Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemical Analysis
2.2. Statistical Analysis Mann-Whitney
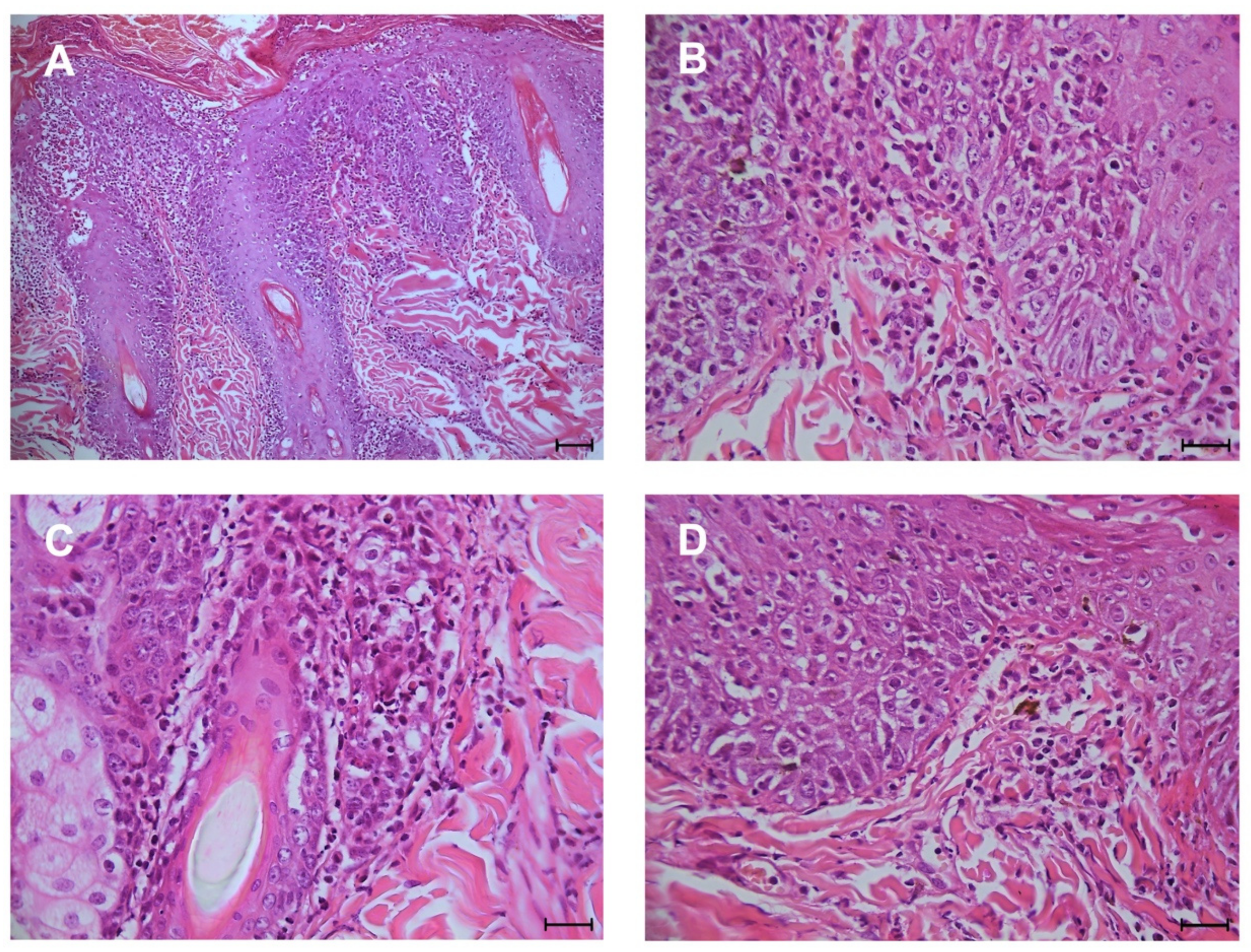
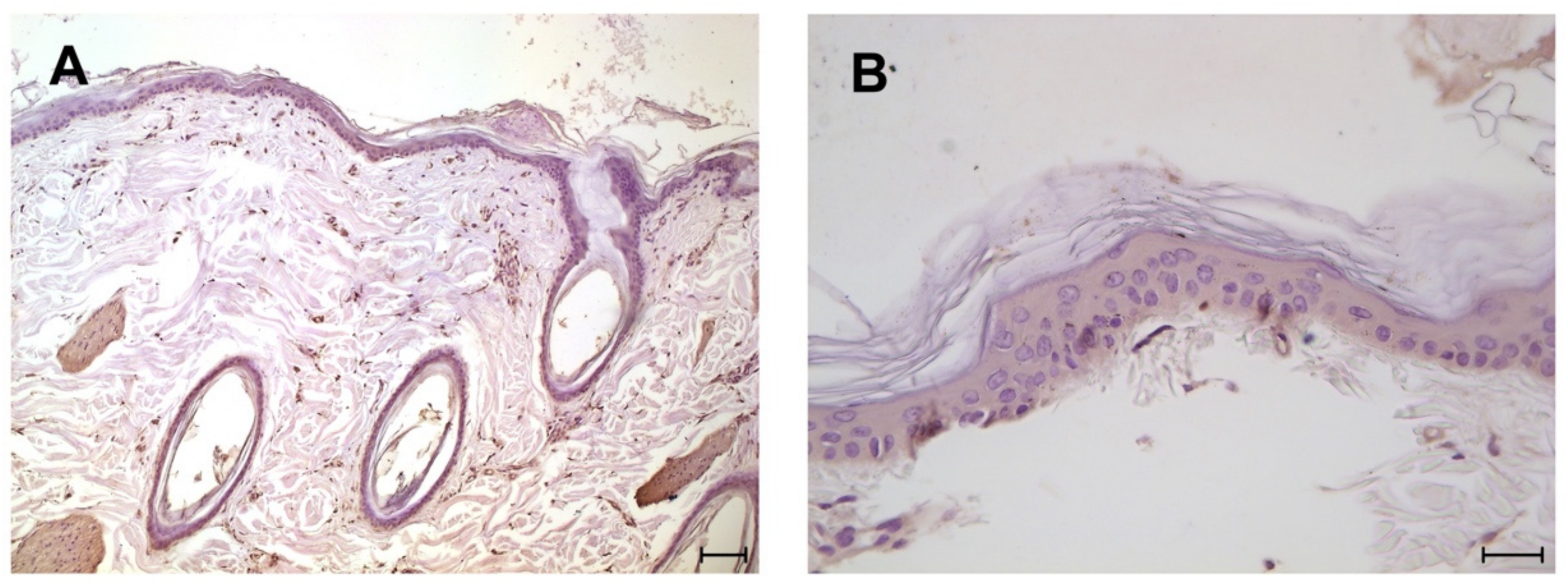
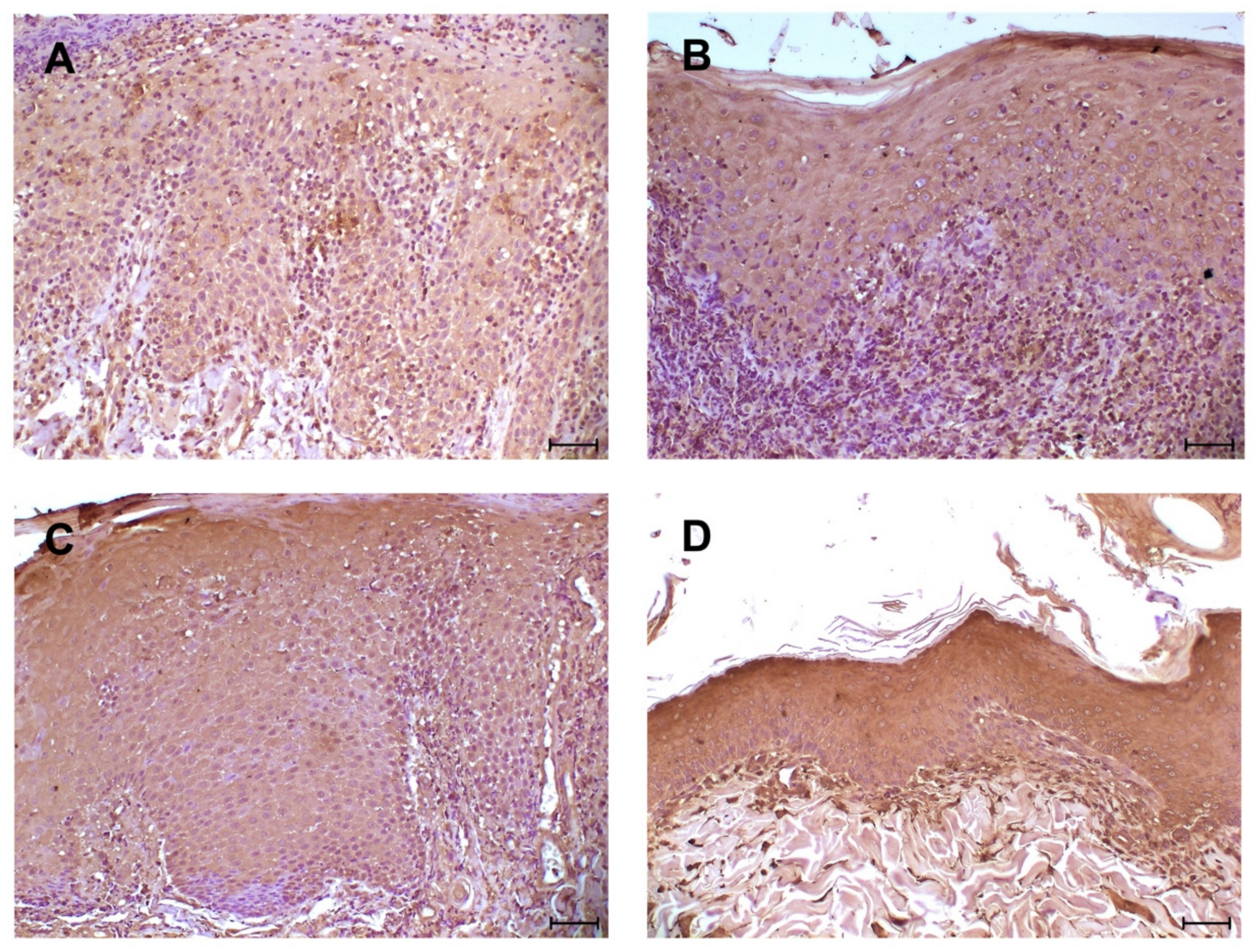
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartzman, R.M.; Orkin, M. A comparative study of canine and human dermatology. AMA Arch. Derm. 1958, 78, 630–636. [Google Scholar] [CrossRef] [PubMed]
- McGavin, M.D.; Carlton, W.; Zachary, J.F.; Thomson, R.G. Thomson’s Special Veterinary Pathology; Mosby: St. Louis, MO, USA, 2000. [Google Scholar]
- Sun, L.; Liu, W.; Zhang, L.J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Lichtman, A.; Pillai, S. Cellular and Molecular Immunology; Saunders: Philadelphia, PA, USA, 2014; p. 544. [Google Scholar]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Lee Gross, T.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Perivascular Diseases of the Dermis. Skin Dis. Dog Cat 2005, 199–237. [Google Scholar] [CrossRef]
- Gilliam, J.N.; Sontheimer, R.D. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J. Am. Acad. Dermatol. 1981, 4, 471–475. [Google Scholar] [CrossRef]
- Griffin, C.E.; Stannard, A.A.; Ihrke, P.J.; Ardans, A.A.; Cello, R.M.; Bjorling, D.R. Canine discoid lupus erythematosus. Vet. Immunol. Immunopathol. 1979, 1, 79–87. [Google Scholar] [CrossRef]
- Banovic, F.; Linder, K.E.; Uri, M.; Rossi, M.A.; Olivry, T. Clinical and microscopic features of generalized discoid lupus erythematosus in dogs (10 cases). Vet. Derm. 2016, 27, 488-e131. [Google Scholar] [CrossRef]
