The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
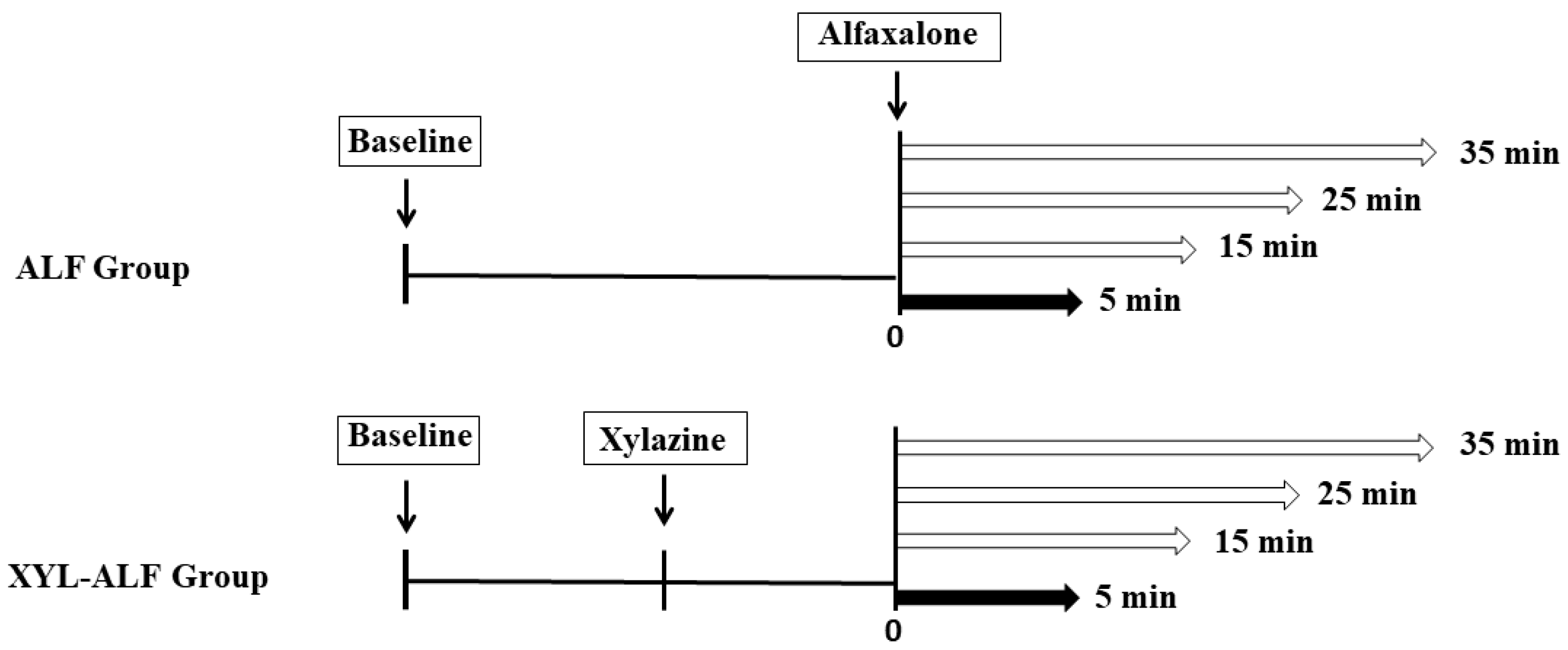
2.2. Experimental Design
2.2.1. Cardiorespiratory Parameters and Rectal Temperature
2.2.2. Echocardiographic Parameters
2.2.3. Nociceptive Thresholds and Flank Muscle Tone
2.3. Statistical Analysis
3. Results
3.1. Sparing Effect of Xylazine on Alfaxalone Induction Dose and Quality
3.2. Effects of Anesthetic Induction with Alfaxalone Alone or Combined with Xylazine
3.2.1. Cardio-Respiratory Parameters
3.2.2. Echocardiographic Parameters
3.2.3. Nociceptive Thresholds and Flank Muscle Tone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Score | Description |
|---|---|
| Smooth (0) | No signs of excitement; no palpebral blinking; may or may not be associated with minimal jaw movement during manipulation; clear laryngeal structure visualization and complete tongue flaccidity with easy intubation performed within ten seconds. |
| Fair (1) | Slight excitement; some rapid blinking, some limb movements and obvious jaw movement during manipulation; intubation is performed within 30–60 s with or without some jaw tone persistence. |
| Poor (2) | Marked excitement; muscle twitching; paddling of limbs; unable to place orotracheal tube. |
Appendix B
| Rating System | Score: Description |
|---|---|
| Pedal withdrawal reflex 1 | (3) Exaggerated response: Active withdrawal of stimulated leg with active movement of head. (2) Weak response: Slow withdrawal of stimulated leg with sluggish head movement. (1) Very weak response: Slow withdrawal of stimulated leg alone. (0) No response: Inactive limb and head reflex |
| Flank muscle tone 2 | (0): Complete relaxation to palpation with minimal or no tone palpable. (1): Detectable relaxation to palpation with less tone palpable. (2): Normal resting tone: flank has some elasticity to palpation. (3): Mild resistance and tone with gentle pressure on the skin and muscle. (4): Strong resistance and tone with strong pressure on the skin. |
References
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal general anesthesia: Theory and practice. Anesth Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.J.; Pasloske, K.; Whittem, T.; Ranasinghe, M.G.; Li, Q.; Lefebvre, H.P. Plasma pharmacokinetics of alfaxalone in dogs after an intravenous bolus of Alfaxan-CD RTU. Vet. Anaesth Analg. 2006, 33, 229–236. [Google Scholar] [CrossRef]
- Whittem, T.; Pasloske, K.S.; Heit, M.C.; Ranasinghe, M.G. The pharmacokinetics and pharmacodynamics of alfaxalone in cats after single and multiple intravenous administration of Alfaxan® at clinical and supraclinical doses. J. Vet. Pharmacol. Ther. 2008, 31, 571–579. [Google Scholar] [CrossRef]
