CT Findings and Histological Evaluation of Red Foxes (Vulpes vulpes) with Chronic Head Trauma Injury: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical Examination
2.3. CT Examination
2.4. Therapy
2.5. Outcome
2.6. Gross Anatomy and Histological Findings
2.7. Statistical Analysis
3. Results
3.1. Population
3.2. Clinical Findings
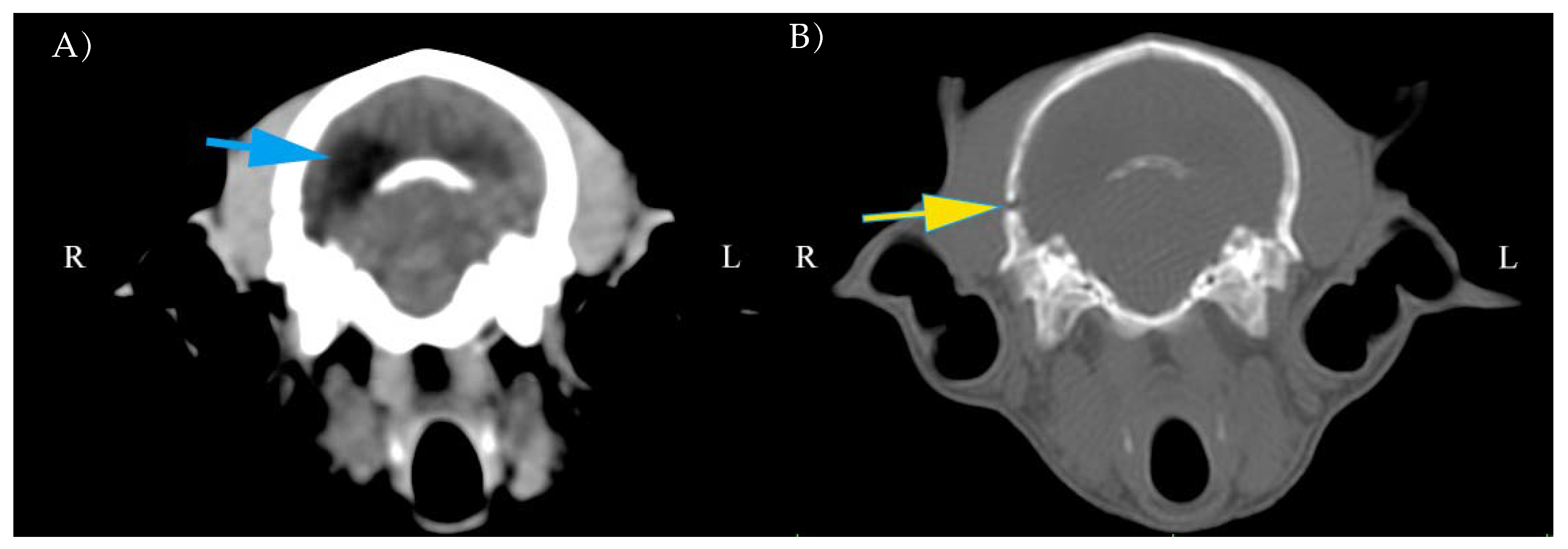
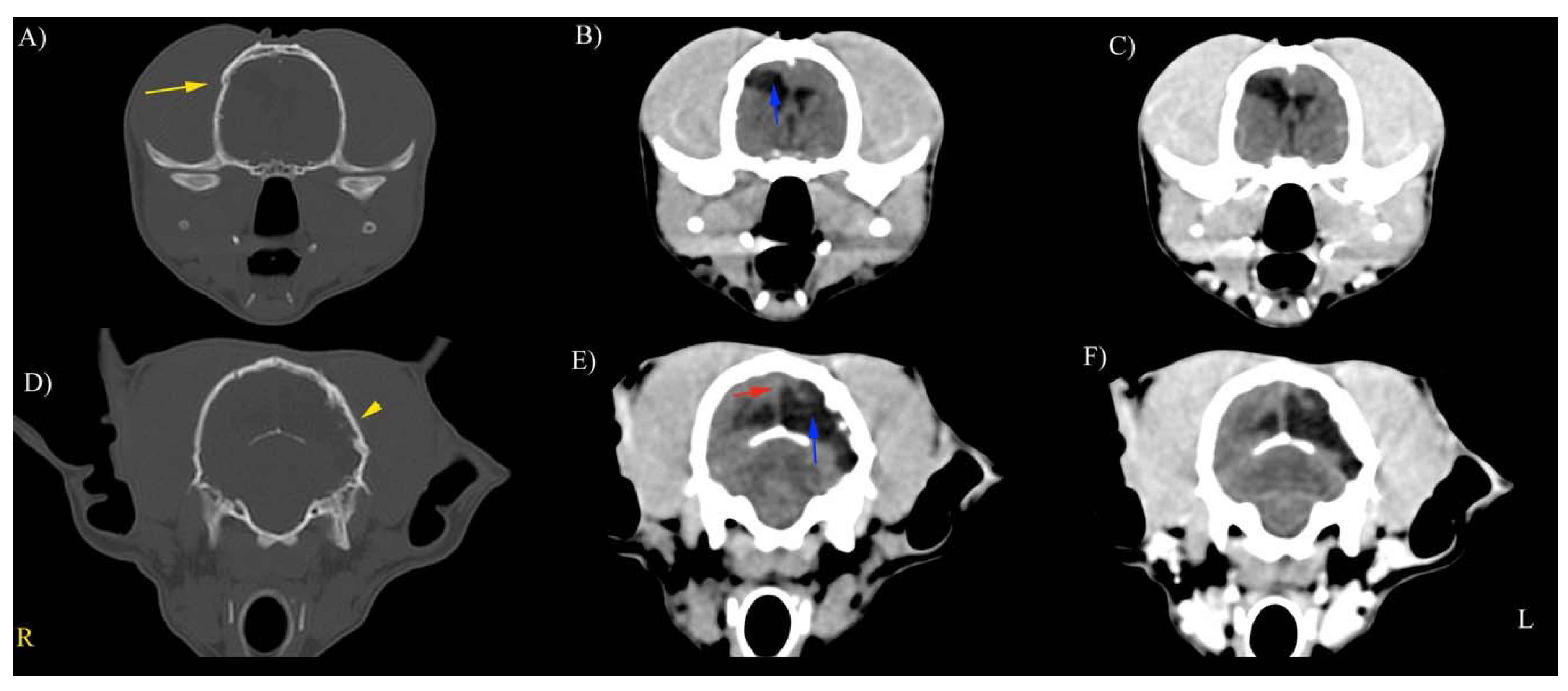
3.3. CT Findings
3.4. Outcome and Survival
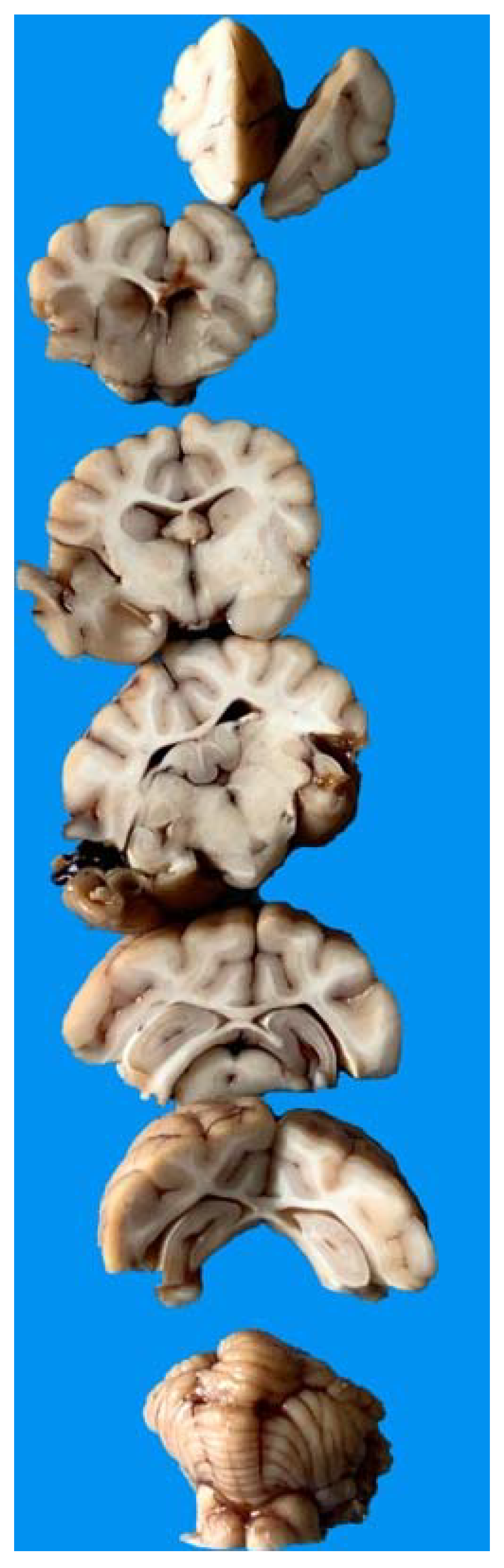
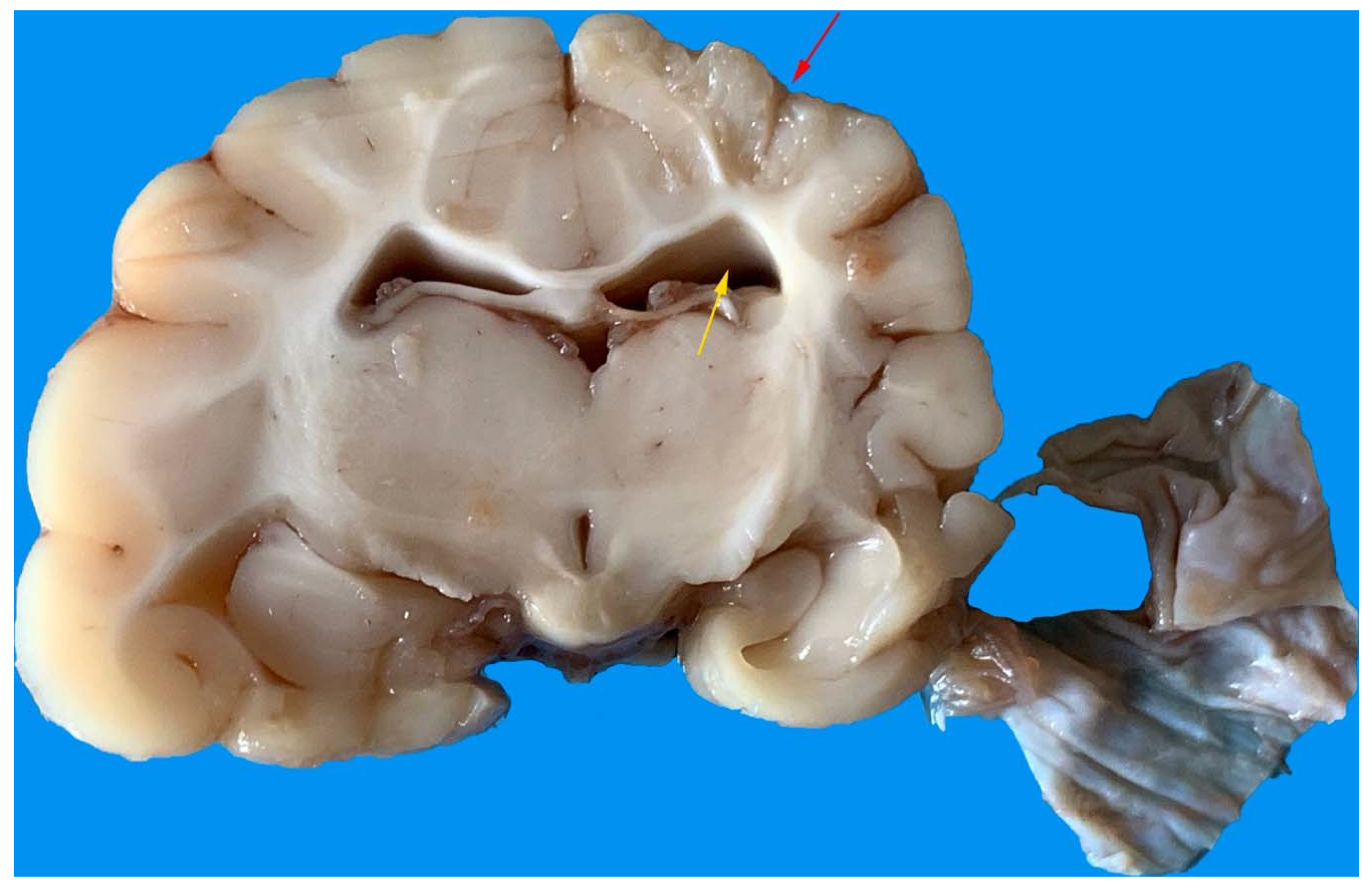
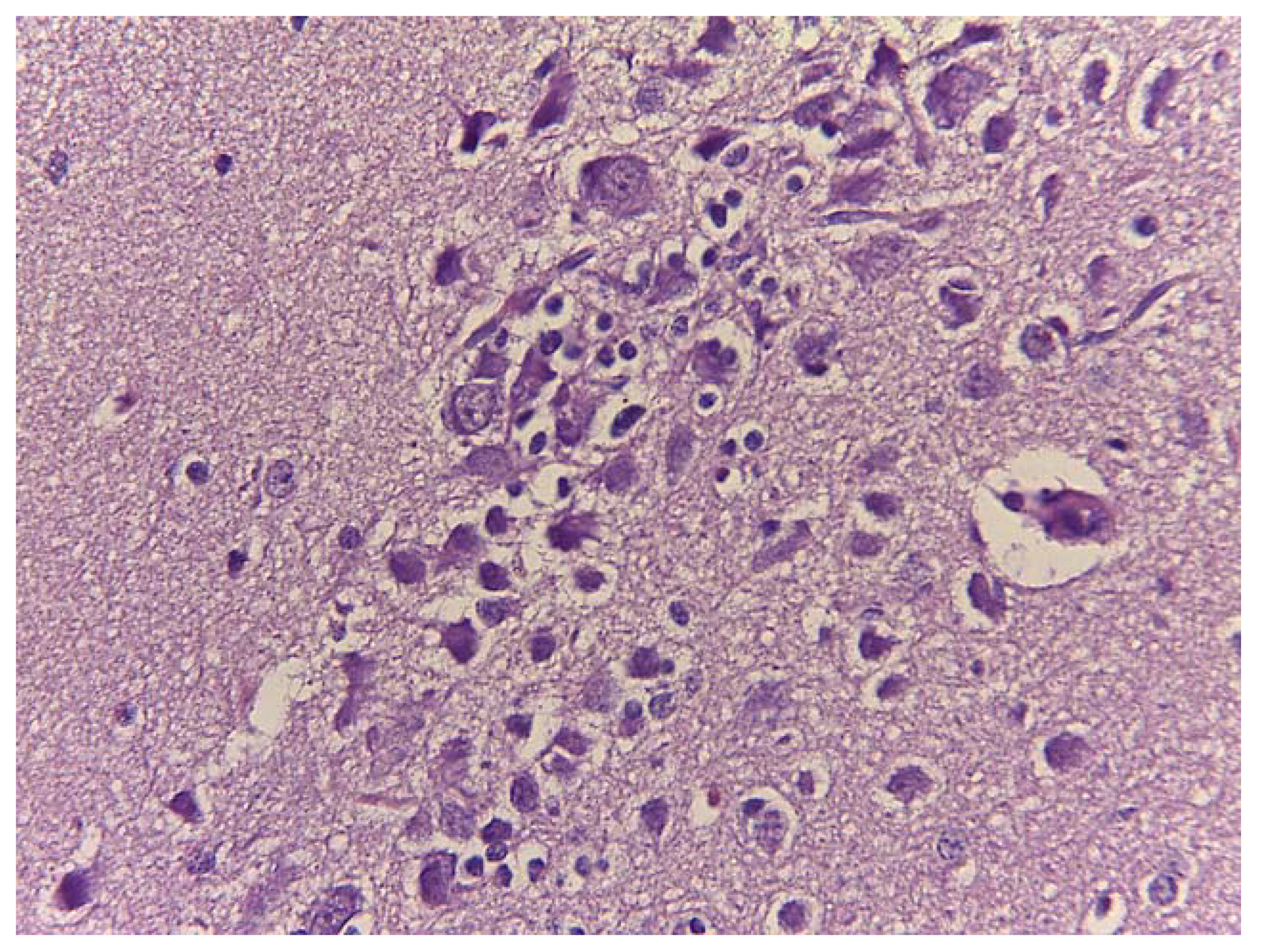
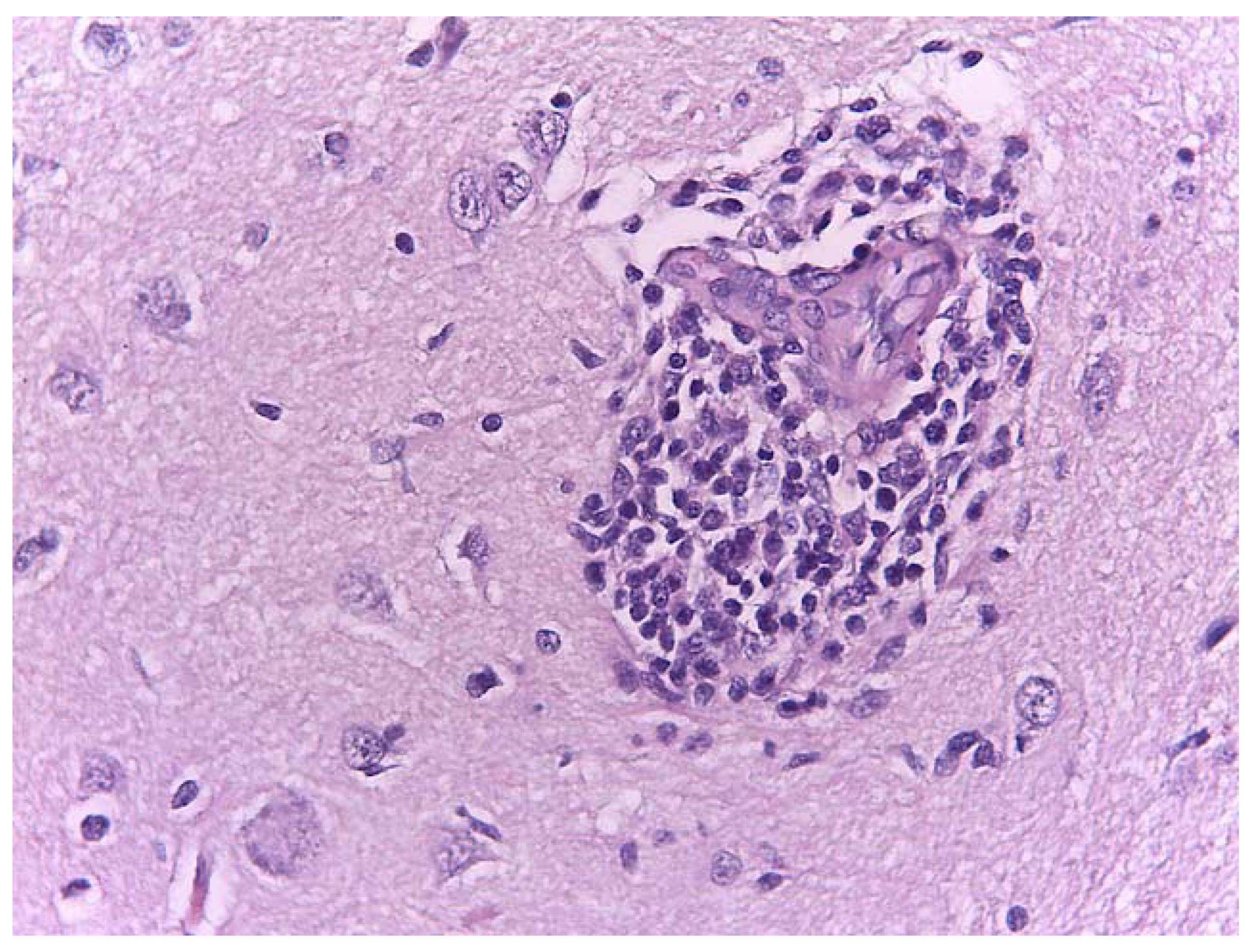
3.5. Gross Anatomy and Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, P.J.; Dowding, C.V.; Molony, S.E.; White, P.C.L.; Harris, S. Activity patterns of urban red foxes (Vulpes vulpes) reduce the risk of traffic-induced mortality. Behav. Ecol. 2007, 18, 716–724. [Google Scholar] [CrossRef]
- Harris, S.; Smith, G.C. Demography of Two Urban Fox (Vulpes vulpes) Populations. J. Appl. Ecol. 1987, 24, 75–86. [Google Scholar] [CrossRef]
- Atkinson, M.W. New perspectives on wildlife rehabilitation. Zoo Biol. 1997, 16, 355–357. [Google Scholar] [CrossRef]
