Commensal Escherichia coli Antimicrobial Resistance and Multidrug-Resistance Dynamics during Broiler Growing Period: Commercial vs. Improved Farm Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. E. coli Isolation
2.4. Antimicrobial Susceptibility Testing
2.5. Statistical Analysis
3. Results
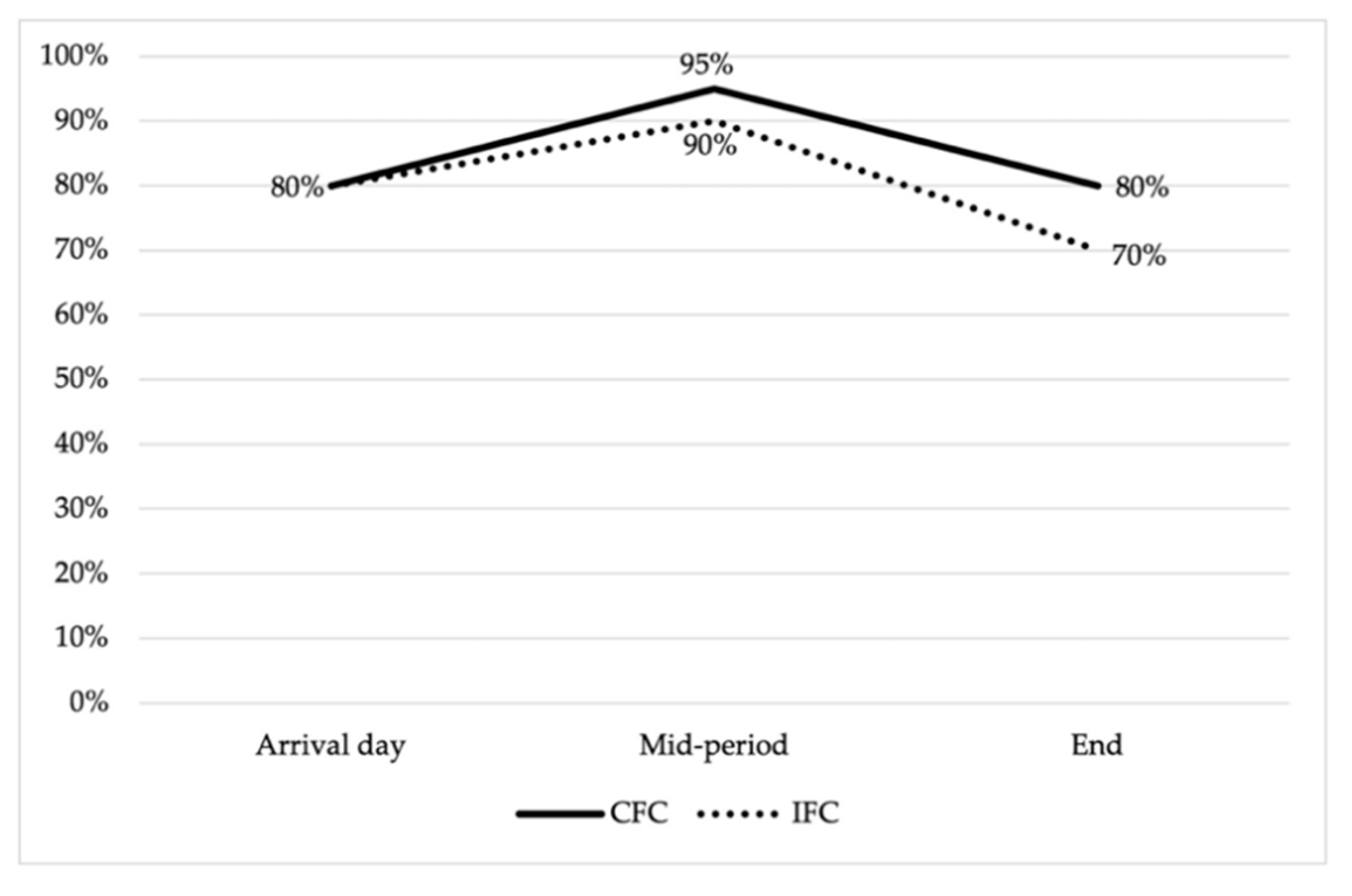
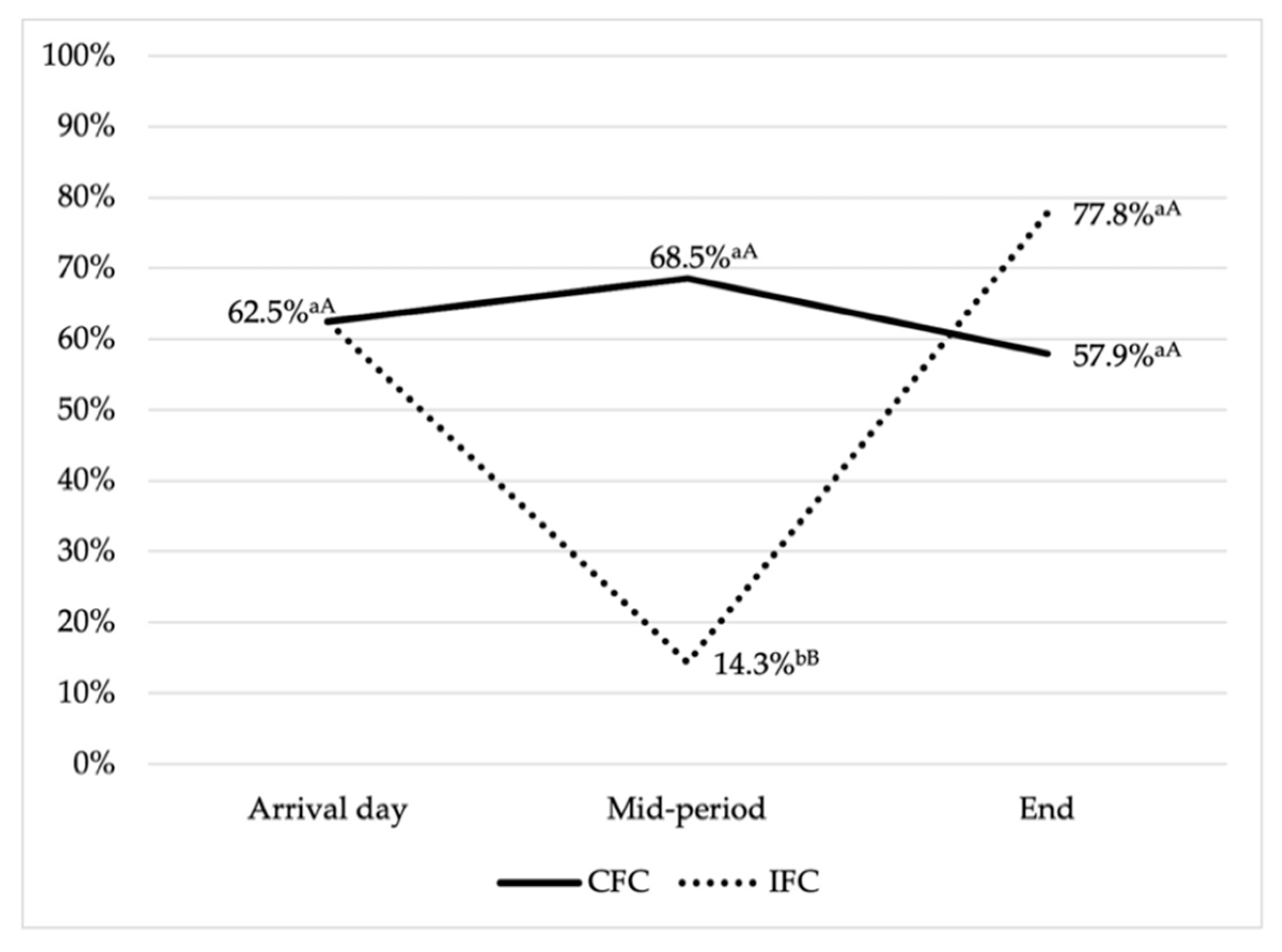
3.1. Prevalence of Antimicrobial Resistance and Multidrug Resistance
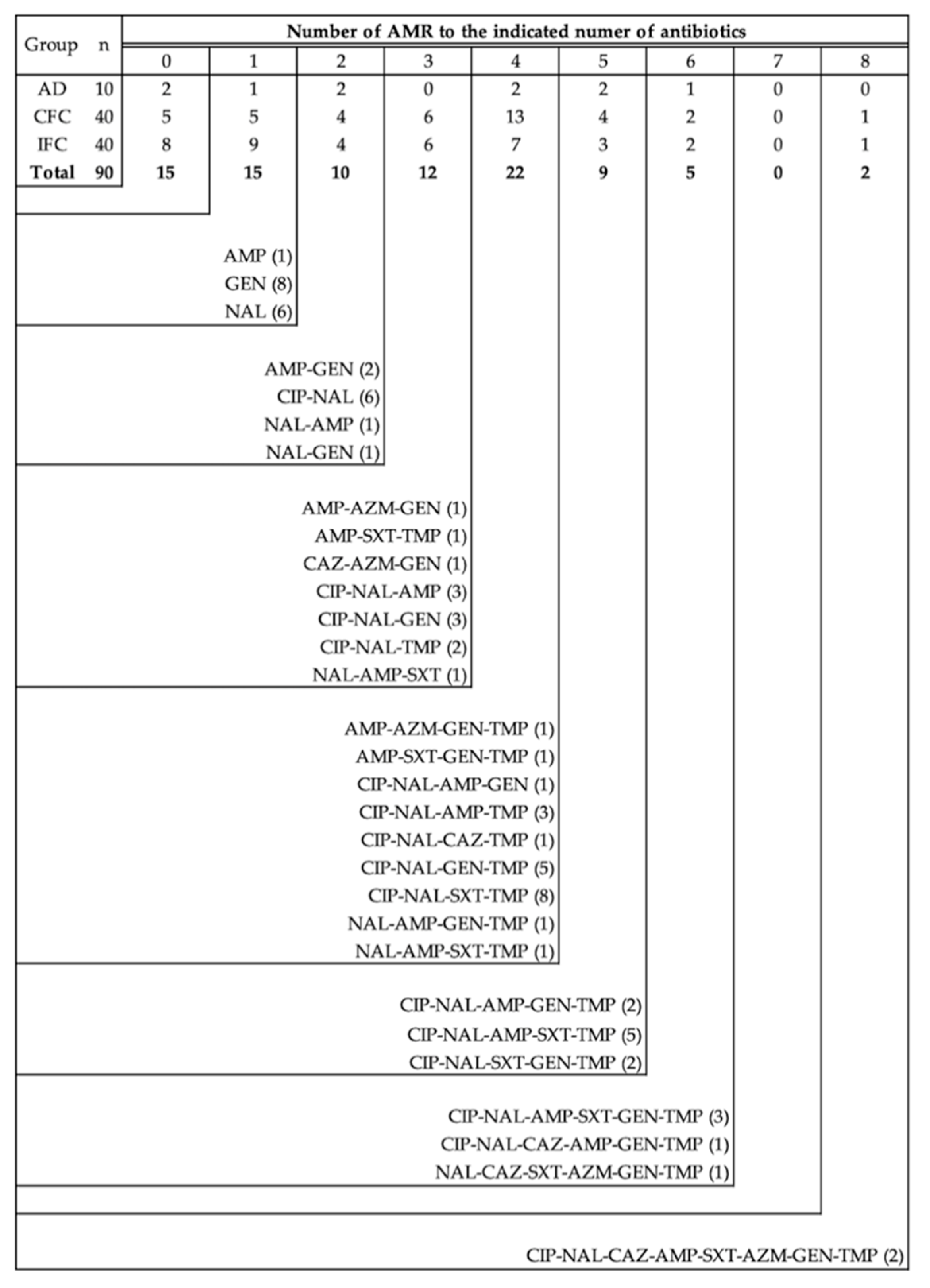
3.2. Antimicrobial Resistance Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- Horigan, V.; Kosmider, R.D.; Horton, R.A.; Randall, L.; Simons, R.R.L. An assessment of evidence data gaps in the investigation of possible transmission routes of extended spectrum β-lactamase producing Escherichia coli from livestock to humans in the UK. Prev. Vet. Med. 2016, 124, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Sharma, C.; Rokana, N.; Chandra, M.; Singh, B.P.; Gulhane, R.D.; Gill, J.P.S.; Ray, P.; Puniya, A.K.; Panwar, H. Antimicrobial resistance: Its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 2018, 4, 237. [Google Scholar] [CrossRef]
