Changes Associated with the Peri-Ovulatory Period, Age and Pregnancy in ACTH, Cortisol, Glucose and Insulin Concentrations in Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of Early Pregnancy and Peri-Ovulatory Period
3.2. Effect of Age and Peri-Ovulatory Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of dietary carbohydrates and time of year on ACTH and cortisol concentrations in adult and aged horses. Domest. Anim. Endocrinol. 2018, 63, 15–22. [Google Scholar] [CrossRef]
- Stewart, A.J.; Hackett, E.; Bertin, F.R.; Towns, T.J. Cortisol and adrenocorticotropic hormone concentrations in horses with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2019, 33, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.; Stewart, A.J.; Jackson, K.V.; Dryburgh, E.L.; Medina-Torres, C.E.; Bertin, F.R. Clinical implications of using adrenocorticotropic hormone diagnostic cutoffs or reference intervals to diagnose pituitary pars intermedia dysfunction in mature horses. J. Vet. Intern. Med. 2021, 35, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Downey, B.R. Regulation of the estrous cycle in domestic animals—A review. Can. Vet. J. 1980, 21, 301–306. [Google Scholar]
- McFarlane, D. Equine pituitary pars intermedia dysfunction. Vet. Clin. N. Am. Equine Pract. 2011, 27, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Moore, G.E.; Bertin, F.R.; Kritchevsky, J.E. What’s New in Old Horses? Postmortem Diagnoses in Mature and Aged Equids. Vet. Pathol. 2016, 53, 390–398. [Google Scholar] [CrossRef]
- McGowan, C.M.; Frost, R.; Pfeiffer, D.U.; Neiger, R. Serum insulin concentrations in horses with equine Cushing’s syndrome: Response to a cortisol inhibitor and prognostic value. Equine Vet. J. 2004, 36, 295–298. [Google Scholar] [CrossRef]
- Horn, R.; Bamford, N.J.; Afonso, T.; Sutherland, M.; Buckerfield, J.; Tan, R.H.H.; Secombe, C.J.; Stewart, A.J.; Bertin, F.R. Factors associated with survival, laminitis and insulin dysregulation in horses diagnosed with equine pituitary pars intermedia dysfunction. Equine Vet. J. 2019, 51, 440–445. [Google Scholar] [CrossRef]
- Tadros, E.M.; Fowlie, J.G.; Refsal, K.R.; Marteniuk, J.; Schott, H.C., 2nd. Association between hyperinsulinaemia and laminitis severity at the time of pituitary pars intermedia dysfunction diagnosis. Equine Vet. J. 2019, 51, 52–56. [Google Scholar] [CrossRef]
