A Retrospective Case Study into the Effect of Hoof Lesions on the Lying Behaviour of Holstein–Friesian in a Loose-Housed System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals and Management
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Lameness and Lesion Prevalence
3.2. Mobility Scores
3.3. Parity and Days in Milk (DIM)
3.4. Before Claw Trimming (BCT)
3.5. After Claw Trimming (ACT)
3.6. Comparison between BCT and ACT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Assessment of the welfare of dairy caftle using animal-based measurements: Direct Obs. and investigation of farm records. Vet. Rec. 2003, 153, 197–202. [Google Scholar] [CrossRef]
- Wilshire, J.; Bell, N. An economic review of cattle lameness. Cattle Pract. 2009, 17, 136–141. [Google Scholar]
- Griffiths, B.E.; White, D.G.; Oikonomou, G. A Cross-Sectional Study into the Prevalence of Dairy Cattle Lameness and Associated Herd-Level Risk Factors in England and Wales. Front. Vet. Sci. 2018, 5, 65. [Google Scholar] [CrossRef]
- Jubb, T.F.; Malmo, J. Lesions causing lameness requiring veterinary treatment in pasture-fed dairy cows in East Gippsland. Aust. Vet. J. 1991, 68, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.; Downham, D.Y.; Clarkson, M.J.; Faull, W.B.; Hughes, J.W.; Manson, F.J.; Merritt, J.B.; Russell, W.B.; Sutherst, J.E.; Ward, W.R. Epidemiology of lameness in dairy cattle: Description and analysis of foot lesions. Vet. Rec. 1996, 138, 586–591. [Google Scholar] [CrossRef]
- Randall, L.V.; Thomas, H.J.; Remnant, J.G.; Bollard, N.J.; Huxley, J.N. Lameness prevalence in a random sample of UK dairy herds. Vet. Rec. 2019, 184, 350. [Google Scholar] [CrossRef]
- Barker, Z.E.; Leach, K.A.; Whay, H.R.; Bell, N.J.; Main, D.C.J. Assessment of lameness prevalence and associated risk factors in dairy herds in england and wales. J. Dairy Sci. 2010, 93, 932–941. [Google Scholar] [CrossRef]
- Blowey, R. Factors associated with lameness in dairy cattle. Practice 2005, 27, 154–162. [Google Scholar] [CrossRef]
- Somers, J.R.; O’Grady, L. Foot lesions in lame cows on 10 dairy farms in Ireland. Ir. Vet. J. 2015, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- O’Callaghan, K.; Cripps, P.; Downham, D.; Murray, R. Subjective and objective assessment of pain and discomfort due to lameness in dairy cattle. Anim. Welf. 2003, 12, 605–610. [Google Scholar]
- Garbarino, E.; Hernandez, J.; Shearer, J.; Risco, C.; Thatcher, W. Effect of Lameness on Ovarian Activity in Postpartum Holstein Cows. J. Dairy Sci. 2004, 87, 4123–4131. [Google Scholar] [CrossRef]
- Hernandez, J.; Shearer, J.K.; Webb, D.W. Effect of lameness on the calving-to-conception interval in dairy cows. J. Am. Vet. Med. Assoc. 2001, 218, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, R.; Vokey, F.; Erb, H.; Guard, C. Visual Locomotion Scoring in the First Seventy Days in Milk: Impact on Pregnancy and Survival. J. Dairy Sci. 2007, 90, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; Garbarino, E.J.; Shearer, J.K.; Risco, C.A.; Thatcher, W.W. Comparison of milk yield in dairy cows with different degrees of lameness. J. Am. Vet. Med. Assoc. 2005, 227, 1292–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Navarro, G.; Green, L.E.; Tadich, N. Effect of lameness and lesion specific causes of lameness on time budgets of dairy cows at pasture and when housed. Vet. J. 2013, 197, 788–793. [Google Scholar] [CrossRef]