- Olivry, T.; Linder, K.E.; Banovic, F. Cutaneous lupus erythematosus in dogs: A comprehensive review. BMC Vet. Res. 2018, 14, 132. [Google Scholar] [CrossRef]
- De Lucia, M.; Mezzalira, G.; Bardagi, M.; Fondevila, D.M.; Fabbri, E.; Fondati, A. A retrospective study comparing histopathological and immunopathological features of nasal planum dermatitis in 20 dogs with discoid lupus erythematosus or leishmaniosis. Vet. Dermatol. 2017, 28, 200-e246. [Google Scholar] [CrossRef]
- Messinger, L.; Strauss, T.; Jonas, L. A Randomized, Double-Blinded Placebo Controlled Crossover Study Evaluating 0.03% Tacrolimus Ointment Monotherapy in the Treatment of Discoid Lupus Erythematosus in Dogs. SOJ Vet. Sci. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Mariotti, F.; Magi, G.E.; Gavazza, A.; Vincenzetti, S.; Komissarov, A.; Shneider, A.; Venanzi, F.M. p62/SQSTM1 expression in canine mammary tumours: Evolutionary notes. Vet. Comp. Oncol. 2019, 17, 570–577. [Google Scholar] [CrossRef]
- Richez, C.; Blanco, P.; Rifkin, I.; Moreau, J.F.; Schaeverbeke, T. Role for toll-like receptors in autoimmune disease: The example of systemic lupus erythematosus. Jt. Bone Spine 2011, 78, 124–130. [Google Scholar] [CrossRef]
- Elloumi, N.; Fakhfakh, R.; Ayadi, L.; Sellami, K.; Abida, O.; Ben Jmaa, M.; Sellami, T.; Kammoun, K.; Masmoudi, H. The Increased Expression of Toll-Like Receptor 4 in Renal and Skin Lesions in Lupus Erythematosus. J. Histochem. Cytochem. 