- Ambros, B.; Duke-Novakovski, T.; Pasloske, K.S. Comparison of the anaesthetic efficacy and cardiopulmonary effects of continuous rate infusions of alfaxalone-2hydroxypropyl-beta-cyclodextrin and propofol in dogs. Am. J. Vet. Res. 2008, 69, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Mocholi, D.; Gasthuys, F.; Vlaminck, L.; Schauvliege, S. Clinical effect of a constant rate infusion of alfaxalone in isoflurane-anesthetized goats undergoing an experimental procedure: A pilot study. Vlaams Diergeneeskd Tijdschr. 2020, 89, 28–33. [Google Scholar] [CrossRef]
- Dzikiti, T.; Zeiler, G.E.; Dzikiti, L.N.; Garcia, E.R. The effects of midazolam and butorphanol, administered alone or combined, on the dose and quality of anaesthetic induction with alfaxalone in goats. J. S. Afr. Vet. Assoc. 2014, 85, 1–8. [Google Scholar] [CrossRef]
- Andaluz, A.; Felez-Ocana, N.; Santos, L.; Fresno, L.; Garcia, F. The effects on cardio-respiratory and acid-base variables of the anaesthetic alfaxalone in a 2-hydroxypropyl-β-cyclodextrin (HPCD) formulation in sheep. Vet. J. 2012, 191, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.P.; Gieseg, M.; Singh, P.M.; Mitchinson, S.L.; Chambers, J.P. A comparison of two different ketamine and diazepam combinations with an alphaxalone and medetomidine combination for induction of anaesthesia in sheep. N. Z. Vet. J. 2012, 60, 136–141. [Google Scholar] [CrossRef]
- Goodwin, W.A.; Keates, H.L.; Pasloske, K.; Pearson, M.; Sauer, B.; Ranasinghe, M.G. The pharmacokinetics and pharmacodynamics of the injectable anaesthetic alfaxalone in the horse. Vet. Anaesth Analg. 2011, 38, 431–438. [Google Scholar] [CrossRef]
- Keates, H.L.; van Eps, A.W.; Pearson, M.R.B. Alfaxalone compared with ketamine for induction of anaesthesia in horses following xylazine and guaifenesin. Vet. Anaesth Analg. 2012, 39, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Maney, J.K.; Durham Jr, H.E.; Goucher, K.P.; Little, E.L. Induction of anesthesia and recovery in donkeys sedated with xylazine: A comparison of midazolam–alfaxalone and midazolam–ketamine. Vet. Anaesth Analg. 2018, 45, 539–544. [Google Scholar] [CrossRef]
- Del Mar Granados, M.; Domínguez, J.M.; Fernández-Sarmiento, A.; Funes, F.J.; Morgaz, J.; Navarrete, R.; Ma Carrillo, J.; Rubio, M.; Muñoz-Rascón, P.; Gómez de Segura, I.A.; et al. Anaesthetic and cardiorespiratory effects of a constant-rate infusion of alfaxalone in desflurane-anaesthetised sheep. Vet. Rec. 2012, 171, 125. [Google Scholar] [CrossRef] [PubMed]
