at Low Doses Stimulates Pituitary GH Whereas It Decreases IGF-I and IGF-Binding Protein-3 in Rats. J. Endocrinol. 2003, 179, 107–117. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.L.; Li, D.F.; Gong, L.M.; Yi, G.F.; Gaines, A.M.; Carroll, J.A. Effects of Fish Oil Supplementation on the Performance and the Immunological, Adrenal, and Somatotropic Responses of Weaned Pigs after an Escherichia coli Lipopolysaccharide Challenge. J. Anim. Sci. 2003, 81, 2758–2765. [Google Scholar] [CrossRef]
- Tsugawa, Y.; Handa, H.; Imai, T. Arginine Induces IGF-1 Secretion from the Endoplasmic Reticulum. Biochem. Biophys. Res. Commun. 2019, 514, 1128–1132. [Google Scholar] [CrossRef]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. Von Willebrand Factor Biosynthesis, Secretion, and Clearance: Connecting the Far Ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Diarra-Mehrpour, M.; Martin, J.P. Inter-Alpha-Trypsin Inhibitor Proteoglycan Family--a Group of Proteins Binding and Stabilizing the Extracellular Matrix. Eur. J. Biochem. 1998, 252, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Liu, G.; Pan, X.; Pang, Y.; Li, Q. L-C1qDC-1, a Novel C1q Domain-Containing Protein from Lethenteron Camtschaticum That Is Involved in the Immune Response. Dev. Comp. Immunol. 2016, 54, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ding, M.; Li, Y.; Zhong, X.; Liu, S.; Guo, Z.; Yin, X.; Fu, S.; Ye, J. The Complement Component 1 q (C1q) in Nile Tilapia (Oreochromis Niloticus): Functional Characterization in Host Defense against Bacterial Infection and Effect on Cytokine Response in Macrophages. Dev. Comp. Immunol. 2018, 87, 98–108. [Google Scholar] [CrossRef]
- Li, L.; Dong, M.; Wang, X.-G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin. Med. J. 2016, 129, 448–455. [Google Scholar] [CrossRef]
- Schmid, H.; Koop, M.; Utermann, S.; Lambacher, L.; Mayer, P.; Schaefer, L. Specific Catalytic Activity of Cathepsin S in Comparison to Cathepsins B and L along the Rat Nephron. Biol. Chem. 1997, 378, 61–69. [Google Scholar] [CrossRef]
- Hobart, M.J.; Fernie, B.A.; DiScipio, R.G. Structure of the Human C7 Gene and Comparison with the C6, C8A, C8B, and C9 Genes. J. Immunol. 1995, 154, 5188–5194. [Google Scholar]
- Wimmers, K.; Khoa, D.V.A.; Schütze, S.; Murani, E.; Ponsuksili, S. The Three-Way Relationship of Polymorphisms of Porcine Genes Encoding Terminal Complement Components, Their Differential Expression, and Health-Related Phenotypes. BMC Proc. 2011, 5, 1–4. [Google Scholar] [CrossRef]

| Ingredients | CTRL and LPS | LPS+MIX |
|---|---|---|
| Wheat | 200.0 | 200.0 |
| Corn | 381.6 | 381.6 |
| Soybean meal (48) | 268.0 | 268.0 |
| Sweet dry whey | 70.0 | 70.0 |
| Soybean oil | 30.0 | 30.0 |
| L-Lys | 6.4 | 6.4 |
| DL-Met | 2.7 | 2.7 |
| L-Thr | 2.8 | 2.8 |
| L-Trp | 1.1 | 1.1 |
| L-Val | 3.2 | 3.2 |
| CaCO3 | 10.0 | 10.0 |
| Dicalcium Phosphate | 16.0 | 16.0 |
| Sodium bicarbonate | 2.2 | 2.2 |
| NaCl | 3.0 | 3.0 |
| Vitamin trace mineral mix (1) | 3.0 | 3.0 |
| L-Arg | 0.0 | 1.25 |
| L-Leu | 0.0 | 0.50 |
| L-Val | 0.0 | 0.25 |
| L-Ile | 0.0 | 0.25 |
| L-Cys2 | 0.0 | 0.75 |
| SID * LYS pig | 13.50 | 13.50 |
| SID THR pig | 8.78 | 8.78 |
| SID MET pig | 5.28 | 5.28 |
| SID CYS pig | 2.83 | 3.58 |
| SID TRP pig | 2.97 | 2.97 |
| SID ILE pig | 7.16 | 7.41 |
| SID VAL pig | 10.80 | 11.05 |
| SID LEU pig | 13.54 | 14.04 |
| SID ARG pig | 10.90 | 12.15 |
| Chemical composition | ||
| Dry matter (%) | 89.7 | 89.2 |
| Ash (%) | 5.56 | 5.71 |
| Crude protein (%) | 19.75 | 20.05 |
| Ether Extract (%) | 5.92 | 5.80 |
| NE (MJ/kg) | 10.53 | 10.53 |
| CTRL | LPS | LPS+MIX | SEM | p-Value | |
|---|---|---|---|---|---|
| Week before LPS challenge (day 0–7) | |||||
| Initial weight (kg) | 8.46 | 8.39 | 7.95 | 0.19 | 0.498 |
| Final weight (kg) | 9.98 | 9.71 | 9.88 | 0.30 | 0.945 |
