Long-Term Recording of Reticulo-Rumen Myoelectrical Activity in Sheep by a Telemetry Method
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Electromyography Recording and Analysis
2.3. Electromyography Recognition of A, B, and C Cycles
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Clauss, M.; Hofmann, R.R.; Fickel, J.; Streich, W.J.; Hummel, J. The intraruminal papillation gradient in wild ruminants of different feeding types: Implications for rumen physiology. J. Morphol. 2009, 270, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Hofmann, R.R.; Streich, W.J.; Fickel, J.; Hummel, J. Convergence in the macroscopic anatomy of the reticulum in wild ruminant species of reticulo-rumen shape in high and low methane sheep different feeding types and a new resulting hypothesis on reticular function. J. Zool. 2010, 281, 26–38. [Google Scholar] [CrossRef]
- Ruckebusch, Y. Gastrointestinal motor functions in ruminants. Comprehensive Physiology. In Handbook of Physiology, The Gastrointestinal System, Motility and Circulation; Oxford University Press: Oxford, UK, 2011; pp. 1225–1282. [Google Scholar]
- Plaza, M.A.; Arruebo, M.P.; Sopena, J.; Bonafonte, J.I.; Murill, M.D. Myoelectrical activity of the gastrointestinal tract of sheep analysed by computer. Res. Vet. Sci. 1996, 60, 55–60. [Google Scholar] [CrossRef]
- Ruckebusch, J.; Bardon, T.; Pairet, M. Opioid control of the ruminant stomach motility: Functional importance of µ, δ and κ receptors. Life Sci. 1984, 35, 1731–1738. [Google Scholar] [CrossRef]
- Grünberg, W.; Constable, P.D. Function and Dysfunction of the Ruminant Forestomach. In Food Animal Practice, 5th ed.; Saunders: London, UK, 2009; pp. 12–19. [Google Scholar]
- Braun, U.; Jacquat, D.; Steininger, K. Ultrasonographic examination of the abdomen of the goat. I. Reticulum, rumen, omasum, abomasum and intestines. Schweiz. Arch. Tierheilkd. 2013, 155, 173–184. [Google Scholar] [CrossRef]