- Frank, N.; Tadros, E.M. Insulin dysregulation. Equine Vet. J. 2014, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bamford, N.J.; Potter, S.J.; Harris, P.A.; Bailey, S.R. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in horses and ponies fed a high fat diet, with or without a once daily high glycaemic meal. Equine Vet. J. 2016, 48, 368–373. [Google Scholar] [CrossRef]
- Bertin, F.R.; Ruffin-Taylor, D.; Stewart, A.J. Insulin dysregulation in horses with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2018, 32, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Bertin, F.R.; Taylor, S.D.; Bianco, A.W.; Sojka-Kritchevsky, J.E. The Effect of Fasting Duration on Baseline Blood Glucose Concentration, Blood Insulin Concentration, Glucose/Insulin Ratio, Oral Sugar Test, and Insulin Response Test Results in Horses. J. Vet. Intern. Med. 2016, 30, 1726–1731. [Google Scholar] [CrossRef]
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet. J. 2018, 50, 249–254. [Google Scholar] [CrossRef]
- Kritchevsky, J.E.; Muir, G.S.; Leschke, D.H.Z.; Hodgson, J.K.; Hess, E.K.; Bertin, F.R. Blood glucose and insulin concentrations after alpha-2-agonists administration in horses with and without insulin dysregulation. J. Vet. Intern. Med. 2020, 34, 902–908. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Carter, R.A.; Geor, R.J.; Staniar, W.B.; Cubitt, T.A.; Harris, P.A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 2009, 179, 204–210. [Google Scholar] [CrossRef]
- Morgan, R.A.; McGowan, T.W.; McGowan, C.M. Prevalence and risk factors for hyperinsulinaemia in ponies in Queensland, Australia. Aust. Vet. J. 2014, 92, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Stewart, A.J.; Yuen, K.Y.; Hinrichsen, S.; Dryburgh, E.L.; Bertin, F.R. The effect of freeze-thaw cycles on determination of immunoreactive plasma adrenocorticotrophic hormone concentrations in horses. J. Vet. Intern. Med. 2020, 34, 1350–1356. [Google Scholar] [CrossRef]
- Leschke, D.H.; Muir, G.S.; Hodgson, J.K.; Coyle, M.; Horn, R.; Bertin, F.R. Immunoreactive insulin stability in horses at risk of insulin dysregulation. J. Vet. Intern. Med. 2019, 33, 2746–2751. [Google Scholar] [CrossRef]