- ESVAC (European Medicines Agency). European Database of Sales of Veterinary Antimicrobial Agents. Available online: https://esvacbi.ema.europa.eu/analytics/saw.dll?PortalPages (accessed on 22 January 2021).
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Khurana, A.; Sinha, R.; Nagaraju, M. Antibiotic Resistance in Poultry Environment. Spread of Resistance from Poultry Farm to Agricultural Field. Cent. Sci. Environ. 2017, 1–36. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Apostolakos, I.; Piccirillo, A. A review on the current situation and challenges of colistin resitance in poultry production. Avian Pathol. 2018, 47, 546–558. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). In Trends from 2010 to 2018 Tenth ESVAC Report; European Medicines Agency: Amsterdam, The Netherlands, 2020; pp. 1–104. [Google Scholar]
- PRAN (Plan Nacional de Resistencia Antibióticos). Informe Anual PRAN Junio 2019—Junio. 2020. Available online: https://resistenciaantibioticos.es/es/publicaciones/informe-anual-2019-2020-plan-nacional-frente-la-resistencia-los-antibioticos (accessed on 10 September 2020). (In Spanish).
- Rojo-Gimeno, C.; Postma, M.; Dewulf, J.; Hogeveen, H.; Lauwers, L.; Wauters, E. Farm-economic analysis of reducing antimicrobial use whilst adopting improved management strategies on farrow-to-finish pig farms. Prev. Vet. Med. 2016, 129, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.V.S.; Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Baskeville, E.; Akamine, A.T.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathol. 2014, 43, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Callaway, T.R.; Kogut, M.H.; Piva, A.; Grilli, E. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms 2019, 7, 65. [Google Scholar] [CrossRef]
- Soleimani, A.F.; Zulkifli, I.; Hair-Bejo, M.; Ebrahimi, M.; Jazayeri, S.D.; Hashemi, S.R.; Meimandipour, A.; Goh, Y.M. Epigenetic modification: Possible approach to reduce Salmonella enterica serovar enteritidis susceptibility under stress conditions. Avian Pathol. 2012, 41, 351–354. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Villagra, A.; Sevilla-Navarro, S.; Pérez-Gracia, M.T.; Vega, S.; Marin, C. The dynamic of antibiotic resistance in commensal Escherichia coli throughout the growing period in broiler chickens: Fast-growing vs. slow-growing breeds. Poult. Sci. 2020, 99, 1591–1597. [Google Scholar] [CrossRef]
- Gocsik, É.; Brooshooft, S.D.; de Jong, I.C.; Saatkamp, H.W. Cost-efficiency of animal welfare in broiler production systems: A pilot study using the Welfare Quality® assessment protocol. Agric. Syst. 2016, 146, 55–69. [Google Scholar] [CrossRef]
- El-Deek, A.; El-Sabrout, K. Behaviour and meat quality of chicken under different housing systems. World Poult. Sci. J. 2019, 75, 105–114. [Google Scholar] [CrossRef]
- Sirri, F.; Castellini, C.; Bianchi, M.; Petracci, M.; Meluzzi, A.; Franchini, A. Effect of fast-, medium- and slow-growing strains on meat quality of chickens reared under the organic farming method. Animal 2011, 91, 312–319. [Google Scholar] [CrossRef]
- Lusk, J.L. Consumer preferences for and beliefs about slow growth chicken. Poult. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- RD (Royal Degree). Spain. Royal Degree 53/2013, 1st of February, Por el Que se Establecen las Normas Básicas Aplicables Para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. 2013. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2013-1337 (accessed on 28 August 2020). (In Spanish).