- Braun, U.; Schweizer, A. Ultrasonographic assessment of reticuloruminal motility in 45 cows. Schweiz. Arch. Tierheilkd. 2015, 157, 87–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arai, S.; Haritani, M.; Sawada, H.; Kimura, K. Effect of mosapride on ruminal motility in cattle. J. Vet. Med. Sci. 2019, 81, 1017–1020. [Google Scholar] [CrossRef]
- Hamilton, A.W.; Davison, C.; Tachtatzis, C.; Andonovic, I.; Michie, C.; Ferguson, H.J.; Somerville, L.; Jonsson, N.N. Identification of the rumination in cattle using support vector machines with motion-sensitive bolus sensors. Sensors 2019, 19, 1165. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.C.W. Motility of the rumen and abomasum during hypocalcaemia. Can. J. Comp. Med. 1983, 47, 276–280. [Google Scholar]
- Arai, S.; Okada, H.; Sawada, H.; Takahashi, Y.; Kimura, K.; Itoh, T. Evaluation of ruminal motility in cattle by a bolus-type wireless sensor. J. Vet. Med. Sci. 2019, 81, 1835–1841. [Google Scholar] [CrossRef]
- Gacsalyi, U.; Zabielski, R.; Pierzynowski, S.G. Telemetry facilitates long-term recording of gastrointestinal myoelectrical activity in pigs. Exp. Physiol. 2000, 85, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Szucs, K.F.; Nagy, A.; Grosz, G.; Tiszai, Z.; Gaspar, R. Correlation between slow-wave myoelectric signals and mechanical contractions in the gastrointestinal tract: Advanced electromyographic method in rats. J. Pharmacol. Toxicol. Methods 2016, 82, 37–44. [Google Scholar] [CrossRef]
- Ruckebusch, Y.; Tomov, T. The sequential contraction of the rumen associated with eructation in sheep. J. Physiol. 1973, 235, 447–458. [Google Scholar] [CrossRef]
- Ruckebusch, Y.; Bueno, L. Pharmacology of the Ruminant Stomach W: Trends in Veterinary Pharmacology and Toxicology Red; van Miert, A.S.J.P.A.M., Frens, J., van der Kreek, F.W., Eds.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1979; pp. 165–178. [Google Scholar]