- Charfeddine, N.; Pérez-Cabal, M. Effect of claw disorders on milk production, fertility, and longevity, and their economic impact in Spanish Holstein cows. J. Dairy Sci. 2017, 100, 653–665. [Google Scholar] [CrossRef]
- Booth, C.; Warnick, L.; Gröhn, Y.; Maizon, D.; Guard, C.; Janssen, D. Effect of Lameness on Culling in Dairy Cows. J. Dairy Sci. 2004, 87, 4115–4122. [Google Scholar] [CrossRef]
- Green, L.; Hedges, V.; Schukken, Y.; Blowey, R.; Packington, A. The Impact of Clinical Lameness on the Milk Yield of Dairy Cows. J. Dairy Sci. 2002, 85, 2250–2256. [Google Scholar] [CrossRef]
- Amory, J.; Barker, Z.; Wright, J.; Mason, S.; Blowey, R.; Green, L. Associations between sole ulcer, white line disease and digital dermatitis and the milk yield of 1824 dairy cows on 30 dairy cow farms in England and Wales from February 2003–November 2004. Prev. Vet. Med. 2008, 83, 381–391. [Google Scholar] [CrossRef]
- Haley, D.; Passillé, A.; Rushen, J. Assessing cow comfort: Effects of two floor types and two tie stall designs on the behaviour of lactating dairy cows. Appl. Anim. Behav. Sci. 2001, 71, 105–117. [Google Scholar] [CrossRef]
- Ito, K.; Chapinal, N.; Weary, D.; Von Keyserlingk, M. Associations between herd-level factors and lying behavior of freestall-housed dairy cows. J. Dairy Sci. 2014, 97, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Olmos, G.; Boyle, L.; Hanlon, A.; Patton, J.; Murphy, J.J.; Mee, J.F. Hoof disorders, locomotion ability and ly-ing times of cubicle-housed compared to pasture-based dairy cows. Livest. Sci. 2009, 125, 199–207. [Google Scholar] [CrossRef]
- Gomez, A.; Cook, N. Time budgets of lactating dairy cattle in commercial freestall herds. J. Dairy Sci. 2010, 93, 5772–5781. [Google Scholar] [CrossRef]
- Thompson, A.; Weary, D.M.; Bran, J.A.; Daros, R.R.; Hötzel, M.J.; von Keyserlingk, M.A.G. Lameness and lying behavior in grazing dairy cows. J. Dairy Sci. 2019, 102, 6373–6382. [Google Scholar] [CrossRef]
- Schütz, K.; Clark, K.V.; Cox, N.R.; Matthews, L.R.; Tucker, C.B. Responses to short-term exposure to simulated rain and wind by dairy cattle: Time budgets, shelter use, body temperature and feed intake. Anim. Welf. 2010, 19, 375. [Google Scholar]
- Overton, M.; Sischo, W.; Temple, G.; Moore, D. Using Time-Lapse Video Photography to Assess Dairy Cattle Lying Behavior in a Free-Stall Barn. J. Dairy Sci. 2002, 85, 2407–2413. [Google Scholar] [CrossRef]
- Maselyne, J.; Pastell, M.; Thomsen, P.T.; Thorup, V.M.; Hänninen, L.; Vangeyte, J.; Van Nuffel, A.; Munksgaard, L. Daily lying time, motion index and step frequency in dairy cows change throughout lacta-tion. Res. Vet. Sci. 2017, 110, 1–3. [Google Scholar] [CrossRef]
