Simple Summary
The gene for Bone Morphogenetic Protein 15 has multiple single-nucleotide polymorphism sites related to fertility in sheep. To increase fertility, a better understanding of the regulation of Bone Morphogenetic Protein 15 in sheep is essential. This work describes the phenotype and molecular characteristics of a mutation identified in the Bone Morphogenetic Protein 15 gene of Cele black sheep in Xinjiang, China. This mutation may affect the fertility of sheep, which is very useful for breeding purposes.
Abstract
The Bone Morphogenetic Protein 15 (BMP15) gene is known to have multiple single-nucleotide polymorphism sites associated with sheep fecundity. This study used gene sequence analysis and mutation detection assays for BMP15 by using 205 blood samples of ewes with known lambing records. Sequence analysis showed that mutation B1 missed the CTT base in exon 1 at positions 28–30, leading to a leucine deletion in the BMP15 protein. Litter size of ewes differed significantly between BB and B+ genotypes of B1 (p < 0.05); however, the differences between wild genotype (++) and homozygous (BB) or wild genotype (++) and heterozygous (B+) were not significant (p > 0.05). Another mutation, T755C, is a T-to-C base change at position 755 of exon 2, resulting in leucine replacement by proline at this position of the BMP15 protein (p.L252P). Two genotypes were identified in the flock: heterozygous (E+) and wild-type genotype (++). Ewes with heterozygous (E+) p.L252P had significantly larger litter sizes than those with the wild-type genotype (p < 0.05). Comprehensive analysis suggests that p.L252P is a mutation that affects fecundity in Cele black sheep.
1. Introduction
Cele black sheep are a prolific sheep breed in Xinjiang, China. This breed produces more lambs per litter and has more estrous cycles within a year than other breeds in the same area. Although the environment of Xinjiang is very harsh, with hot summers, cold winters, and windy as well as dry conditions all year round, ewes of this breed produce 2–4 lambs per litter.
In recent years, substantial progress has been made in the understanding of major genes for the prolificacy of sheep throughout the world. A number of studies showed that major genes offer the potential to significantly increase the reproductive performance of sheep flocks [1,2,3]. The following three classes of fecundity genes (Fec) have been identified in sheep: bone morphogenetic protein receptor type IB (BMPR-IB), known as FecB on chromosome 6, growth differentiation factor 9 (GDF9), known as FecG on chromosome 5, and Bone Morphogenetic Protein 15 (BMP15), known as FecX on the X chromosome. Interestingly, all three Fec genes belong to the TGF-β super family [4,5,6].
We previously identified mutations in FecB of the BMPR-IB gene associated with increased litter sizes in Cele black sheep. However, the litter sizes of wild-type genotypes (++) of the BMPR-IB gene were higher than (−/+) and (−/−), reaching 162%. Other genes must, therefore, also control ovulation in Cele black sheep [7].
The expression of the BMP15 gene was confirmed in the sheep ovary by RT-PCR. In situ hybridization showed that BMP15 mRNA in the sheep ovary is only expressed in oocytes, and its encoded product plays an important role in oocyte development [8]. The BMP15 gene is located in chromosome X of sheep and consists of two exons encoded by 1179 nucleotides. This gene encodes a prepropeptide of 393 amino acid residues. Active mature BMP15 peptide has a length of 125 amino acids.