| Average daily gain (g/d) | 217.5 | 188.8 | 275.3 | 25.7 | 0.385 |
| Feed intake (g/d) | 366.2 | 333.3 | 301 | 30.7 | 0.694 |
| Week after LPS challenge (day 7–14) | |||||
| Final weight (kg) | 13.05 | 11.85 | 12.11 | 0.42 | 0.512 |
| Average daily gain (g/day) | 438.9 | 306.1 | 318.8 | 32.0 | 0.184 |
| Feed intake (g/day) | 484.2 | 581.7 | 453.2 | 42.0 | 0.540 |
| Last three weeks of the study (day 14–35) | |||||
| Final weight (kg) | 27.07 | 25.79 | 27.08 | 0.75 | 0.772 |
| Average daily gain (g/day) | 667.5 | 663.9 | 712.8 | 20.3 | 0.542 |
| Feed intake (g/ day) | 1054.6 | 1149.2 | 1110.9 | 48.3 | 0.686 |
| CTRL | LPS | LPS+MIX | SEM | p-Value | |
|---|---|---|---|---|---|
| Inflammatory markers | |||||
| Day 10 | |||||
| TNF-α (pg/mL) | 129.9 | 134.8 | 142.4 | 10.08 | 0.886 |
| Haptoglobin (mg/dL) | 23.16 a | 31.91 b | 32.08 b | 0.996 | <0.001 |
| Day 35 | |||||
| TNF-α (pg/mL) | 61.38 | 78.60 | 69.63 | 4.996 | 0.446 |
| Haptoglobin (mg/dL) | 20.64 a | 19.84 ab | 13.59 b | 1.317 | <0.05 |
| Hormones | |||||
| Day 10 | |||||
| Cortisol (µg/dL) | 2.700 a | 3.463 c | 1.711 b | 0.158 | <0.001 |
| IGF-1 (µg/L) | 59.07 a | 92.51 b | 110.6 c | 4.489 | <0.001 |
| Day 35 | |||||
| Cortisol (µg/dL) | 3.167 | 3.243 | 2.690 | 0.1922 | 0.447 |
| IGF-1 (µg/L) | 159.8 a | 143.8 a | 231.3 b | 9.708 | <0.001 |
| Accession Number | Protein Name | Peptide Count | Unique Peptides | ANOVA | Fold Change (LPS/CTRL) |
|---|---|---|---|---|---|
| p-Value | |||||
| CTRL vs. LPS | |||||
| A0A5G2QEV5 | Inter-alpha-trypsin inhibitor heavy chain H2 | 21 | 21 | 0.03 | 0.876 |
| F1SL22 | von Willebrand factor | 9 | 9 | 0.04 | 0.4 |
| A0A4x1U519 | C1q domain-containing protein | 2 | 2 | 0.04 | 0.59 |
| A0A287AAW7 | C1q domain-containing protein | 1 | 1 | 0.02 | 0.642 |
| LPS vs. LPS+MIX | |||||
| A0A287A1M4 | Uncharacterised protein | 11 | 7 | 0.04 | 0.734 |
| A0SEH1 | Complement component | 9 | 1 | 0.05 | 1.686 |
| A0A5G2QLJ8 | Complement C1q B chain | 2 | 2 | 0.03 | 0.704 |
| A0A4x1VG41 | Beta-2-microglobulin | 1 | 1 | 0.03 | 0.01 |
| A0A287A359 | Uncharacterised protein | 1 | 1 | 0.03 | 0.814 |
| B2CNZ7 | Cathepsin B | 1 | 1 | 0.04 | 0.716 |
| A0A286ZKE0 | Uncharacterised protein | 1 | 1 | 0.03 | 0.867 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prates, J.A.M.; Freire, J.P.B.; de Almeida, A.M.; Martins, C.; Ribeiro, D.M.; Osório, H.; Pinho, M.A.S.; Lopes, P.A.; Correia, J.M.J.; Pinto, R.M.A.; et al. Influence of Dietary Supplementation with an Amino Acid Mixture on Inflammatory Markers, Immune Status and Serum Proteome in LPS-Challenged Weaned Piglets. Animals 2021, 11, 1143. https://doi.org/10.3390/ani11041143
Prates JAM, Freire JPB, de Almeida AM, Martins C, Ribeiro DM, Osório H, Pinho MAS, Lopes PA, Correia JMJ, Pinto RMA, et al. Influence of Dietary Supplementation with an Amino Acid Mixture on Inflammatory Markers, Immune Status and Serum Proteome in LPS-Challenged Weaned Piglets. Animals. 2021; 11(4):1143. https://doi.org/10.3390/ani11041143
Chicago/Turabian StylePrates, José A. M., João P. B. Freire, André M. de Almeida, Cátia Martins, David M. Ribeiro, Hugo Osório, Mário A. S. Pinho, Paula A. Lopes, Jorge M. J. Correia, Rui M. A. Pinto, and et al. 2021. "Influence of Dietary Supplementation with an Amino Acid Mixture on Inflammatory Markers, Immune Status and Serum Proteome in LPS-Challenged Weaned Piglets" Animals 11, no. 4: 1143. https://doi.org/10.3390/ani11041143
APA StylePrates, J. A. M., Freire, J. P. B., de Almeida, A. M., Martins, C., Ribeiro, D. M., Osório, H., Pinho, M. A. S., Lopes, P. A., Correia, J. M. J., Pinto, R. M. A., Costa, T., Corrent, E., & Chalvon-Demersay, T. (2021). Influence of Dietary Supplementation with an Amino Acid Mixture on Inflammatory Markers, Immune Status and Serum Proteome in LPS-Challenged Weaned Piglets. Animals, 11(4), 1143. https://doi.org/10.3390/ani11041143