- Westin, R.; Vaughan, A.; De Passillé, A.; Devries, T.; Pajor, E.; Pellerin, D.; Siegford, J.; Vasseur, E.; Rushen, J. Lying times of lactating cows on dairy farms with automatic milking systems and the relation to lameness, leg lesions, and body condition score. J. Dairy Sci. 2016, 99, 551–561. [Google Scholar] [CrossRef]
- Thorup, V.M.; Nielsen, B.L.; Robert, P.-E.; Giger-Reverdin, S.; Konka, J.; Michie, C.; Friggens, N.C. Lameness Affects Cow Feeding but Not Rumination Behavior as Characterized from Sensor Data. Front. Vet. Sci. 2016, 3, 37. [Google Scholar] [CrossRef]
- Norring, M.; Haggman, J.; Simojoki, H.; Tamminen, P.; Winckler, C.; Pastell, M. Short communication: Lameness impairs feeding behavior of dairy cows. J. Dairy Sci. 2014, 97, 4317–4321. [Google Scholar] [CrossRef] [PubMed]
- Solano, L.; Barkema, H.; Pajor, E.; Mason, S.; Leblanc, S.; Nash, C.; Haley, D.; Pellerin, D.; Rushen, J.; De Passillé, A.; et al. Associations between lying behavior and lameness in Canadian Holstein-Friesian cows housed in freestall barns. J. Dairy Sci. 2016, 99, 2086–2101. [Google Scholar] [CrossRef] [PubMed]
- Blackie, N.; Amory, J.; Bleach, E.; Scaife, J. The effect of lameness on lying behaviour of zero grazed Holstein dairy cattle. Appl. Anim. Behav. Sci. 2011, 134, 85–91. [Google Scholar] [CrossRef]
- Sepúlveda-Varas, P.; Weary, D.; Von Keyserlingk, M. Lying behavior and postpartum health status in grazing dairy cows. J. Dairy Sci. 2014, 97, 6334–6343. [Google Scholar] [CrossRef]
- Nechanitzky, K.; Starke, A.; Vidondo, B.; Müller, H.; Reckardt, M.; Friedli, K.; Steiner, A. Analysis of behavioral changes in dairy cows associated with claw horn lesions. J. Dairy Sci. 2016, 99, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Chapinal, N.; de Passillé, A.; Weary, D.; von Keyserlingk, M.; Rushen, J. Using gait score, walking speed, and lying behavior to detect hoof lesions in dairy cows. J. Dairy Sci. 2009, 92, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, A.; Bergsten, C.; Ekesbo, I.; Kaart, T.; Aland, A.; Lidfors, L. Influence of digital dermatitis and sole ulcer on dairy cow behaviour and milk production. Animal 2011, 5, 1259–1269. [Google Scholar] [CrossRef]
- Sepúlveda-Varas, P. Claw horn lesions in mid-lactation primiparous dairy cows under pasture-based systems: Association with behavioral and metabolic changes around calving. J. Dairy Sci. 2018, 101, 9439–9450. [Google Scholar] [CrossRef]
- Rulquin, H.; Caudal, J. Effects of lying or standing on mammary blood flow and heart rate of dairy cows. Ann. Zootech. INRA/EDP Sci. 1992, 41, 101. [Google Scholar] [CrossRef]
- Delamaire, E.; Guinard, F. Increasing milking intervals decreases the mammary blood flow and mammary uptake of nutrients in dairy cows. J. Dairy Sci. 2006, 89, 3439–3446. [Google Scholar] [CrossRef]
- Munksgaard, L.; Jensen, M.; Pedersen, L.; Hansen, S.; Matthews, L. Quantifying behavioural priorities—Effects of time constraints on behaviour of dairy cows, bos taurus. Appl. Anim. Behav. Sci. 2005, 92, 3–14. [Google Scholar] [CrossRef]
- Jensen, M.; Pedersen, L.; Munksgaard, L. The effect of reward duration on demand functionsfor rest in dairy heifers and lying requirementsas measured by demand functions. Appl. Anim. Behav. Sci. 2005, 90, 207–217. [Google Scholar] [CrossRef]