- Cunneen, A.; Wood, K.A.; Mathison, K.; Herndon, A.M.; Bertin, F.R. Comparison of a continuous indwelling glucometer with a point-of-care device in healthy adult horses. Vet. Rec. 2020, 187, e21. [Google Scholar] [CrossRef]
- Satue, K.; Fazio, E.; Munoz, A.; Medica, P. Endocrine and Electrolyte Balances during Periovulatory Period in Cycling Mares. Animals 2021, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Medica, P.; Cravana, C.; Ferlazzo, A.A. Pituitary-adrenocortical adjustments to transport stress in horses with previous different handling and transport conditions. Vet. World 2016, 9, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Vazzana, I.; Giudice, E.; Fazio, F.; Piccione, G. Relationship between serum cortisol levels and some physiological parameters following reining training session in horse. Anim. Sci. J. 2016, 87, 729–735. [Google Scholar] [CrossRef]
- Burgess, L.H.; Handa, R.J. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 1992, 131, 1261–1269. [Google Scholar] [CrossRef]
- Bertin, F.R.; Pader, K.S.; Lescun, T.B.; Sojka-Kritchevsky, J.E. Short-term effect of ovariectomy on measures of insulin sensitivity and response to dexamethasone administration in horses. Am. J. Vet. Res. 2013, 74, 1506–1513. [Google Scholar] [CrossRef]
- Hedberg, Y.; Dalin, A.M.; Forsberg, M.; Lundeheim, N.; Sandh, G.; Hoffmann, B.; Ludwig, C.; Kindahl, H. Effect of ACTH (tetracosactide) on steroid hormone levels in the mare. Part B: Effect in ovariectomized mares (including estrous behavior). Anim. Reprod. Sci. 2007, 100, 92–106. [Google Scholar] [CrossRef]
- Asa, C.S.; Goldfoot, D.A.; Garcia, M.C.; Ginther, O.J. Sexual behavior in ovariectomized and seasonally anovulatory pony mares (Equus caballus). Horm. Behav. 1980, 14, 46–54. [Google Scholar] [CrossRef]
- Satue, K.; Domingo, R.; Redondo, J.I. Relationship between progesterone, oestrone sulphate and cortisol and the components of renin angiotensin aldosterone system in Spanish purebred broodmares during pregnancy. Theriogenology 2011, 76, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Rendle, D.I.; Litchfield, E.; Heller, J.; Hughes, K.J. Investigation of rhythms of secretion and repeatability of plasma adrenocorticotropic hormone concentrations in healthy horses and horses with pituitary pars intermedia dysfunction. Equine Vet. J. 2014, 46, 113–117. [Google Scholar] [CrossRef]