At present, multiple mutations related to the fecundity of the BMP15 gene have been identified in sheep. Ewes of mutant heterozygous individuals achieved a higher ovulation number, while homozygous mutant individuals were completely infertile because of the absence of primary follicles in the ovary [8,9,10,11] (e.g., FecXI, FecXB, FecXL, FecXH, FecXG, and FecXR). Galloway identified two different independent point mutations in BMP15: Inverdale (FecXL) and Hanna (FecXH) [8]. Cambridge and Belclare sheep have the FecXG for BMP15 (B2, g. C718T) and FecXB for BMP15 (B4, g. G1100T), and mutations B1 (CTT missed) and B3 (g. T747C) were also identified [11]. FecXI and FecXH change the amino residue of the mature BMP15 protein, thus changing the function of BMP15 and increasing ovulation of ewes [8]. Mutations in the BMP15 gene have also been reported in Lacaune sheep [12]. This mutation leads to five naturally occurring mutations in the BMP15 gene that have been associated with the modulation of ovarian function in Lacaune sheep. Indeed, FecXL are similar to other FecX mutations, which have been associated with increased ovulation rates or sterility. A 17-bp deletion in BMP15 has been identified in Rasa Aragonesa sheep of Spain [13,14]. Exon 2 site 525–541 of the BMP15 gene was missing 17 nucleotides and was named FecXR. In contrast, homozygous ewes with two mutations of FecXGr and FecX0 are hyperprolific [15]. A novel mutation Fex Bar of BMP15 exhibited streaky ovaries with a blockade at the primary stage of folliculogenesis, as shown by histochemistry [2].
This study firstly verified the polymorphism of the four sites of FecXI, FecXH, FecXG, and FecXB of the BMP15 gene in a population of Cele black sheep. Furthermore, other mutations in the BMP15 gene were explored and their effects on litter size were tested. This work describes the phenotypic and molecular characterization of a mutation identified in the BMP15 gene in Cele black sheep.
2. Materials and Methods
2.1. Experimental Animals
A total of 205 healthy Cele black sheep ewes, 1–2 years old, were chosen in Cele County, Xinjiang Province, China. All of these Cele black sheep ewes were lambing second borns and lambing in the spring. Briefly, 205 blood samples were collected from the jugular vein directly into tubes containing heparin sodium. Samples were then transferred to the laboratory freezer (−20 °C). At the start of the breeding season, ewes were randomly mated with rams. The ewes were directly obtained from farmers and nearby villages of Cele County during 2008–2010 and were not treated with hormones to synchronize estrus.
2.2. DNA Extraction
DNA was extracted from blood using a slightly modified standard phenol chloroform procedure [16]. DNA concentrations were assessed using a BioPhotometer plus (Eppendorf, Hamburg, Germany) and stored at −20 °C before use.
2.3. Detection of FecXI, FecXH, FecXG, and FecXB Mutation Sites of the BMP15 Gene
With reference to previous research [6,8,10], four pairs of primers were designed for FecXI, FecXH, FecXG, and FecXB to detect the BMP15 gene. These primers are listed in Table 1.

Table 1.
Primers of FecXI, FecXH, FecXG, and FecXB mutation sites of the Bone Morphogenetic Protein 15 (BMP15) gene.
The PCR reaction volume of 20 µL contained Taq DNA polymerase 1.5 U, 10 × buffer 2 µL, MgCl2 (25 mmol/L) 1.2 µL, dNTP (2.5 mmol/L) 2 µL, genomic DNA ~50 ng, as well as upstream and downstream primers (each 10 pM). Water was added at 20 µL.
The above sites were detected using the Touch-Down PCR reaction program. The reaction conditions were as follows: (1) 95 °C pre-denaturation for 5 min; (2) Annealing temperature for PCR was performed by gradient PCR which 95 °C denaturation for 30 s, 65 °C–51 °C (65 °C, 63 °C, 61 °C, 59 °C, 57 °C, 55 °C, 53 °C, 51 °C) annealing for 40 s, 72 °C extension for 30 s (each annealing temperature of 65 °C–53 °C for two cycles respectively and 51 °C for 19 cycles); (3) finally, extension at 72 °C for 5 min. The product was detected by 2% agarose gel electrophoresis.
Restriction enzyme digestion of the PCR product BMP15 gene FecXH mutation (CT) was amplified with the Spe I enzyme. The BMP15 gene FecXI mutation (TA) was amplified via Xba I endonuclease digestion. The BMP15 gene FecXG mutation amplification product was digested with the Hinf I enzyme. The FecXB mutation was amplified and digested with Dde I endonuclease. The digestion reaction system used 4 μL PCR product, 1 μL 10× buffer, and 5 U endonuclease filled to 10 μL with double-distilled water. The digestion used a water bath at 37 °C for 12 h. The digested product was detected by 3% agarose electrophoresis.