- Leach, K.; Tisdall, D.; Bell, N.; Main, D.; Green, L. The effects of early treatment for hindlimb lameness in dairy cows on four commercial UK farms. Vet. J. 2012, 193, 626–632. [Google Scholar] [CrossRef]
- Whay, H.; Main, D.; Green, L.; Webster, A. Farmer perception of lameness prevalence. In Proceedings of the 12th International Symposium on Lameness in Ruminants, Orlando, FL, USA, 9–13 January 2002; pp. 355–358. [Google Scholar]
- Archer, S.C.; Bell, N.; Huxley, J. Lameness in UK dairy cows: A review of the current status. Practice 2010, 32, 492–504. [Google Scholar] [CrossRef]
- Tadich, N.; Flor, E.; Green, L. Associations between hoof lesions and locomotion score in 1098 unsound dairy cows. Vet. J. 2010, 184, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Flower, F.; Weary, D. Effect of Hoof Pathologies on Subjective Assessments of Dairy Cow Gait. J. Dairy Sci. 2006, 89, 139–146. [Google Scholar] [CrossRef]
- Manske, T.; Hultgren, J.; Bergsten, C. Prevalence and interrelationships of hoof lesions and lameness in swedish dairy cows. Prev. Vet. Med. 2002, 54, 247–263. [Google Scholar] [CrossRef]
- Charlton, G.L.; Bouffard, V.; Gibbons, J.; Vasseur, E.; Haley, D.B.; Pellerin, D.; Rushen, J.; de Passillé, A.M. Can automated measures of lying time help assess lameness and leg lesions on tie-stall dairy farms? Appl. Anim. Behav. Sci. 2016, 175, 14–22. [Google Scholar] [CrossRef]
- O’Leary, N.; Byrne, D.; O’Connor, A.; Shalloo, L. Invited review: Cattle lameness detection with accelerometers. J. Dairy Sci. 2020, 103, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Kaniyamattam, K.; Hertl, J.; Lhermie, G.; Tasch, U.; Dyer, R.; Gröhn, Y. Cost benefit analysis of automatic lameness detection systems in dairy herds: A dynamic programming approach. Prev. Vet. Med. 2020, 178, 104993. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.; Medrano-Galarza, C.; Passillé, A.; Rushen, J. Lying laterality and the effect of icetag data loggers on lying behaviour of dairy cows. Appl. Anim. Behav. Sci. 2012, 136, 104–107. [Google Scholar] [CrossRef]
- Mackay, J.R.; Deag, J.M.; Haskell, M.J. Establishing the extent of behavioural reactions in dairy cattle to a leg mounted activity monitor. Appl. Anim. Behav. Sci. 2012, 139, 35–41. [Google Scholar] [CrossRef]
- Blackie, N.; Scaife, J.; Bleach, E. Lying behaviour and activity of early lactation holstein dairy cattle measured using an activity monitor. Cattle Pract. 2006, 14, 139. [Google Scholar]
- Beer, G.; Alsaaod, M.; Starke, A.; Schuepbach-Regula, G.; Müller, H.; Kohler, P.; Steiner, A. Use of Extended Characteristics of Locomotion and Feeding Behavior for Automated Identification of Lame Dairy Cows. PLoS ONE 2016, 11, e0155796. [Google Scholar] [CrossRef]
- Chapinal, N.; de Passillé, A.; Rushen, J. Correlated changes in behavioral indicators of lameness in dairy cows following hoof trimming. J. Dairy Sci. 2010, 93, 5758–5763. [Google Scholar] [CrossRef]