- Kania, B.F.; Wielgosz, M. Role of central µ-opioid receptors in the wall of gastric myoelectrical spike burst activity in sheep. Med. Wet. 2007, 63, 1478–1481. [Google Scholar]
- Sarna, S.; Stoddard, C.; Belbeck, L.; McWade, D. Intrinsic nervous control of migrating myoelectric complexes. Am. J. Physiol. 1981, 241, 16–23. [Google Scholar] [CrossRef]
- Burden, A.; Bartlett, R. Normalisation of EMG amplitude: An evaluation and comparison of old and new methods. Med. Eng. Phys. 1999, 21, 247–257. [Google Scholar] [CrossRef]
- Gajewski, Z.; Pawliński, B.; Zięcik, A.; Zabielski, R. The using of the DSI telemetry implants in the reproductive tract EMG recording in the sows in relation to LH, P4, E2. In Proceedings of the 16th ICAR, Budapest, Hungary, 13–17 July 2008; p. 853. [Google Scholar]
- Domino, M.; Pawlinski, B.; Gajewska, M.; Jasinski, T.; Sady, M.; Gajewski, Z. Uterine EMG activity in the non-pregnant sow during estrous cycle. BMC Vet. Res. 2018, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Pawliński, B.; Domino, M.; Aniołek, O.; Ziecik, A.; Gajewski, Z. Bioelectrical activity of porcine oviduct and uterus during spontaneous and induced estrus associated with cyclic hormone changes. Theriogenology 2016, 86, 2312–2322. [Google Scholar] [CrossRef]
- Yao, G.; Woliński, J.; Korczyński, W.; Zabielski, R. Daily changes in antro-duodenal myoelectric activity in weaned pigs. Anim. Sci. 2003, 76, 273–281. [Google Scholar] [CrossRef]
- Gajewski, Z.; Blitek, M.; Klos, J.; Gromadzka-Hliwa, K.; Pawlinski, B.; Andrzejczak, A.; Ziecik, A. Oviductal and uterine myometrial activity during periovulatory period in the pig. Rep. Dom. Anim. 2004, 1, 41–47. [Google Scholar]