- Andaluz, A.; Santos, L.; García, F.; Ferrer, R.I.; Fresno, L.; Moll, X. Maternal and foetal cardiovascular effects of the anaesthetic alfaxalone in 2-hydroxypropyl-β-cyclodextrin in the pregnant ewe. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Dzikiti, B.T.; Ndawana, P.S.; Zeiler, G.; Ferreira, J.P.; Dzikiti, L.N. Determination of the minimum infusion rate of alfaxalone during its co-administration with fentanyl at three different doses by constant rate infusion intravenously in goats. Vet. Anaesth Analg. 2016, 43, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Del Álamo, A.M.; Mandsager, R.E.; Riebold, T.W.; Payton, M.E. Evaluation of intravenous administration of alfaxalone, propofol, and ketamine-diazepam for anesthesia in alpacas. Vet. Anaesth Analg. 2015, 42, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Posner, L.P. Sedatives and tranquilizers. In Veterinary Pharmacology and Therapeutics, 10th ed.; Riviere, J.E., Papich, M.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 324–350. [Google Scholar]
- Clarke, K.W.; Hall, L.W. “Xylazine”—A new sedative for horses and cattle. Vet. Rec. 1969, 85, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Hu, M.L.; Qiu, Z.Y.; Zhou, F.Y.; Zeng, J.; Wan, J.; Wang, S.W.; Zhang, W.; Ding, M.X. Physiologic and biochemical effects of electroacupuncture combined with intramuscular administration of dexmedetomidine to provide analgesia in goats. Am. J. Vet. Res. 2016, 77, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Dzikiti, T.B.; Ndawana, P.S.; Zeiler, G.; Bester, L.; Dzikiti, L.N. Determination of the minimum infusion rate of alfaxalone during its co-administration with midazolam in goats. Vet. Rec open. 2015, 2, e000065. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Guo, N.N.; Li, Y.L.; Li, M.; Ding, M.X. Analgesic and physiological effect of electroacupuncture combined with epidural lidocaine in goats. Vet. Anaesth Analg. 2017, 44, 959–967. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Sharkey, I.; Boddy, A.V.; Wallace, H.; Mycroft, J.; Hollis, R.; Picton, S. Body surface area estimation in children using weight alone: Application in paediatric oncology. Br. J. Cancer. 2001, 85, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Mocholí, D.; Belda, E.; Bosmans, T.; Laredo, F.G. Clinical efficacy and cardiorespiratory effects of intramuscular administration of alfaxalone alone or in combination with dexmedetomidine in cats. Vet. Anaesth Analg. 2016, 43, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Oakleaf, M.; Mama, K.; Mangin, L.; Lebsock, K.; Bisazza, K.; Easley, J. Comparison of ketamine and alfaxalone for induction of anesthesia in goats. Vet. Anaesth Analg. 2018, 45, 855. [Google Scholar] [CrossRef]