- AHDB. Dairy Mobility Scoresheet. Available online: https://ahdb.org.uk/knowledge-library/dairy-mobility-scoresheet (accessed on 18 March 2021).
- Holzhauer, M.; Bartels, C.; Döpfer, D.; Schaik, G. Clinical course of digital dermatitis lesions in an endemically infected herd without preventive herd strategies. Vet. J. 2008, 177, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Enevoldsen, C.; Gröhn, Y.; Thysen, I. Heel erosion and other interdigital disorders in dairy cows: Associations with season, cow characteristics, disease, and production. J. Dairy Sci. 1991, 74, 1299–1309. [Google Scholar] [CrossRef]
- Russell, A.M.; Rowlands, G.J.; Shaw, S.R.; Weaver, A.D. Survey of lameness in British dairy cattle. Vet. Rec. 1982, 111, 155–160. [Google Scholar] [CrossRef]
- Cook, N.; Bennett, T.; Nordlund, K. Effect of Free Stall Surface on Daily Activity Patterns in Dairy Cows with Relevance to Lameness Prevalence. J. Dairy Sci. 2004, 87, 2912–2922. [Google Scholar] [CrossRef]
- Shepley, E.; Lensink, J.; Leruste, H.; Vasseur, E. The effect of free-stall versus strawyard housing and access to pasture on dairy cow locomotor activity and time budget. Appl. Anim. Behav. Sci. 2020, 224, 104928. [Google Scholar] [CrossRef]
- Fregonesi, A.J.; Leaver, J. Behaviour, performance and health indicators of welfare for dairy cows housed in strawyard or cubicle systems. Livest. Prod. Sci. 2001, 68, 205–216. [Google Scholar] [CrossRef]
- Endres, M.; Barberg, A. Behavior of Dairy Cows in an Alternative Bedded-Pack Housing System. J. Dairy Sci. 2007, 90, 4192–4200. [Google Scholar] [CrossRef]
- Galindo, F.; Broom, D. The relationships between social behaviour of dairy cows and the occurrence of lameness in three herds. Res. Vet. Sci. 2000, 69, 75–79. [Google Scholar] [CrossRef]
- Walker, S.L.; Smith, R.F.; Routly, J.E.; Jones, D.N.; Morris, M.J.; Dobson, H. Lameness, Activity Time-Budgets, and Estrus Expression in Dairy Cattle. J. Dairy Sci. 2008, 91, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ward, W.; Lautenbach, K.; Hughes, J.; Murray, R. Behaviour of first lactation and adult dairy cows while housed and at pasture and its relationship with sole lesions. Vet. Rec. 1993, 133, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Weary, D.M.; von Keyserlingk, M.A.G. Lying behavior: Assessing within- and between-herd variation in free-stall-housed dairy cows. J. Dairy Sci. 2009, 92, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Bichalo, R.; Machado, V.; Caixeta, L. Lameness in dairy cattle: A debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence and thickness of the digital cushion. J. Dairy Sci. 2009, 92, 3175–3184. [Google Scholar] [CrossRef]
- Thomsen, P.; Munksgaard, L.; Sørensen, J. Locomotion scores and lying behaviour are indicators of hoof lesions in dairy cows. Vet. J. 2012, 193, 644–647. [Google Scholar] [CrossRef]
- Chapinal, N.; Passillé, A.; Rushen, J.; Wagner, S. Effect of analgesia during hoof trimming on gait, weight distribution, and activity of dairy cattle. J. Dairy Sci. 2010, 93, 3039–3046. [Google Scholar] [CrossRef]