- Woliński, J.; Zięcik, A.; Gajewski, Z.; Korczyński, W.; Zabielski, R. A method of recording the oviduct and uterus myoelectrical activity in gilts using implantable telemetry. J. Anim. Feed Sci. 2003, 12, 359–367. [Google Scholar] [CrossRef]
- Domino, M.; Pawlinski, B.; Gajewski, Z. The linear synchronization measures of uterine EMG signals: Evidence of synchronized action potentials during propagation. Theriogenology 2016, 86, 1873–1878. [Google Scholar] [CrossRef]
- Foster, D. Disorders of Rumen Distension and Dysmotility. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 499–512. [Google Scholar] [CrossRef]
- Jorgensen, R.J.; Nyengaard, N.R.; Hara, S.; Enemark, J.M.; Andersen, P.H. Rumen motility during induced hyper- and hypocalcaemia. Acta Vet. Scand. 1998, 39, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.; Benedito, J.L.; Abuelo, A.; Castillo, C. Ruminal Acidosis in Feedlot: From Aetiology to Prevention. Sci. World J. 2014, 2014, 702572. [Google Scholar] [CrossRef]
- Forbes, J.M.; Barrio, J.P. Abdominal chemo- and mechanosensitivity in ruminants and its role in the control of food intake. Exp. Physiol. 1992, 77, 27–50. [Google Scholar] [CrossRef]
- Andersen, P.H. Bovine endotoxicosis—Some aspects of relevance to production diseases. A review. Acta Vet. Scand. 2003, 44, S141. [Google Scholar] [CrossRef]
- Leek, B.F. Clinical diseases of the rumen: A physiologist’s view. Vet. Rec. 1983, 113, 10–14. [Google Scholar] [CrossRef]
- Bayne, J.E.; Edmondson, M.A. Diseases of the gastrointestinal system. Sheep Goat Cervid Med. 2021, 63–96. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.A.; Talukder, A.K.; Parvin, M.S.; Islam, M.T. Clinical diseases of ruminants recorded at the Patuakhali Science and Technology University Veterinary Clinic. Bangladesh J. Vet. Med. 2012, 10, 63–73. [Google Scholar] [CrossRef]