2017, 65, 389–398. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Wassef, A.; Janardhan, K.; Pearce, J.W.; Singh, B. Toll-like receptor 4 in normal and inflamed lungs and other organs of pig, dog and cattle. Histol. Histopathol. 2004, 19, 1201–1208. [Google Scholar] [CrossRef]
- Czapski, G.A.; Zhao, Y.; Lukiw, W.J.; Strosznajder, J.B. Acute Systemic Inflammatory Response Alters Transcription Profile of Genes Related to Immune Response and Ca(2+) Homeostasis in Hippocampus; Relevance to Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 7838. [Google Scholar] [CrossRef]
- Blair, J.A.; Wang, C.; Hernandez, D.; Siedlak, S.L.; Rodgers, M.S.; Achar, R.K.; Fahmy, L.M.; Torres, S.L.; Petersen, R.B.; Zhu, X.; et al. Individual Case Analysis of Postmortem Interval Time on Brain Tissue Preservation. PLoS ONE 2016, 11, e0151615. [Google Scholar] [CrossRef]
- Kollisch, G.; Kalali, B.N.; Voelcker, V.; Wallich, R.; Behrendt, H.; Ring, J.; Bauer, S.; Jakob, T.; Mempel, M.; Ollert, M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005, 114, 531–541. [Google Scholar] [CrossRef]
- Panzer, R.; Blobel, C.; Folster-Holst, R.; Proksch, E. TLR2 and TLR4 expression in atopic dermatitis, contact dermatitis and psoriasis. Exp. Dermatol. 2014, 23, 364–366. [Google Scholar] [CrossRef]