2.4. Exons 1 and 2 of BMP15 Gene Sequence Analysis
The sheep BMP15 genes were amplified using PCR with primers designed by Oligo version 6.0, using gene sequences published on GenBank (sheep genomic BMP15 exon 1, AF236078; sheep genomic BMP15 exon 2, AF236079).
The following PCR primers were used:
BMP15 exon 1:
Forward 5′-TTTCATTTTTCCTTGCCCTATCC-3′;
Reverse 5′CCTGACAGAAAACTGACAGATCC-3′.
BMP15 exon 2:
Forward 5′-TTCTCTGAGCTTCAGTTTCCTCG-3′;
Reverse 5′-TGCACCTTTGCCGTCACCTGCAT-3′.
Amplification was conducted for 35 cycles in a 20-μL reaction mixture, using 50 ng of DNA templates, 50 mm KCl, 10 mm Tris-HCL, 1.5 mm MgCl2, 200 μm of each dNTP, 5% DMSO, 10 pM of each primer, and 1.5 U of Taq DNA polymerase. Touchdown amplification was conducted using the following program: 95 °C for 5 min, followed by a gradient over 16 cycles of 95 °C for 45 s, 65 °C annealing for 60 s, and 72 °C extension for 90 s. The annealing temperature was subsequently decreased by 2 °C every second cycle; 19 cycles at 94 °C for 45 s, at 51 °C for 60 s, and at 72 °C for 90 s. The final extension at 72 °C lasted for 6 min. The PCR products were separated by electrophoresis at 85 V for 45 min in 1.5% agarose gels, and the results were visualized on a VDS system (UV and video).
Twelve samples from the flock were amplified; the size of BMP15 exon 1 was 550 bp, and that of BMP15 exon 2 was 1116 bp. The resulting PCR products were sequenced on an ABI 373 sequencer (Shanghai Biological Engineering Co., Ltd., Shanghai, China).
Two mutations were identified in the BMP15 gene. In exon 1, the 28–30 CTT base was missing, which resulted in a predicted leucine deletion in BMP15 protein (B1). At position 755 in exon 2, one T was changed to C, resulting in a predicted change from leucine to proline in BMP15 protein (T755C).
2.5. Mutation Detection Assays
The sequences of forward and reverse primers for the amplification of the fragment that includes new mutants of BMP15 genes are listed in Table 2. PCR was performed in 20 μL reaction mixture with 50 ng template DNA, 50 mM KCl, 10 mm Tris-HCL, 1.5 mm MgCl2, 200 μm of each dNTP, 5% DMSO, 10 pM of each primer, and 1.5 U of Taq DNA polymerase.

Table 2.
Primers of mutational sites in BMP15.
Tetra-primer amplification refractory polymorphism system PCR (Tetra-ARMS-PCR) was used to amplify B1 to identify the missing CTT using the four primers listed in Table 1. PCR products were separated on a 3% agarose gel and visualized with the VDS system. The fragment size showed fragments of 189 and 286 bp that were missing CTT (homozygous); 134, 189, and 286 bp (heterozygous); 134 and 286 bp that were the wild-type genotype (no missing CTT), and a fragment of 286 bp indicated the control size in each array.
To design the exon 2 forward primer, A was changed into G at the second end of the 3′ cleavage site to enhance recognition by the restriction enzyme Apa I. A total of 4 μL of PCR products was digested with 10 U Apa I, 1 μL 10× buffer, and ultra-pure water was added to a 10-μL final volume. This mixture was incubated at 37 °C for at least 12 h. The digested products were separated in an 8% polyacrylamide gel (29:1) for 8 h at 120 V. The gels were stained with silver nitrate and then observed in the VDS system. The homozygous sizes were 120 and 20 bp, heterozygous sizes were 140, 120, and 20 bp, and wild-type genotype (no mutation) size was 140 bp.
2.6. Statistical Analysis
Analysis of the association between genotypes and litter size was conducted by applying a general linear model (GLM) procedure using SAS software version 9.0 (SAS Institute Inc.). The model was described as:
where Y represents the value of litter size, μ represents the overall mean, a represents the effect of the genotype, and e represents the random residual error.