- Vettorato, E.; Schöffmann, G.; Burke, J.G.; Gibson, A.J.; Clutton, E.R. Clinical effects of isoflurane and sevoflurane in lambs. Vet. Anaesth. Analg. 2012, 39, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.; Boyle, M.B.; Garfield, R.E. Electrical activity of the human uterus during pregnancy as recorded from the abdominal surface. Obstet. Gynecol. 1997, 90, 102–111. [Google Scholar] [CrossRef]
- Fahadi, N.; Suryono, S.; Suseno, D.E. Electromyogram signal analysis in frequency domain of uterine muscle contraction during childbirth. Int. J. Innov. Res. Adv. Eng. 2017, 4, 10–14. [Google Scholar]
- Domino, M.; Domino, K.; Gajewski, Z. An application of higher order multivariate cumulants in modelling of myoelectrical activity of porcine uterus during early pregnancy. BioSystems 2019, 175, 30–38. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Reid, C.S.W. Rumen motility in sheep and cattle given different diets. N. Z. J. Agric. Res. 1983, 26, 289–295. [Google Scholar] [CrossRef]
- Wyburn, R.S. The mixing and propulsion of the stomach contents of ruminants. In Digestive Physiology and Metabolism of Ruminants; Ruckebusch, Y., Thivend, P., Eds.; MTP: Lancaster, UK, 1980; pp. 35–51. [Google Scholar] [CrossRef]
- Ruckebusch, Y. Pharmacology of the reticulo-ruminal motor functions. J. Vet. Pharmacol. Ther. 1983, 6, 245–272. [Google Scholar] [CrossRef]
- Kania, B.F.; Wielgosz, M. Role of central δ-opioid receptors in the wall of gastric myoelectrical spike burst activity in sheep. Med. Wet. 2009, 65, 344–348. [Google Scholar]
- Kania, B.F.; Wielgosz, M. Role of central κ-opioid receptors in the myoelectrical spike burst activity of the stomach wall in sheep. Med. Wet. 2010, 66, 196–200. [Google Scholar]
- Braun, U. Ultrasonography of the gastrointestinal tract in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 567–590. [Google Scholar] [CrossRef]
- Braun, U.; Jacquat, D. Ultrasonography of the omasum in 30 Saanen goats. BMC Vet. Res. 2011, 7, 11. [Google Scholar] [CrossRef]