- Seiler, A.; Helldin, J.-O.; Seiler, C. Road mortality in Swedish mammals: Results of a drivers’ questionnaire. Wildl. Biol. 2004, 10, 225–233, 229. [Google Scholar] [CrossRef]
- Kuo, K.W.; Bacek, L.M.; Taylor, A.R. Head Trauma. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 111–128. [Google Scholar] [CrossRef]
- Simpson, S.A.; Syring, R.; Otto, C.M. Severe blunt trauma in dogs: 235 cases (1997–2003). J. Vet. Emerg. Crit. Care 2009, 19, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.O.; Caldas, G.G.; Santos, C.R.O.; Junior, D.B. Traumatic brain injury in dogs and cats: A systematic review. Vet. Med. 2018, 63, 345–357. [Google Scholar] [CrossRef]
- O’Connor, W.T.; Smyth, A.; Gilchrist, M.D. Animal models of traumatic brain injury: A critical evaluation. Pharmacol. Ther. 2011, 130, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.H. The effect of associated injuries, blood loss, and oxygen debt on death and disability in blunt traumatic brain injury: The need for early physiologic predictors of severity. J. Neurotrauma 1995, 12, 579–590. [Google Scholar] [CrossRef]
- Sande, A.; West, C. Traumatic brain injury: A review of pathophysiology and management. J. Vet. Emerg. Crit. Care 2010, 20, 177–190. [Google Scholar] [CrossRef]
- Davies, E.S.; Volk, H.A.; Behr, S.; Summers, B.; de Lahunta, A.; Syme, H.; Jull, P.; Garosi, L. Porencephaly and hydranencephaly in six dogs. Vet. Rec. 2012, 170, 179. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Klumpp, S.; Amort, K.; Jawinski, S.; Kramer, M. Porencephaly in dogs and cats: Magnetic resonance imaging findings and clinical signs. Vet. Radiol. Ultrasound 2012, 53, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.; Matiasek, K.; Henke, D.; Grevel, V. Cerebellar cortical degeneration with selective granule cell loss in Bavarian mountain dogs. J. Small Anim. Pr. 2007, 48, 462–465. [Google Scholar] [CrossRef]
- Machado, G.F.; Laranjeira, M.G.; Schweigert, A.; de Melo, G.D. Porencephaly and cortical dysplasia as cause of seizures in a dog. BMC Vet. Res. 2012, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.; Gernone, F.; Simone, A.; Giannuzzi, P. Central vestibular syndrome in a red fox (Vulpes vulpes) with