- Abida, O.; Bouzid, D.; Krichen-Makni, S.; Kharrat, N.; Masmoudi, A.; Abdelmoula, M.; Ben Ayed, M.; Turki, H.; Sellami-Boudawara, T.; Masmoudi, H. Potential role of TLR ligand in aethiopathogenesis of Tunisian endemic pemphigus foliaceus. Biochem. Physiol. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Begon, E.; Michel, L.; Flageul, B.; Beaudoin, I.; Jean-Louis, F.; Bachelez, H.; Dubertret, L.; Musette, P. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur. J. Dermatol. 2007, 17, 497–506. [Google Scholar] [CrossRef]
- Hayashi, F.; Means, T.K.; Luster, A.D. Toll-like receptors stimulate human neutrophil function. Blood 2003, 102, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liang, Y.; Shao, F.; Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 20054–20059. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Zhou, M.; Wang, S.; Huang, Y.; Hu, H.; Zhao, J.; Gaskin, F.; Yang, N.; Fu, S.M. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 2019, 103, 102286. [Google Scholar] [CrossRef]
- Lauffer, F.; Jargosch, M.; Krause, L.; Garzorz-Stark, N.; Franz, R.; Roenneberg, S.; Bohner, A.; Mueller, N.S.; Theis, F.J.; Schmidt-Weber, C.B.; et al. Type I Immune Response Induces Keratinocyte Necroptosis and Is Associated with Interface Dermatitis. J. Investig. Dermatol. 2018, 138, 1785–1794. [Google Scholar] [CrossRef]
| Case N° | Sex | Age (Years) | Breed | Leishmania Elisa-Test | Ana-Test |
|---|---|---|---|---|---|
| 1 | Female | 5 | German Shepperd | N | N |
| 2 | Male | 10 | Cocker | N | N |
| 3 | Male | 2 | Akita Inu | N | N |
| 4 | Male | 2 | Maremma Shepperd | N | N |
| 5 | Male | 7 | Crossbred | N | N |
| 6 | Female | 4 | Collie | N | N |
| 7 | Male | 3 | Collie | N | N |