Y = μ + a + e
3. Results
3.1. Detection of FecXI, FecXH, FecXG, and FecXB Mutation Sites of the BMP15 Gene
Genomic DNA was PCR-amplified with primers at the FecXH site to obtain a target band with a size of 240 bp. After digestion with Spe I enzyme, it was detected by 8% non-denaturing PAGE electrophoresis. The results showed that the PCR product was not cleaved by the enzyme, and its size still remained at 240 bp (see Supplementary Figure S1A,B).
PCR amplification of genomic DNA with FecXI primers resulted in a target band with a size of 150 bp, and primer dimer bands also formed. However, this did not affect the enzymatic digestion. After Xba1 digestion, detection was performed by 8% non-denaturing PAGE electrophoresis, which identified multiple bands; however, their size was not the target band. The PCR product could not be cleaved by the enzyme. The type still retained its size of 150 bp, indicating a lack of polymorphism at this site in Cele black sheep (see Supplementary Figure S2A,B).
Genomic DNA was PCR-amplified with FecXG primers to obtain a 141-bp target band. After digestion with Hinf I endonuclease, the digested product was detected by 3% agarose electrophoresis. The result was only a 111-bp band, indicating that there was no polymorphism at this site in Cele black sheep (see Supplementary Figure S3A,B).
PCR amplification of genomic DNA with FecXB primers identified a target band of 153 bp. Although primer dimers were also found, this did not affect endonuclease digestion. After digestion, only a 122-bp band remained, indicating that there was no polymorphism at this site in Cele black sheep (see Supplementary Figure S4A,B).
3.2. Sequencing Results of the BMP15 Gene Coding Region
3.2.1. Exon 1 of the BMP15 Gene Sequence
From each of the 12 samples, exon 1 of the BMP15 gene was amplified and sequenced by an automated ABI DNA sequencer (model 373, PE Applied Biosystems). Comparison and blast data showed that the E1 site had a CTT deletion at 28–30 (Figure 1A–C).
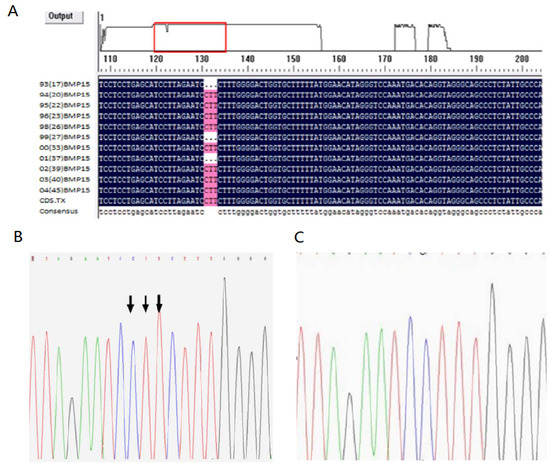
Figure 1.
Sequencing results of exon 1 of the BMP15 gene. (A) Comparison of sequence exon 1; (B) sequence of wild-type (without mutation) at E1+28-30 site of exon 1; (C) sequence of mutation (missing CTT) at E1+28-30 site of exon 1.
3.2.2. Exon 2 of the BMP15 Gene Sequence
Exon 2 of the BMP15 PCR products was sequenced for each of the 12 samples. Position 755 of exon 2 was changed, and T was replaced by C. This was predicted to result in a change from leucine to proline (Figure 2A,B).
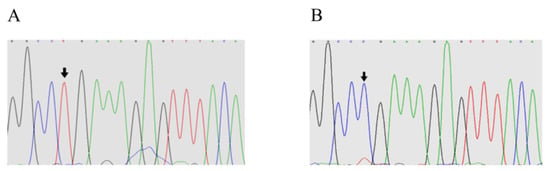
Figure 2.
Sequencing results of exon 2 of the BMP15 gene. (A) Sequence of wild-type E2+755 at the site of BMP15 exon 2; (B) sequence of mutation (T–C) at the E2+755 site of BMP15.