presumptive right caudal cerebral artery ischemic infarct and prevalent midbrain involvement. Open Vet. J. 2017, 7, 197–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Platt, S.R.; Radaelli, S.T.; McDonnell, J.J. The prognostic value of the modified Glasgow Coma Scale in head trauma in dogs. J. Vet. Intern. Med. 2001, 15, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Ash, K.; Hayes, G.M.; Goggs, R.; Sumner, J.P. Performance evaluation and validation of the animal trauma triage score and modified Glasgow Coma Scale with suggested category adjustment in dogs: A VetCOT registry study. J. Vet. Emerg. Crit. Care 2018, 28, 192–200. [Google Scholar] [CrossRef]
- Chai, O.; Peery, D.; Bdolah-Abram, T.; Moscovich, E.; Kelmer, E.; Klainbart, S.; Milgram, J.; Shamir, M.H. Computed tomographic findings in dogs with head trauma and development of a novel prognostic computed tomography-based scoring system. Am. J. Vet. Res. 2017, 78, 1085–1090. [Google Scholar] [CrossRef]
- Platt, S.R.; Radaelli, S.T.; McDonnell, J.J. Computed tomography after mild head trauma in dogs. Vet. Rec. 2002, 151, 243. [Google Scholar] [CrossRef]
- Hodgson, N.; Walters, A.; Lawson, C.; Hague, D.; Joslyn, S.; McMichael, M. Acute surgical intervention for a depressed skull fracture causing a laceration to the brain parenchyma from a bite wound in a dog. Can. Vet. J. 2018, 59, 31–35. [Google Scholar]
- Nykamp, S.; Scrivani, P.; DeLahunta, A.; Yu-Speight, A.; Riis, R. Chronic subdural hematomas and hydrocephalus in a dog. Vet. Radiol. Ultrasound 2001, 42, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Gielen, I.; Kromhout, K.; Gavin, P.; Van Ham, L.; Polis, I.; van Bree, H. Agreement between low-field MRI and CT for the detection of suspected intracranial lesions in dogs and cats. J. Am. Vet. Med. Assoc. 2013, 243, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Di, G.; Shao, X.; Zhou, W.; Jiang, X. Predictors Associated With Post-Traumatic Hydrocephalus in Patients With Head Injury Undergoing Unilateral Decompressive Craniectomy. Front. Neurol. 2018, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.R.; Galbraith, S. Posttraumatic hydrocephalus—A retrospective review. Surg. Neurol. 1985, 23, 261–264. [Google Scholar] [CrossRef]