- Satue, K.; Marcilla, M.; Medica, P.; Ferlazzo, A.; Fazio, E. Testosterone, androstenedione and dehydroepiandrosterone concentrations in pregnant Spanish Purebred mare. Theriogenology 2019, 123, 62–67. [Google Scholar] [CrossRef]
- Marcilla, M.; Munoz, A.; Satue, K. Longitudinal changes in serum catecholamines, dopamine, serotonin, ACTH and cortisol in pregnant Spanish mares. Res. Vet. Sci. 2017, 115, 29–33. [Google Scholar] [CrossRef]
- Flisinska-Bojanowska, A.; Komosa, M.; Gill, J. Influence of pregnancy on diurnal and seasonal changes in cortisol, T3 and T4 levels in the mare blood serum. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 98, 23–30. [Google Scholar] [CrossRef]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef]
- Ellis, M.J.; Livesey, J.H.; Donald, R.A. Horse plasma corticotrophin-releasing hormone (CRH): Characterisation and lack of a late gestational rise or a plasma CRH-binding protein. J. Endocrinol. 1994, 143, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Rapson, J.L.; Schott, H.C., 2nd; Nielsen, B.D.; McCutcheon, L.J.; Harris, P.A.; Geor, R.J. Effects of age and diet on glucose and insulin dynamics in the horse. Equine Vet. J. 2018, 50, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Beythien, E.; Wulf, M.; Ille, N.; Aurich, J.; Aurich, C. Effects of sex, pregnancy and season on insulin secretion and carbohydrate metabolism in horses. Anim. Reprod. Sci. 2017, 184, 86–93. [Google Scholar] [CrossRef]
- George, L.A.; Staniar, W.B.; Cubitt, T.A.; Treiber, K.H.; Harris, P.A.; Geor, R.J. Evaluation of the effects of pregnancy on insulin sensitivity, insulin secretion, and glucose dynamics in Thoroughbred mares. Am. J. Vet. Res. 2011, 72, 666–674. [Google Scholar] [CrossRef]
- Hart, K.A.; Wochele, D.M.; Norton, N.A.; McFarlane, D.; Wooldridge, A.A.; Frank, N. Effect of Age, Season, Body Condition, and Endocrine Status on Serum Free Cortisol Fraction and Insulin Concentration in Horses. J. Vet. Intern. Med. 2016, 30, 653–663. [Google Scholar] [CrossRef]
- de Laat, M.A.; Sillence, M.N. The repeatability of an oral glucose test in ponies. Equine Vet. J. 2016, 49, 238–243. [Google Scholar] [CrossRef]