3.3. Mutation of the BMP15 Gene Polymorphism
A total of 205 ewes with reproduction records were tested using Tetra-ARMS-PCR methods for site E1+28-30. The 3% agarose gel showed that heterozygous (B+) samples for the mutation had fragments of all three sizes (134, 189, and 286 bp), homozygous (BB) had two sizes (189 and 286 bp), and the wild-type genotype (++) also had two sizes (134 and 286 bp, Figure 3).
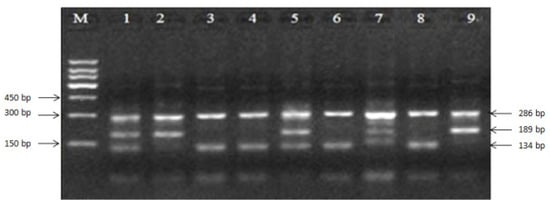
Figure 3.
Tetra-primer amplification refractory polymorphism system PCR (Tetra-ARMS-PCR) products of E1+28-30 site of exon 1 in BMP15. M: 150-bp marker; wild-type genotype (++): 3, 4, 6, and 8; heterozygous (B+): 1, 5, and 7; homozygous (BB): 2 and 9.
PCR-RFLP methods were used to identify E2+755 sites. PCR was conducted using primers with single mismatches to generate products containing restriction enzyme sites. The mismatch that was created in the appropriate primer to create the restriction enzyme Apa1 cleavage site was mentioned above (also see Figure 4A). The digested fragments were separated on an 8% polyacrylamide gel and were visualized with the VDS system. The fragments for the homozygous genotype (EE) were 120 and 20 bp; for the heterozygous genotype (E+), these were 140, 120, and 20 bp, and for the wild-type genotype (++), this was 140 bp, as the 20 bp was too small and moved out of the gel (Figure 4B).
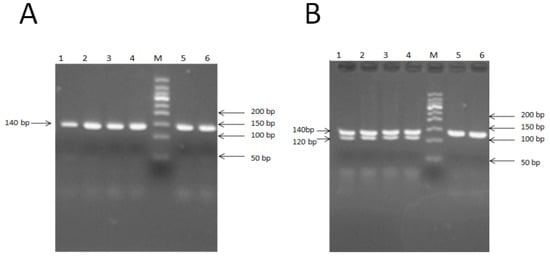
Figure 4.
Mutation in BMP15 gene exon 2. (A) PCR products of exon 2, E2+755 site of BMP15. M: 50-bp marker; 1–6: 140-bp M and 150-bp marker; wild-type genotype (++): 3, 4, 6, and 8; heterozygous genotype (B+): 1, 5, and 7; homozygous genotype (BB): 2 and 9. (B) PCR-RFLP 8% polyacrylamide gel result of exon 2, E2+755 site of BMP15. M: 100-bp marker; wild-type genotype (++): 5 and 6; heterozygous genotype (B+): 1, 2, 3 and 4.
3.4. Genotypic and Allelic Frequencies of the Mutation of the BMP15 Gene in Cele Black Sheep
The main genotypes in the investigated flock are the wild-type genotypes of E1+28-30 and E2+755 sites (Table 3). Both X2 values (p < 0.01) suggest that these genotypes significantly departed from the Hardy–Weinberg equilibrium, indicating that the flock was influenced by breeding or sampling.

Table 3.
Genotypic and allelic frequencies of E1 = 28-30 and E2 = 755 sites in Cele black sheep.
3.5. Effect of Genotypes on Litter Size
The results of litter sizes of different genotypes are shown in Table 4. Both the heterozygous genotypes of E1+28-30 and E2+755 had larger litter sizes (2.15 and 2.20). The litter size of E1+28-30 wild-type (++) ewes was higher than that of homozygous (BB) ewes. For E2+755, only heterozygous (E+) and wild-type (++) genotypes were identified, and heterozygous (E+) ewes produced 0.33 more offspring on average than wild-type (++) ewes (p < 0.05).

Table 4.