- Oakleaf, M.H.; Mama, K.R.; Mangin, L.M.; Lebsock, K.J.; Bisazza, K.T.; Hess, A.M.; Easley, J.T. Comparison of intravenous anesthetic induction doses and physiologic effects of ketamine or alfaxalone in goats undergoing surgery with isoflurane anesthesia. Am. J. Vet. Res. 2019, 80, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Pinelas, R.; Alibhai, H.I.; Mathis, A.; Lozano, A.J.; Brodbelt, D.C. Effects of different doses of dexmedetomidine on anaesthetic induction with alfaxalone—A clinical trial. Vet. Anaesth Analg. 2014, 41, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Mocholí, D.; Escudero, E.; Belda, E.; Laredo, F.G.; Hernandis, V.; Marín, P. Pharmacokinetics and effects of alfaxalone after intravenous and intramuscular administration to cats. N. Z. Vet. J. 2018, 66, 172–177. [Google Scholar] [CrossRef]
- Prassinos, N.N.; Galatos, A.D.; Raptopoulos, D. A comparison of propofol, thiopental or ketamine as induction agents in goats. Vet. Anaesth Analg. 2005, 32, 289–296. [Google Scholar] [CrossRef]
- Muir, W.; Lerche, P.; Wiese, A.; Nelson, L.; Pasloske, K.; Whittem, T. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet. Anaesth Analg. 2008, 35, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Morgaz, J.; Muñoz, P.; Fernández, J.; Navarrete, R.; Quiros, S.; López, I.; Gómez-Villamandos, R.; Granados, M. Comparison of cardiopulmonary parameters after induction of anaesthesia with alfaxalone or etomidate in dogs. Vet. Anaesth Analg. 2011, 38, 9–10. [Google Scholar]
- Changmin, H.; Jianguo, C.; Dongming, L.; Guohong, L.; Mingxing, D. Effects of xylazole alone and in combination with ketamine on the metabolic and neurohumoral responses in healthy dogs. Vet. Anaesth Analg. 2010, 37, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Benedetti, I.C.; Bublot, I.; Ribas, T.; Fourel, I.; Vogl, C.; Dubois, C.; Milani, M.; Ida, K.K.; Portier, K. Pharmacokinetics of intramuscular alfaxalone and its echocardiographic, cardiopulmonary and sedative effects in healthy dogs. PLoS ONE 2018, 13, e0204553. [Google Scholar] [CrossRef]
- Maney, J.K.; Shepard, M.K.; Braun, C.; Cremer, J.; Hofmeister, E.H. A comparison of cardiopulmonary and anesthetic effects of an induction dose of alfaxalone or propofol in dogs. Vet. Anaesth Analg. 2013, 40, 237–244. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, T.; Hyun, C. Effects of alfaxalone on echocardiographic examination in healthy dogs. Korean J. Vet. Res. 2015, 55, 221–225. [Google Scholar] [CrossRef][Green Version]
- El-Hawari, S.F.; Sakata, H.; Oyama, N.; Tamura, J.; Higuchi, C.; Endo, Y.; Miyoshi, K.; Sano, T.; Suzuki, K.; Yamashita, K. Anesthetic and cardiorespiratory effects of single-bolus intravenous alfaxalone with or without intramuscular xylazine-premedication in calves. J. Vet. Med. Sci. 2018, 80, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.; Proost, K.; Pardon, B.; De Cremer, L.; Schauvliege, S. General anesthesia for surgical treatment of urethral obstruction in nine goats. Vlaams Diergeneeskd. Tijdschr. 2018, 87, 314–325. [Google Scholar] [CrossRef]