- De Lahunta, A. Cerebrospinal fluid and hydrocephalus. In Veterinary Neuroanatomy and Clinical Neurology; De Lahunta, A., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1983; pp. 30–52. [Google Scholar]
- Thomas, W.B. Hydrocephalus in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 143–159. [Google Scholar] [CrossRef]
- Marmarou, A.; Foda, M.A.A.-E.; Bandoh, K.; Yoshihara, M.; Yamamoto, T.; Tsuji, O.; Young, H.F. Posttraumatic ventriculomegaly: Hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J. Neurosurg. 1996, 85, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- MacKillop, E. Magnetic resonance imaging of intracranial malformations in dogs and cats. Vet. Radiol. Ultrasound 2011, 52, S42–S51. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chen, Y.-F.; Xiao, F. Brain Midline Shift Measurement and Its Automation: A Review of Techniques and Algorithms. Int. J. Biomed. Imaging 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, K.; Mateo, I.; Peery, D.; Mazaki-Tovi, M.; Klainbart, S.; Kelmer, E.; Ruggeri, M.; Shamir, M.H.; Chai, O. The prognostic value of the Koret CT score in dogs following traumatic brain injury. Vet. J. 2020, 266, 105563. [Google Scholar] [CrossRef] [PubMed]



| Mental Status | ||||
|---|---|---|---|---|
| Unremarkable | Depressed | Delirium | Stupor or Semi-Comatose | Comatose |
| 1 | 0 | 2 | 2 | 4 |
| Mild | Moderate | Severe | |
|---|---|---|---|
| Cranial vault fractures (depression) | 3 (50%) | 1 (17%) | 2 (33%) |
| Abnormalities of the parenchyma | |||
| Mild | Moderate | Severe | |
| Cerebral hemisphere or cerebellum as a single lesion | 3 (50%) | 2 (33%) | 1 (17%) |
| Multiple lesions | 2 (33%) | 3 (50%) | 0 |
| Intracranial hemorrhage | |||
| Mild | Moderate | Severe | |
| Intra-axial | 3 (50%) | 3 (50%) | 0 |
| Extra-axial | 2 (33%) | 1 (17%) | 1 (17%) |
| Other findings | |||
| Midline shift | 2 (33%) | 1 (17%) | 0 |
| Lateral ventricle asymmetry | 2 (33%) | 1 (17%) | 2 (33%) |
| Hydrocephalus | 1 (33%) | 1 (17%) | 2 (33%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacitignola, L.; Samarelli, R.; Zizzo, N.; Circella, E.; Acquafredda, C.; Stabile, M.; Lombardi, R.; Staffieri, F.; Camarda, A. CT Findings and Histological Evaluation of Red Foxes (Vulpes vulpes) with Chronic Head Trauma Injury: A Retrospective Study. Animals 2021, 11, 1010. https://doi.org/10.3390/ani11041010
Lacitignola L, Samarelli R, Zizzo N, Circella E, Acquafredda C, Stabile M, Lombardi R, Staffieri F, Camarda A. CT Findings and Histological Evaluation of Red Foxes (Vulpes vulpes) with Chronic Head Trauma Injury: A Retrospective Study. Animals. 2021; 11(4):1010. https://doi.org/10.3390/ani11041010
Chicago/Turabian StyleLacitignola, Luca, Rossella Samarelli, Nicola Zizzo, Elena Circella, Claudia Acquafredda, Marzia Stabile, Roberto Lombardi, Francesco Staffieri, and Antonio Camarda. 2021. "CT Findings and Histological Evaluation of Red Foxes (Vulpes vulpes) with Chronic Head Trauma Injury: A Retrospective Study" Animals 11, no. 4: 1010. https://doi.org/10.3390/ani11041010
APA StyleLacitignola, L., Samarelli, R., Zizzo, N., Circella, E., Acquafredda, C., Stabile, M., Lombardi, R., Staffieri, F., & Camarda, A. (2021). CT Findings and Histological Evaluation of Red Foxes (Vulpes vulpes) with Chronic Head Trauma Injury: A Retrospective Study. Animals, 11(4), 1010. https://doi.org/10.3390/ani11041010