- Deming, J.A.; Bergeron, R.; Leslie, K.E.; Devries, T.J. Associations of cow-level factors, frequency of feed delivery, and standing and lying behaviour of dairy cows milked in an automatic system. Can. J. Anim. Sci. 2013, 93, 427–433. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Thomas, H.J.; Kaler, J.; Craigon, J.; Huxley, J.N. Effects of lameness treatment for claw horn lesions on lying behaviour in dairy cows. Appl. Anim. Behav. Sci. 2016, 179, 11–16. [Google Scholar] [CrossRef]
- Cutler, J.H.; Shearer, J.K.; Kelton, D.F.; Cramer, G.; Gorden, P.J.; Millman, S.T. An Observational Study of the Effects of Therapeutic Hoof Blocks on the Locomotion, Behavior, and Production of Healthy Dairy Cattle. J. Appl. Anim. Welf. Sci. 2015, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bichalho, R.; Oikonomou, G. Control and prevention of lameness associated with claw lesions in dairy cows. Livest. Sci. 2013, 156, 96–105. [Google Scholar] [CrossRef]
- Offer, J.; Fisher, G.; Kempson, S.; Logue, D. The Effect of Feeding Grass Silage in Early Pregnancy on Claw Health During First Lactation. Vet. J. 2001, 161, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Frankena, K.; Somers, J.G.C.J.; Schouten, W.G.P.; van Stek, J.V.; Metz, J.; Stassen, E.N.; Graat, E.A.M. The effect of digital lesions and floor type on locomotion score in dutch dairy cows. Prev. Vet. Med. 2009, 88, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.; Leach, K.; Main, D.; Whay, H. Can lameness/mobility scoring be used to identify cows with digital dermatitis? In Proceedings of the Cattle Lameness Conference, Nottingham, UK, 25 March 2009; pp. 41–42. [Google Scholar]
- Schlageter-Tello, A.; Van Hertem, T.; Bokkers, E.A.; Viazzi, S.; Bahr, C.; Lokhorst, K. Performance of human observers and an automatic 3-dimensional computer-vision-based locomotion scoring method to detect lameness and hoof lesions in dairy cows. J. Dairy Sci. 2018, 101, 6322–6335. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.; Green, M.; Chagunda, M.; Mason, C.; Archer, S.; Green, L.; Huxley, J. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. J. Dairy Sci. 2015, 98, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Stambuck, C.; McArt, J.; Bicalho, R.M.A.; Huson, H. A longitudinal study of digital cushion thickness and its function as a predictor for compromised locomotion and hoof lesions in holstein cows. Transl. Anim. Sci. 2019, 3, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Huxley, J.; Banks, C.; Green, M. Temporal associations between low body condition, lameness and milk yield in a UK dairy herd. Prev. Vet. Med. 2014, 113, 63–71. [Google Scholar] [CrossRef]


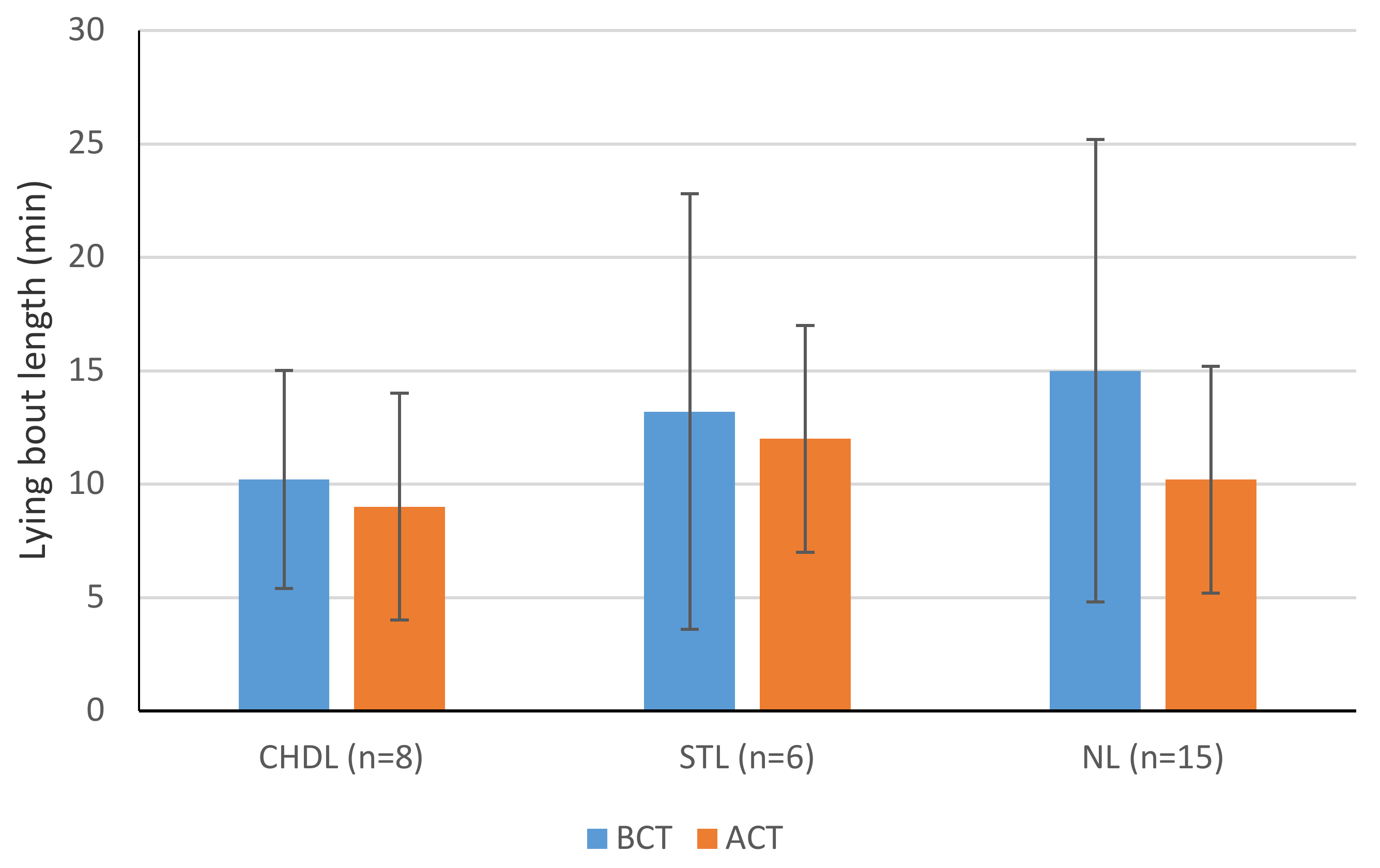
| Category | Score | Description of Behaviour |
|---|---|---|
| Good mobility | 0 | Walks with even weight bearing and rhythm on all four feet, with a flat back. Long, fluid strides possible. |
| Imperfect mobility | 1 | Steps uneven (rhythm or weight bearing) or strides shortened; affected limb or limbs not immediately identifiable. |