- Castro, W.J.R.; Zanine, A.M.; Ferreira, D.J.; Souza, A.L.; Pinho, R.M.A.; Parente, M.O.M.; Parente, H.N.; Santos, E.M. Delinted cottonseed in diets for finishing sheep. Trop. Anim. Health Prod. 2020, 52, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, R.; Kiela, P.; Leśniewska, V.; Krzemiński, R.; Mikołajczyk, M.; Barej, W. Kinetics of pancreatic juice secretion in relation to duodenal migrating myoelectric complex in preruminant and ruminant calves feed twice daily. Br. J. Nutr. 1997, 78, 427–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Julia, C.; Latour, A. Analyse automatique par minicalculateur des contractions du reseau chez les ruminants. Ann. Res. Vet. 1975, 6, 23–34. [Google Scholar]
- Neethirajan, S. Transforming the Adaptation Physiology of Farm Animals through Sensors. Animals 2020, 10, 1512. [Google Scholar] [CrossRef]
- Ma, H.; Lundy, J.D.; Cottle, E.L.; O’Malley, K.J.; Trichel, A.M.; Klimstra, W.B.; Hartman, A.L.; Reed, D.S.; Teichert, T. Applications of minimally invasive multimodal telemetry for continuous monitoring of brain function and intracranial pressure in macaques with acute viral encephalitis. PLoS ONE 2020, 15, e0232381. [Google Scholar] [CrossRef] [PubMed]
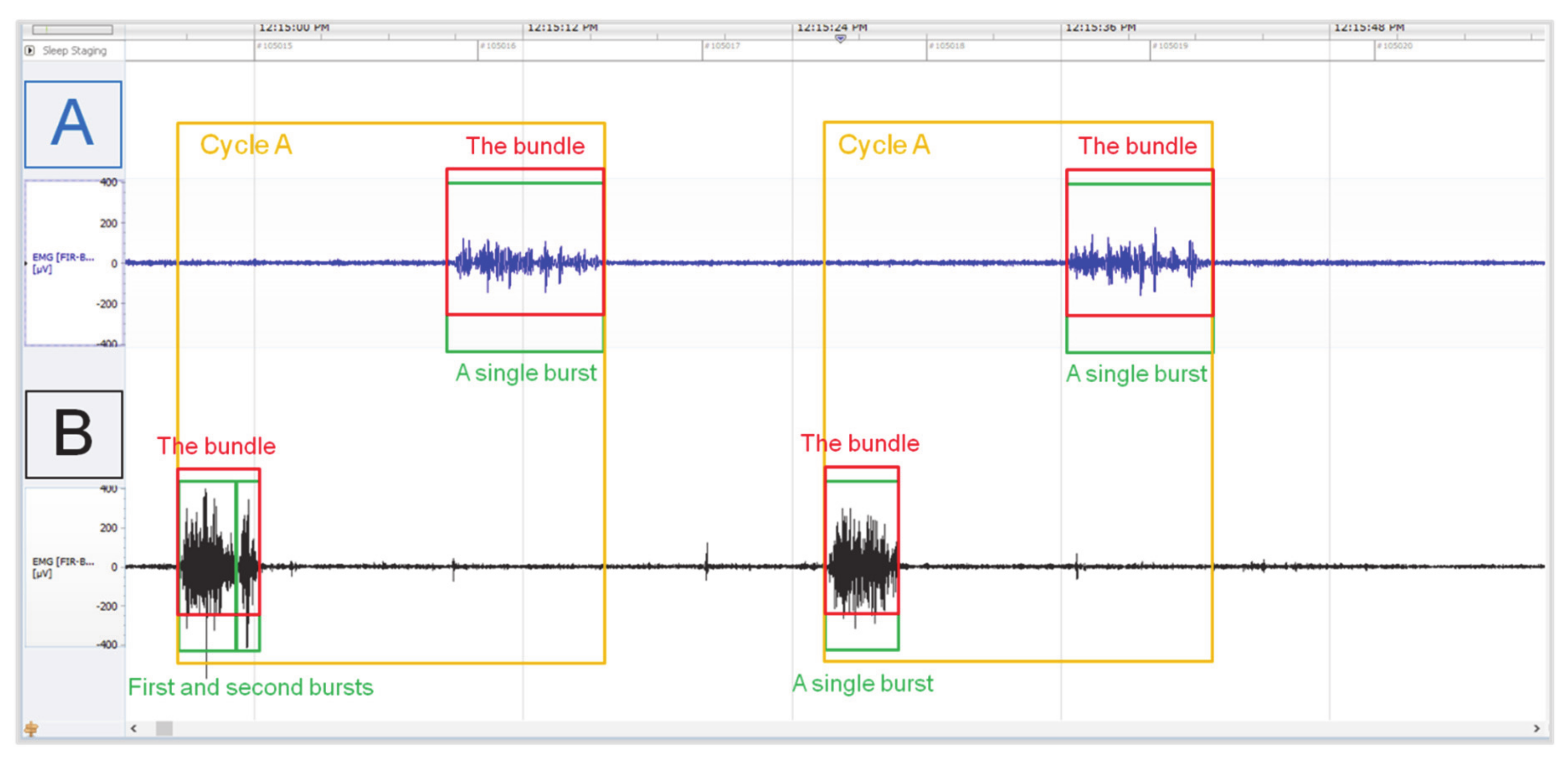
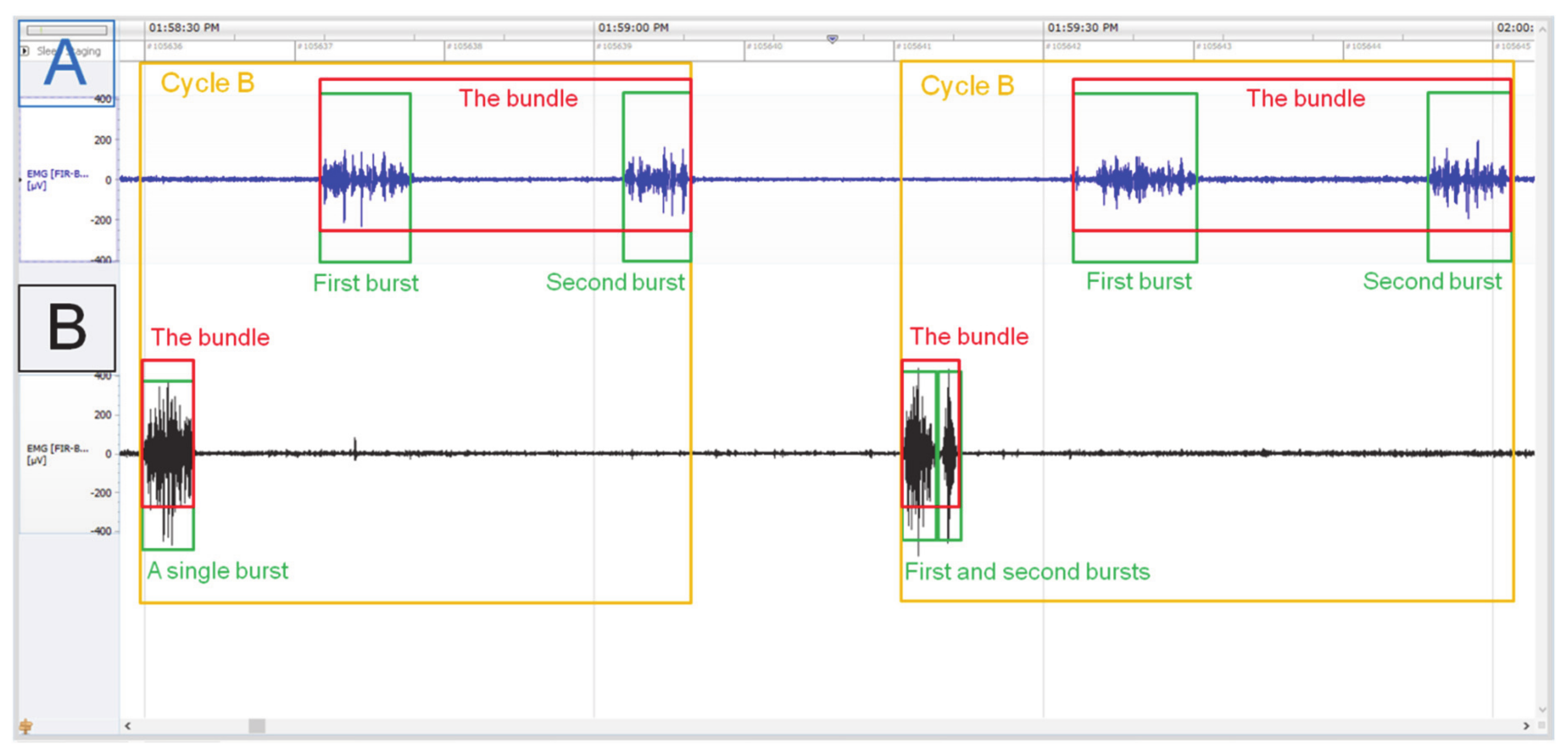
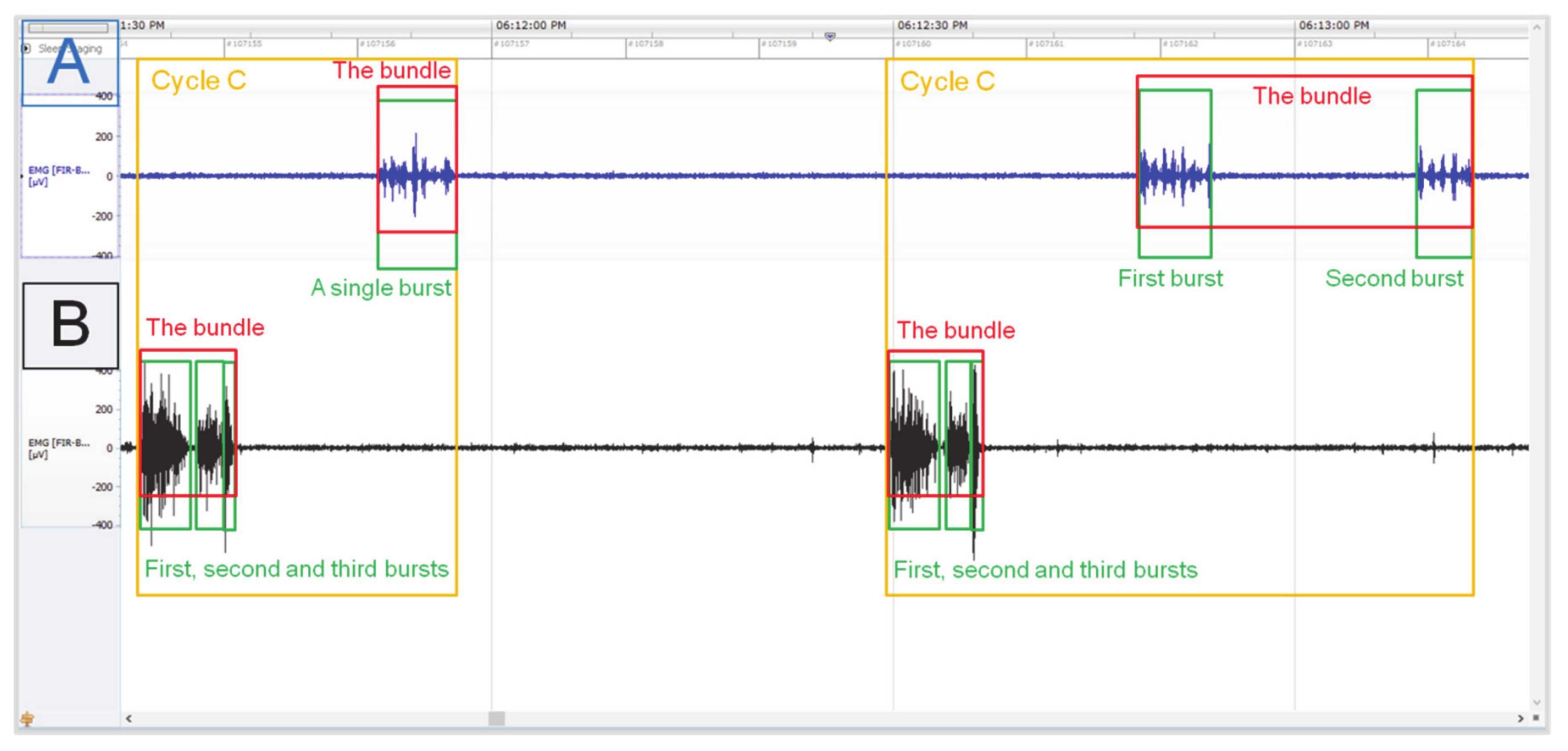

| Reticulo-Rumen | EMG | Cycle A | Cycle B | Cycle C | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Rumen | Reticulum | Rumen | Reticulum | Rumen | Reticulum | ||
| A 1 | (mV) | 0.72 ± 0.14 a | 2.01 ± 0.28 b | 0.85 ± 0.16 a | 1.98 ± 0.30 b | 0.80 ± 0.17 a | 2.11 ± 0.41 b | |
| Bundle | RMS 2 | (mV) | 0.10 ± 0.02 m | 0.51 ± 0.22 n | 0.13 ± 0.02 m | 0.50 ± 0.001 n | 0.12 ± 0.02 m | 0.61 ± 0.001 n |
| D 3 | (s) | 7.71 ± 1.13 u | 3.24 ± 0.37 v | 26.14 ± 2.68 w | 3.25 ± 0.22 v | 16.79 ± 8.94 x | 6.48 ± 0.39 y | |