- Bertin, F.R.; de Laat, M.A. The diagnosis of equine insulin dysregulation. Equine Vet. J. 2017, 49, 570–576. [Google Scholar] [CrossRef]
- Horn, R.; Bertin, F.R. Evaluation of combined testing to simultaneously diagnose pituitary pars intermedia dysfunction and insulin dysregulation in horses. J. Vet. Intern. Med. 2019, 33, 2249–2256. [Google Scholar] [CrossRef]
- Bertin, F.R.; Sojka-Kritchevsky, J.E. Comparison of a 2-step insulin-response test to conventional insulin-sensitivity testing in horses. Domest. Anim. Endocrinol. 2013, 44, 19–25. [Google Scholar] [CrossRef]
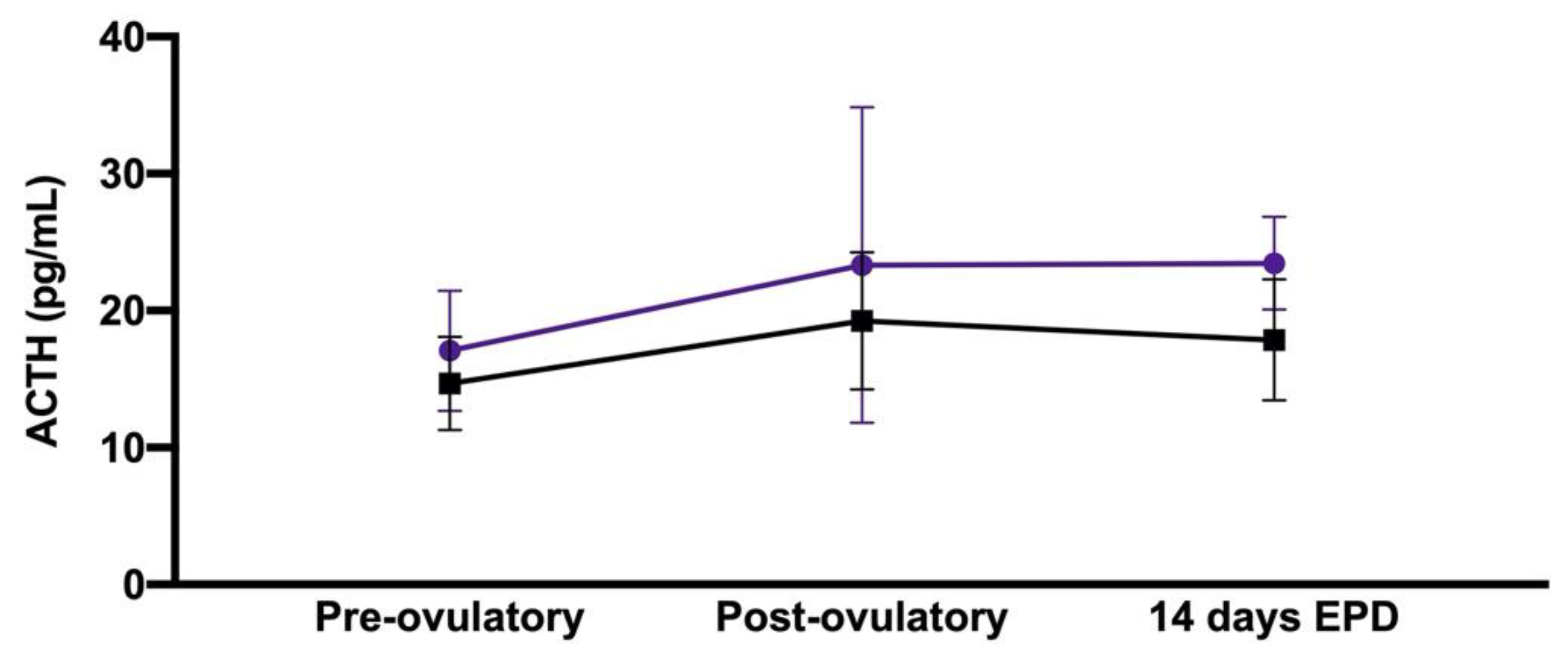
| Peri-Ovulatory Period | Pregnant Mares (n = 11) | Non-Pregnant Mares (n = 7) |
|---|---|---|
| Pre-ovulatory | 17.1 + 4.4 pg/mL | 14.7 + 3.4 pg/mL |
| Post-ovulatory | 23.3 + 11.5 pg/mL | 19.2 + 5.0 pg/mL |
| 14-day EPD | 23.5 + 3.4 pg/mL | 17.9 + 4.4 pg/mL |
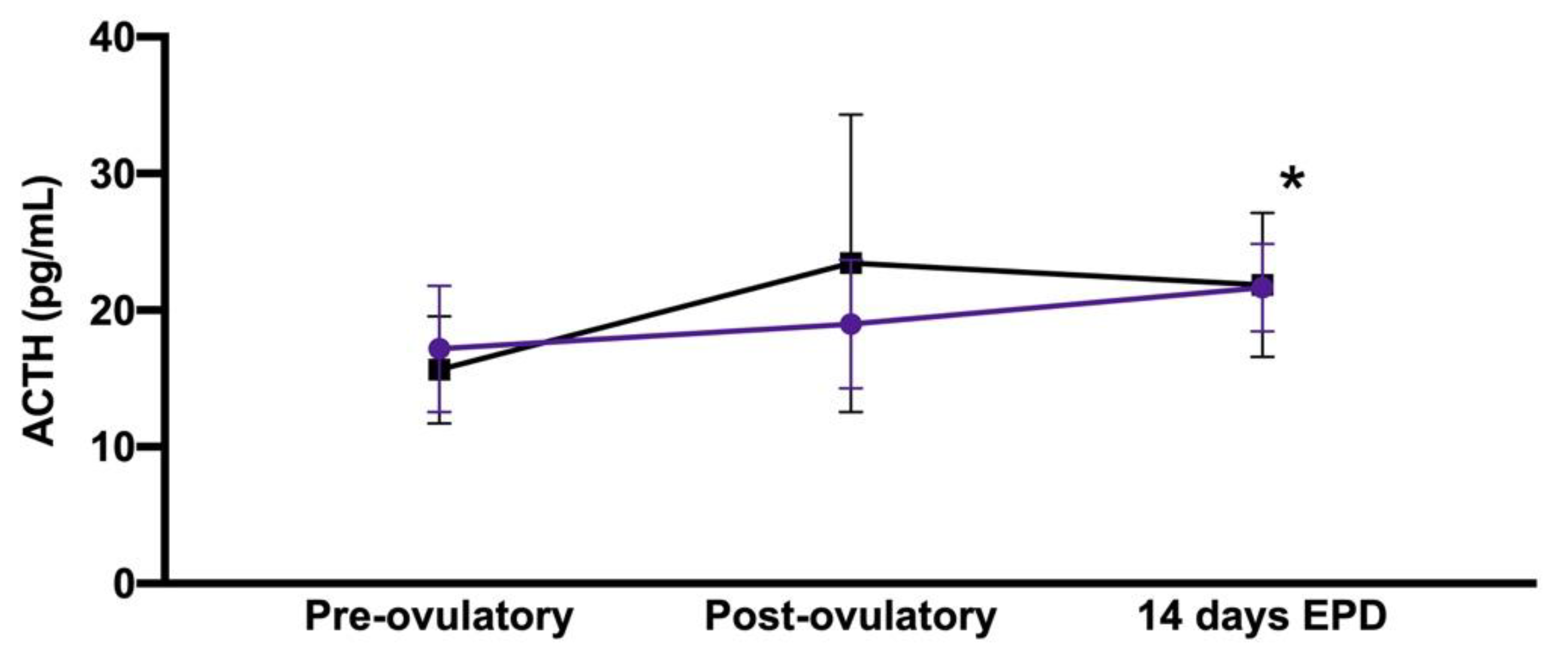
| Peri-Ovulatory Period | Older Mares (n = 6) | Younger Mares (n = 12) |
|---|---|---|
| Pre-ovulatory a | 17.2 + 4.6 pg/mL | 15.6 + 3.9 pg/mL |
| Post-ovulatory | 19.0 + 4.7 pg/mL | 23.4 + 10.9 pg/mL |
| 14-day EPD a | 21.7 + 3.2 pg/mL | 21.9 + 5.2 pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hicks, G.R.; Fraser, N.S.; Bertin, F.-R. Changes Associated with the Peri-Ovulatory Period, Age and Pregnancy in ACTH, Cortisol, Glucose and Insulin Concentrations in Mares. Animals 2021, 11, 891. https://doi.org/10.3390/ani11030891
Hicks GR, Fraser NS, Bertin F-R. Changes Associated with the Peri-Ovulatory Period, Age and Pregnancy in ACTH, Cortisol, Glucose and Insulin Concentrations in Mares. Animals. 2021; 11(3):891. https://doi.org/10.3390/ani11030891
Chicago/Turabian StyleHicks, Gemma R., Natalie S. Fraser, and François-René Bertin. 2021. "Changes Associated with the Peri-Ovulatory Period, Age and Pregnancy in ACTH, Cortisol, Glucose and Insulin Concentrations in Mares" Animals 11, no. 3: 891. https://doi.org/10.3390/ani11030891
APA StyleHicks, G. R., Fraser, N. S., & Bertin, F.-R. (2021). Changes Associated with the Peri-Ovulatory Period, Age and Pregnancy in ACTH, Cortisol, Glucose and Insulin Concentrations in Mares. Animals, 11(3), 891. https://doi.org/10.3390/ani11030891