- Kersia Group. Pig & Poultry Farming. Available online: https://www.kersia-group.com/activities/pig-poultry-farming/ (accessed on 20 March 2021).
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- EC (European Commission). Commission Implementing Decission, of 12 November 2013, on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria (2013/652/EU). 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013D0652 (accessed on 20 August 2020).
- Aviagen Ross 308: Broiler Performance Objectives; Aviagen Inc.: Huntsville, AL, USA, 2019; pp. 1–15.
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; Elmougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; et al. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: A risk to public health and food safety. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Marin, C.; Sevilla-Navarro, S.; Lonjedo, R.; Catalá-Gregori, P.; Ferrús, M.A.; Vega, S.; Jiménez-Belenguer, A. Genotyping and molecular characterization of antimicrobial resistance in thermophilic Campylobacter isolated from poultry breeders and their progeny in Eastern Spain. Poult. Sci. 2020, 99, 5096–5104. [Google Scholar] [CrossRef]
- Oikarainen, P.E.; Pohjola, L.K.; Pietola, E.S.; Heikinheimo, A. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet. Microbiol. 2019, 231, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, L.L.; Thøfner, I.; Bisgaard, M.; Christensen, J.P.; Olsen, R.H.; Christensen, H. Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Vet. Microbiol. 2017, 207, 13–18. [Google Scholar] [CrossRef]
- Dame-Korevaar, A.; Fischer, E.A.J.; van der Goot, J.; Velkers, F.; van den Broek, J.; Veldman, K.; Ceccarelli, D.; Mevius, D.; Stegeman, A. Effect of challenge dose of plasmid-mediated extended-spectrum β-lactamase and AmpC β-lactamase producing Escherichia coli on time-until-colonization and level of excretion in young broilers. Vet. Microbiol. 2019, 239, 108446. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Börjesson, S.; Landén, A.; Bengtsson, B. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J. Antimicrob. Chemother. 2014, 69, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; Daehre, K.; Roesler, U.; Friese, A. Extended-spectrum-beta-lactamaseand plasmid-encoded cephamycinaseproducing enterobacteria in the broiler hatchery as a potential mode of pseudovertical transmission. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; Daehre, K.; Semmler, T.; Guenther, S.; Roesler, U.; Friese, A. Environmental adaptation and vertical dissemination of ESBL-/pAmpC-producing Escherichia coli in an integrated broiler production chain in the absence of an antibiotic treatment. Microb. Biotechnol. 2018, 11, 1017–1026. [Google Scholar] [CrossRef]
- Dierikx, C.M.; van der Goot, J.A.; Smith, H.E.; Kant, A.; Mevius, D.J. Presence of ESBL/AmpC -Producing Escherichia coli in the Broiler Production Pyramid: A Descriptive Study. PLoS ONE 2013, 8, e79005. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). European Strategic Action Plan on Antibiotic Resistance 2011. Available online: http://www.euro.who.int/__data/assets/pdf_file/0008/147734/wd14E_AntibioticResistance_111380.pdf (accessed on 25 August 2020).