| 8 | Female | 4 | Retriever | N | N |
| 9 | Female | 4 | Crossbred | N | N |
| 10 | Female | 2 | American Akita | N | N |
| 11 | Male | 3 | German Shepperd | N | N |
| 12 | Male | 2 | Labrador | N | N |
| 13 | Female | 6 | Crossbred | N | N |
| 14 | Male | 5 | Pinscher | N | N |
| 15 | Male | 5 | German Shepperd | N | N |
| 16 | Male | 7 | Golden Retriever | N | N |
| Mean Score | Minimum Score | Maximum Score | SD | % of Positive Cases | |
|---|---|---|---|---|---|
| DLE (n = 16) | 11.25 **** | 8 | 12 | 1.6 | 100 (16/16) |
| Normal skin (n = 8) | 4.5 | 4 | 6 | 1 | 100 (8/8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cerbo, A.; Giusti, S.; Mariotti, F.; Spaterna, A.; Rossi, G.; Magi, G.E. Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE). Animals 2021, 11, 1044. https://doi.org/10.3390/ani11041044
Di Cerbo A, Giusti S, Mariotti F, Spaterna A, Rossi G, Magi GE. Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE). Animals. 2021; 11(4):1044. https://doi.org/10.3390/ani11041044
Chicago/Turabian StyleDi Cerbo, Alessandro, Sara Giusti, Francesca Mariotti, Andrea Spaterna, Giacomo Rossi, and Gian Enrico Magi. 2021. "Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE)" Animals 11, no. 4: 1044. https://doi.org/10.3390/ani11041044
APA StyleDi Cerbo, A., Giusti, S., Mariotti, F., Spaterna, A., Rossi, G., & Magi, G. E. (2021). Increased Expression of Toll-Like Receptor 4 in Skin of Dogs with Discoid Lupus Erythematous (DLE). Animals, 11(4), 1044. https://doi.org/10.3390/ani11041044