| A 1 | (mV) | 0.72 ± 0.14 a | 1.93 ± 0.29 b | 0.75 ± 0.15 a | 1.89 ± 0.31 b | 0.76 ± 0.18 a | 1.76 ± 0.28 b,c | |
| First burst | RMS 2 | (mV) | 0.10 ± 0.02 m | 0.35 ± 0.05 o | 0.11 ± 0.02 m | 0.37 ± 0.05 o | 0.11 ± 0.02 m | 0.33 ± 0.07 o |
| D 3 | (s) | 7.71 ± 1.13 u | 1.95 ± 0.26 v | 7.85 ± 1.23 u | 2.01 ± 0.19 v | 7.25 ± 0.99 u | 2.77 ± 0.14 v | |
| A 1 | (mV) | - | 1.74 ± 0.30 b,c | 0.71 ± 0.19 a | 1.73 ± 0.36 b,c | 0.66 ± 0.13 a | 1.78 ± 0.41 b,c | |
| Second burst | RMS 2 | (mV) | - | 0.48 ± 0.06 n | 0.13 ± 0.02 m | 0.51 ± 0.07 n | 0.22 ± 0.02 m | 0.51 ± 0.17 n |
| D 3 | (s) | - | 0.82 ± 0.49 z | 5.27 ± 0.75 y | 0.73 ± 0.12 z | 5.46 ± 0.75 y | 1.06 ± 0.27 z | |
| A 1 | (mV) | - | - | - | - | - | 1.60 ± 0.28 c | |
| Third burst | RMS 2 | (mV) | - | - | - | - | - | 0.45 ± 0.16 n,o |
| D 3 | (s) | - | - | - | - | - | 0.78 ± 0.08 z | |
| Reticulo-Rumen | EMG | Cycle A | Cycle B | Cycle C | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Rumen | Reticulum | Rumen | Reticulum | Rumen | Reticulum | ||
| A 1 | (mV) | 22.16% | 11.13% | 14.34% | 20.55% | 26.15% | 15.49% | |
| Bundle | RMS 2 | (mV) | 19.29% | 44.88% | 10.44% | 46.30% | 19.23% | 21.93% |
| D 3 | (s) | 18.53% | 17.14% | 16.21% | 13.40% | 52.69% | 16.08% | |
| A 1 | (mV) | 22.16% | 12.11% | 14.74% | 19.66% | 29.00% | 10.83% | |
| First burst | RMS 2 | (mV) | 19.29% | 26.05% | 11.74% | 27.60% | 17.94% | 17.73% |
| D 3 | (s) | 18.53% | 18.96% | 17.54% | 16.51% | 12.80% | 14.27% | |
| A 1 | (mV) | - | 14.30% | 20.16% | 24.55% | 10.66% | 20.82% | |
| Second burst | RMS 2 | (mV) | - | 47.62% | 15.09% | 51.57% | 13.68% | 27.86% |
| D 3 | (s) | - | 12.26% | 13.34% | 13.66% | 16.69% | 18.24% | |
| A 1 | (mV) | - | - | - | - | 18.85% | ||
| Third burst | RMS 2 | (mV) | - | - | - | - | - | 36.22% |
| D 3 | (s) | - | - | - | - | - | 12.47% | |