- Davies, R.; Wales, A. Antimicrobial Resistance on Farms: A Review Including Biosecurity and the Potential Role of Disinfectants in Resistance Selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Balasch, S.; Vega, S.; Lainez, M. Sources of Salmonella contamination during broiler production in Eastern Spain. Prev. Vet. Med. 2011, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chuppava, B.; Keller, B.; Abd El-Wahab, A.; Sürie, C.; Visscher, C. Resistance Reservoirs and Multi-Drug Resistance of Commensal Escherichia coli from Excreta and Manure Isolated in Broiler Houses with Different Flooring Designs. Front. Microbiol. 2019, 10, 2633. [Google Scholar] [CrossRef]
- Luiken, R.E.C.; Van Gompel, L.; Bossers, A.; Munk, P.; Joosten, P.; Hansen, R.B.; Knudsen, B.E.; García-Cobos, S.; Dewulf, J.; Aarestrup, F.M.; et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020, 143. [Google Scholar] [CrossRef]
- Carrique-Mas, J.J.; Marín, C.; Breslin, M.; McLaren, I.; Davies, R. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathol. 2009, 38, 419–424. [Google Scholar] [CrossRef]
- Maertens, H.; De Reu, K.; Meyer, E.; Van Coillie, E.; Dewulf, J. Limited association between disinfectant use and either antibiotic or disinfectant susceptibility of Escherichia coli in both poultry and pig husbandry. BMC Vet. Res. 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef]
- Dawkins, M.S. Animal welfare as preventative medicine. Anim. Welf. 2019, 28, 137–141. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Mandal, R.K.; Jiang, T.; Wideman, R.F.; Lohrmann, T.; Kwon, Y.M. Microbiota Analysis of Chickens Raised Under Stressed Conditions. Front. Vet. Sci. 2020, 7, 482637. [Google Scholar] [CrossRef] [PubMed]
- Burbarelli, M.F.C.; Merseguel, C.E.B.; Ribeiro, P.A.P.; Lelis, K.D.; Polycarpo, G.V.; Carão, A.C.P.; Bordin, R.A.; Fernandes, A.M.; Souza, R.L.M.; Moro, M.E.G.; et al. The effects of two different cleaning and disinfection programs on broiler performance and microbiological status of broiler houses. Rev. Bras. Cienc. Avic. 2015, 17, 575–580. [Google Scholar] [CrossRef]
- Marin, C.; Lainez, M. Salmonella detection in feces during broiler rearing and after live transport to the slaughterhouse. Poult. Sci. 2009, 88, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Navarro, S.; Marin, C.; Cortés, V.; García, C.; Catalá-Gregori, P. Campylobacter prevalence and risk factors associated with exceeding allowable limits in poultry slaughterhouses in Spain. Vet. Rec. 2020, 186. [Google Scholar] [CrossRef] [PubMed]
- Althaus, D.; Zweifel, C.; Stephan, R. Analysis of a poultry slaughter process: Influence of process stages on the microbiological contamination of broiler carcasses. Ital. J. Food Saf. 2017, 6, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gregova, G.; Kmetova, M.; Kmet, V.; Venglovsky, J.; Feher, A. Antibiotic resistance of Escherichia coli isolated from a poultry slaughterhouse. Ann. Agric. Environ. Med. 2012, 19, 75–77. [Google Scholar]
| Analytical Constituents | Diet | |
|---|---|---|