- Sinclair, M.D. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar] [PubMed]
- Dzikiti, B.T.; Ndawana, P.S.; Dzikiti, L.N.; Stegmann, F.G. The minimum infusion rate of alfaxalone during its co-administration with lidocaine at three different doses by constant rate infusion in goats. Vet. Anaesth Analg. 2018, 45, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lakhdhir, S.; Caulkett, N.; Duke-Novakovski, T.; Woodbury, M.; Boysen, S. Evaluation of intramuscular sodium nitroprusside injection to improve oxygenation in white-tailed deer (Odocoileus virginianus) anesthetized with medetomidine–alfaxalone–azaperone. Vet. Anaesth Analg. 2021, 48, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.S.; Samir, H.; Yoshida, T.; Matsuura, K.; Abdelmageed, H.A.; Elbadawy, M.; Al-Rejaie, S.; El-Husseiny, H.M.; Elfadadny, A.; Ma, D.; et al. Assessment of the Cardiac Functions Using Full Conventional Echocardiography with Tissue Doppler Imaging before and after Xylazine Sedation in Male Shiba Goats. Animals 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.; Johnson, M.H. The reliability and validity of iontophoretically applied potassium as an experimental pain stimulus. Pain 1990, 41, S316. [Google Scholar] [CrossRef]
- Liu, D.M.; Zhou, Z.Y.; Ding, Y.; Chen, J.G.; Hu, C.M.; Chen, X.; Ding, M.X. Physiologic effects of electroacupuncture combined with intramuscular administration of xylazine to provide analgesia in goats. Am. J. Vet. Res. 2009, 70, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
| ALF Group (n = 6) | XYL-ALF Group (n = 6) | p Value | |
|---|---|---|---|
| Induction dose (mg/kg bw) | 4.0 ± 0.30 | 2.3 ± 0.25 * | p < 0.001 |
| Induction score | 1(0–1) | 0.5(0–1) | p = 0.3202 |
| Time to extubation (min) | 6.6 ±1.9 | 9.4 ± 3.2 | p = 0.0781 |
| Parameter | Group | Baseline | (5) | (15) | (25) | (35) |
|---|---|---|---|---|---|---|
| HR (beats/min) | ALF | 103 ± 10 | 140 ± 17 * | 138 ± 16 * | 124 ± 13.3 | 117 ± 14.4 |
| XYL-ALF | 97 ± 17.2 | 118 ± 26 * | 98 ± 10.2 † | 98 ± 23 † | 94 ± 14 † | |
| SAP (mmHg) | ALF XYL-ALF | 114 ± 10 115 ± 8 | 124 ± 7 99 ± 15 † p = 0.0085 | 108 ± 12 101 ± 13 | 111 ± 8 106 ± 10 | 113 ± 17 102 ± 9 |
| MAP (mm Hg) | ALF | 87 ± 7.9 | 93 ± 6 | 83 ± 9.7 | 85 ± 14 | 79 ± 18 |
| XYL-ALF | 82 ± 10 | 68 ± 13.5 † | 70 ± 8 † | 76 ± 13 | 73 ± 9.6 | |
| DAP (mmHg) | ALF XYL-ALF | 70 ± 12 62 ± 8 | 76 ± 8 53 ± 11 † p = 0.0011 | 66 ± 9 55 ± 9 † p = 0.0209 | 72 ± 16 60 ± 18 | 62 ± 15 67 ± 9 |
| DAP (mmHg) | ALF XYL-ALF | 70 ± 12 62 ± 8 | 76 ± 8 53 ± 11 † p = 0.0011 | 66 ± 9 55 ± 9 † p = 0.0209 | 72 ± 16 60 ± 18 | 62 ± 15 67 ± 9 |