| Reticulo-Rumen | EMG | Cycle A | Cycle B | Cycle C | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Rumen | Reticulum | Rumen | Reticulum | Rumen | Reticulum | ||
| A 1 | (mV) | 20.38% | 13.19% | 18.74% | 15.33% | 21.12% | 16.07% | |
| Bundle | RMS 2 | (mV) | 18.57% | 42.85% | 11.24% | 39.83% | 17.54% | 21.69% |
| D 3 | (s) | 14.96% | 11.86% | 10.91% | 16.82% | 53.22% | 16.46% | |
| A 1 | (mV) | 20.38% | 15.08% | 18.82% | 16.18% | 24.11% | 15.91% | |
| First burst | RMS 2 | (mV) | 18.57% | 24.57% | 13.94% | 24.77% | 16.97% | 23.25% |
| D 3 | (s) | 14.96% | 13.23% | 15.66% | 19.36% | 13.71% | 15.05% | |
| A 1 | (mV) | - | 16.87% | 26.53% | 20.66% | 19.79% | 22.86% | |
| Second burst | RMS 2 | (mV) | - | 45.69% | 16.33% | 50.54% | 17.21% | 33.84% |
| D 3 | (s) | - | 13.74% | 14.22% | 15.62% | 13.70% | 17.17% | |
| A 1 | (mV) | - | - | - | - | - | 17.47% | |
| Third burst | RMS 2 | (mV) | - | - | - | - | - | 35.61% |
| D 3 | (s) | - | - | - | - | - | 13.09% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, M.; Domino, M.; Zabielski, R.; Gajewski, Z. Long-Term Recording of Reticulo-Rumen Myoelectrical Activity in Sheep by a Telemetry Method. Animals 2021, 11, 1052. https://doi.org/10.3390/ani11041052
Wierzbicka M, Domino M, Zabielski R, Gajewski Z. Long-Term Recording of Reticulo-Rumen Myoelectrical Activity in Sheep by a Telemetry Method. Animals. 2021; 11(4):1052. https://doi.org/10.3390/ani11041052
Chicago/Turabian StyleWierzbicka, Małgorzata, Małgorzata Domino, Romuald Zabielski, and Zdzisław Gajewski. 2021. "Long-Term Recording of Reticulo-Rumen Myoelectrical Activity in Sheep by a Telemetry Method" Animals 11, no. 4: 1052. https://doi.org/10.3390/ani11041052
APA StyleWierzbicka, M., Domino, M., Zabielski, R., & Gajewski, Z. (2021). Long-Term Recording of Reticulo-Rumen Myoelectrical Activity in Sheep by a Telemetry Method. Animals, 11(4), 1052. https://doi.org/10.3390/ani11041052