| Starter (d 1–21) (%) | Grower (d 22–42) (%) | |
| Crude Fat | 3.5 | 3.1 |
| Crude Protein | 20.5 | 19.4 |
| Crude Fibre | 2.6 | 3.1 |
| Crude Ash | 6.6 | 5.0 |
| Lysine | 1.14 | 1.13 |
| Methionine | 0.62 | 0.51 |
| Calcium | 1.00 | 0.78 |
| Phosphorus Available | 0.69 | 0.51 |
| Sodium | 0.15 | 0.14 |
| Ingredients | Corn, soy flour, wheat, soy oil, calcium carbonate, monocalcium phosphate, sodium chloride | Corn, soy flour, rice bran, calcium carbonate, sodium chloride |
| Days of Life | CFC | IFC | ||||||
|---|---|---|---|---|---|---|---|---|
| MR (%) | BW (g) | FI (Kg) | FCR | MR (%) | BW (g) | FI (Kg) | FCR | |
| 7 | 1.47 | 157.73 | 0.13 | 1.19 | 0.98 | 160.42 | 0.13 | 1.12 |
| 14 | 0.50 | 413.17 | 0.37 | 1.35 | 1.49 | 428.59 | 0.36 | 1.38 |
| 21 | 0 | 788.25 | 0.71 | 2.02 | 0 | 789.15 | 0.67 | 1.89 |
| 28 | 0 | 1234.59 | 1.17 | 2.66 | 0 | 1233.51 | 1.09 | 2.46 |
| 35 | 0 | 1810.06 | 1.45 | 2.54 | 0 | 1788.30 | 1.27 | 2.27 |
| 42 | 0 | 2471.14 | 1.51 | 2.25 | 0 | 2461.13 | 1.36 | 2.09 |
| Experimental Group | Sampling Moment | n | CIP | NAL | CTX | CAZ | AMP | CHL | SXT | CST | AZM | TGC | GEN | TMP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFC | Arrival day | 10 | 70 a | 70 | 0 | 0 | 50 | 0 | 30 ab | 0 | 0 b | 0 | 30 bc | 40 a |
| Mid-period | 20 | 60 a | 75 | 0 | 0 | 30 | 0 | 55 a | 0 | 0 b | 0 | 10 c | 55 a | |
| End | 20 | 60 a | 65 | 0 | 2 | 45 | 0 | 10 b | 0 | 1 ab | 0 | 75 a | 60 a | |
| IFC | Arrival day | 10 | 70 a | 70 | 0 | 0 | 50 | 0 | 30 ab | 0 | 0 b | 0 | 30 bc | 40 a |
| Mid-period | 20 | 30 b | 55 | 0 | 0 | 20 | 0 | 10 b | 0 | 0 b | 0 | 15 c | 10 b | |
| End | 20 | 50 a | 65 | 0 | 4 | 35 | 0 | 35 a | 0 | 5 a | 0 | 65 ab | 55 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoro-Dasi, L.; Villagra, A.; Sevilla-Navarro, S.; Pérez-Gracia, M.T.; Vega, S.; Marin, C. Commensal Escherichia coli Antimicrobial Resistance and Multidrug-Resistance Dynamics during Broiler Growing Period: Commercial vs. Improved Farm Conditions. Animals 2021, 11, 1005. https://doi.org/10.3390/ani11041005
Montoro-Dasi L, Villagra A, Sevilla-Navarro S, Pérez-Gracia MT, Vega S, Marin C. Commensal Escherichia coli Antimicrobial Resistance and Multidrug-Resistance Dynamics during Broiler Growing Period: Commercial vs. Improved Farm Conditions. Animals. 2021; 11(4):1005. https://doi.org/10.3390/ani11041005
Chicago/Turabian StyleMontoro-Dasi, Laura, Arantxa Villagra, Sandra Sevilla-Navarro, Maria Teresa Pérez-Gracia, Santiago Vega, and Clara Marin. 2021. "Commensal Escherichia coli Antimicrobial Resistance and Multidrug-Resistance Dynamics during Broiler Growing Period: Commercial vs. Improved Farm Conditions" Animals 11, no. 4: 1005. https://doi.org/10.3390/ani11041005
APA StyleMontoro-Dasi, L., Villagra, A., Sevilla-Navarro, S., Pérez-Gracia, M. T., Vega, S., & Marin, C. (2021). Commensal Escherichia coli Antimicrobial Resistance and Multidrug-Resistance Dynamics during Broiler Growing Period: Commercial vs. Improved Farm Conditions. Animals, 11(4), 1005. https://doi.org/10.3390/ani11041005