| Impaired mobility | 2 | Uneven weight bearing on a limb that is immediately identifiable and/or obviously shortened strides (usually with an arch to the centre of the back). |
| Severely impaired mobility | 3 | Unable to walk as fast as a brisk human pace (cannot keep up with the healthy herd). Lame leg easy to identify—limping; may barely stand on lame leg/s; back arched when standing and walking. Very lame. |
| Measure | Description |
|---|---|
| Lying time | Time in hours (h) that the sensor is positioned horizontally. |
| Standing time | Time in hours (h) that the sensor is positioned vertically. |
| Lying bout length | Period between the sensor changing from vertical to horizontal, then back to vertical. |
| Step count | The number of times the cow lifts her leg, based on the amount of force used. |
| Leg | SH | SU | Laminitis | WLD | Sole Separation | DD | IH | Other * |
|---|---|---|---|---|---|---|---|---|
| Front: | ||||||||
| Left | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Right | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (1.5%) |
| Hind: | ||||||||
| Left | 4 (6.3%) | 3 (5%) | 1 (1.5%) | 0 (0%) | 1 (1.5%) | 7 (11.1%) | 5 (8%) | 7 (11.1%) |
| Right | 1 (1.5%) | 5 (8%) | 1 (1.5%) | 1 (1.5%) | 1 (1.5%) | 9 (14.2%) | 11 (17.5%) | 2 (3.2%) |
| CHDL (n = 8) | STL (n = 6) | NL (n = 15) | p-Value | |
|---|---|---|---|---|
| Lying time (h/d) BCT | 15.00 ± 1.04 a,c | 11.30 ± 2.44 b | 11.77 ± 1.67 b,x | 0.003 |
| Standing time (h/d) * BCT | 9.68 ± 2.38 a | 12.69 ± 2.43 a,b | 12.21 ±1.67 b | 0.021 |
| Min lying bout (min) BCT | 10.2 ± 4.8 | 13.20 ± 9.6 | 15.0 ± 10.2 x | 0.483 |
| Max lying bout (min) BCT | 169.2 ± 21.0 | 141.0 ± 21.0 | 147.0 ± 30 | 0.085 |
| Lying bouts/day BCT | 11.8 ± 2.3 | 10.0 ± 2.2 | 10.0 ± 2.2 | 0.193 |
| Lying time (h/d) ACT * | 13.66 ± 0.98 d | 11.67 ± 2.12 | 12.57 ± 1.90 y | 0.176 |
| Standing time (h/d) ACT | 10.28 ± 0.97 | 12.32 ± 2.11 | 11.32 ± 1.68 | 0.083 |
| Min lying bout (min) * ACT | 9.0 ± 4.8 | 12.0 ± 9.0 | 10.2 ± 5.4 y | 0.878 |
| Max lying bout (min) * ACT | 177.6 ± 24.6 | 142.8 ± 33 | 165 ± 43.8 | 0.126 |
| Lying bouts/day ACT | 11.3 ± 0.7 | 11.0 ± 2.8 | 10.9 ± 1.9 | 0.927 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, K.J.; Booth, R.E.; Blackie, N. A Retrospective Case Study into the Effect of Hoof Lesions on the Lying Behaviour of Holstein–Friesian in a Loose-Housed System. Animals 2021, 11, 1120. https://doi.org/10.3390/ani11041120
Ji KJ, Booth RE, Blackie N. A Retrospective Case Study into the Effect of Hoof Lesions on the Lying Behaviour of Holstein–Friesian in a Loose-Housed System. Animals. 2021; 11(4):1120. https://doi.org/10.3390/ani11041120
Chicago/Turabian StyleJi, Karen Jiewei, Richard E. Booth, and Nicola Blackie. 2021. "A Retrospective Case Study into the Effect of Hoof Lesions on the Lying Behaviour of Holstein–Friesian in a Loose-Housed System" Animals 11, no. 4: 1120. https://doi.org/10.3390/ani11041120
APA StyleJi, K. J., Booth, R. E., & Blackie, N. (2021). A Retrospective Case Study into the Effect of Hoof Lesions on the Lying Behaviour of Holstein–Friesian in a Loose-Housed System. Animals, 11(4), 1120. https://doi.org/10.3390/ani11041120