| fR (breaths/min) | ALF | 20 ± 2.9 | 22 ± 3.8 | 21 ± 4.5 | 21 ± 9.8 | 22 ± 6.6 |
| XYL-ALF | 23 ± 6.8 | 16 ± 6 | 18 ± 4.3 | 19 ± 8.5 | 16 ± 2.4 † | |
| SpO2 (%) | ALF | 95 ± 2.4 | 90 ± 1.7 | 92 ± 1.9 | 93 ± 3 | 92 ± 3.4 |
| XYL-ALF | 94 ± 2.3 | 82 ± 1.7 *† | 84 ± 2.7 †* | 87 ± 3 †* | 91 ± 2 | |
| PE’CO2 (mmHg) | ALF XYL-ALF | 35 ± 1.9 40 ± 2.9 † | ||||
| RT (°C) | ALF | 39.4 ± 0.3 | 39 ± 0.5 * | 39 ± 0.4 * | 39.1 ± 0.4 | 39.2 ± 0.4 |
| XYL-ALF | 39.4 ± 0.2 | 40 ± 0.2 * | 38.5 ± 0.3 * | 38.4 ± 0.3 * | 38.4 ± 0.2 * |
| Parameter | Group | Baseline | (5) | (15) | (25) | (35) |
|---|---|---|---|---|---|---|
| EF (%) | ALF | 76 ± 6 | 71 ± 8 | 73 ± 9 | 75 ± 7 | 76 ± 8 |
| XYL-ALF | 80 ± 6 | 84 ± 9 † | 78 ± 6 | 72 ± 9 | 81 ± 11 | |
| FS (%) | ALF | 38 ± 8 | 34 ± 6 | 36 ± 7 | 37 ± 6 | 39 ± 7 |
| XYL-ALF | 42 ± 7 | 47 ± 9 † | 39 ± 6 | 36 ± 7 | 44 ± 9 | |
| SV (mL) | ALF | 18 ± 5 | 17 ± 9 | 12 ± 9 | 16 ± 8 | 16 ± 3 |
| XYL-ALF | 19 ± 4 | 14 ± 7 | 14 ± 8 | 14 ± 2 | 18 ± 8 | |
| CO (L/min) | ALF | 1.8 ± 0.5 | 2.4 ± 1.3 | 1.8 ±1.4 | 2 ± 0.9 | 2 ± 0.9 |
| XYL-ALF | 1.7 ± 0.2 | 1.6 ± 0.6 | 1.3 ± 0.7 | 1.4 ± 0.4 | 1.4 ± 0.5 | |
| CI (L/min/m2) | ALF | 2.4 ± 0.6 | 3 ± 1.5 | 2.3 ± 1.7 | 2.5 ± 1 | 2.5 ± 0.4 |
| XYL-ALF | 2.4 ± 0.3 | 2.3 ± 0.9 | 1.8 ± 1 | 1.9 ± 0.4 | 1.9 ± 0.8 |
| Parameter | Group | Baseline | (5) | (15) | (25) | (35) |
|---|---|---|---|---|---|---|
| Nociceptive threshold | ALF | 33 ± 14 | 43 ± 23 | 36 ± 21 | 28 ± 5 | 28 ± 3 |
| XYL-ALF | 29 ± 7 | 87 ± 52 *† | 45 ± 28 | 38 ± 21 | 35 ± 11 | |
| Flank muscle tone | ALF XYL-ALF | 2(2) 2(2) | 1 (1–2) 0.5 (0–1) *† † p = 0.0198 | 2 (2–3) 1(0–2) † † p = 0.0104 | 2 (2–3) 2 (1–2) p = 0.2184 | 2 (2–3) 2 (2) p = 0.5000 |
| Nociceptive response | ALF XYL-ALF | 2(1–2) 1(0–2) † † p = 0.0163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouelfetouh, M.M.; Liu, L.; Salah, E.; Sun, R.; Nan, S.; Ding, M.; Ding, Y. The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats. Animals 2021, 11, 723. https://doi.org/10.3390/ani11030723
Abouelfetouh MM, Liu L, Salah E, Sun R, Nan S, Ding M, Ding Y. The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats. Animals. 2021; 11(3):723. https://doi.org/10.3390/ani11030723
Chicago/Turabian StyleAbouelfetouh, Mahmoud M., Lingling Liu, Eman Salah, Rui Sun, Sha Nan, Mingxing Ding, and Yi Ding. 2021. "The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats" Animals 11, no. 3: 723. https://doi.org/10.3390/ani11030723
APA StyleAbouelfetouh, M. M., Liu, L., Salah, E., Sun, R., Nan, S., Ding, M., & Ding, Y. (2021). The Effect of Xylazine Premedication on the Dose and Quality of Anesthesia Induction with Alfaxalone in Goats. Animals, 11(3), 723. https://doi.org/10.3390/ani11030723