Least squares mean and standard errors for litter sizes of different BMP15 mutations.
4. Discussion
In mice, BMP15 is specifically expressed in the oocyte, initially at the one-layer primary follicle stage, which continues through ovulation. Interestingly, BMP15 is most closely related to and shares a coincident expression pattern with the mouse GDF9 gene, which is essential for female fertility [17]. In mammals with a low ovulation rate phenotype, both oocyte-derived GDF9 and BMP15 proteins are essential for normal follicular development, both at the early and later stages of oocyte growth. Regulation of BMP15, GDF9, or both is potentially a new technique to enhance fecundity in mammals [18,19]. In ewes immunized with different BMP15 or GDF9 peptide sequences, antibodies generated against the N-terminal region of BMP15 or GDF9 were potent inhibitors of the paracrine actions of these oocyte-secreted factors both in vivo and in vitro. This induced an ovulation phenotype that blocks the biological actions of BMP15 or GDF9 at their N-termini, suggesting potential as contraceptives or sterilizing agents [20]. Similarly, in cattle, immunization against BMP15 and/or GDF9 altered follicle development [21], and BMP15 appears to be important in promoting follicle growth at the early stages while restraining the number of dominant preovulatory follicles. BMP15 is a key determinant of both ovulation number and litter size in sheep and cattle [22].
Mutations of the BMP15 gene have been identified in several sheep breeds across the world, indicating that the heterozygous genotype (B+) influences the additive effect on ovulation rate. Apparently, recombination or mutations are frequent within the BMP15 gene in sheep. FecXI, FecXH, FecXG, and FecXB of the BMP15 gene are closely related to the fetal sex of several sheep. No polymorphisms were found in the investigated population of Cele black sheep, indicating that these are not associated with the number of high-yielding lambs in this breed.
Dube discovered two mutations in the BMP15 gene, both of which result in high yields of Inverdale sheep [17]. In their report, the Inverdale (FecXI) allele or the Hanna (FecXH) allele increased litter size, while homozygous ewes that inherited alleles from both parents had small, undeveloped ovaries and were infertile. B1 mutation has also been identified at the 28-30 position of exon 1 with a three-base deletion of CTT, which leads to BMP15 protein deletion of leucine. This locus has previously been shown to be polymorphic (without any phenotypic effect) in the predicted signal sequence, while a subset of sheep have two leucine codons (CTT) at this position and other sheep have only one. The present study also found B1 deletion, and three genotypes were detected. Analysis showed that the homozygous genotype (BB) had the smallest litter size compared with the heterozygous genotype (B+) with the highest litter size and the wild-type genotype (++). The differences between homozygous (BB) and wild-type (++) phenotypes were not significant (p > 0.05) and neither were the differences between heterozygous (B+) and wild-type (++) phenotypes (p > 0.05). Furthermore, two leucine codons were detected in Cele black sheep. These results suggest that B1 deletion does not increase ovulation in Cele black sheep.
The presented results show that BMP15 has a mutation at position 755 of exon 2 in Cele black sheep, which results in proline instead of leucine formation (p.L252P). Only heterozygous (E+) and wild-type (++) genotypes were detected in the 205 tested ewes. No homozygous genotype (EE) was observed. A possible reason is that only the mutations in ewes with offspring were tested, and the allele frequency of mutation E was low (0.102). This may have resulted in a particularly low frequency of the homozygous genotype (EE) or EE may be sterile. Consequently, ewes with offspring records were unavailable, and more samples are needed in the future. This study showed that heterozygous (E+) ewes have larger litter sizes than wild-type (++) ewes (p < 0.05), suggesting that this mutation may have influenced the litter size by a change in the structure and function of the BMP15 protein.
5. Conclusions
According to many studies of the BMP15 gene in sheep, three characteristics of changes in gene function can be summarized: (1) Heterozygous mutations of BMP15 increase the ovulation rate and ewes have more progeny; (2) homozygous individuals show abnormal ovarian development, rudimentary “streak” ovaries, and sterility; (3) all mutations are within the BMP15 gene coding region, which disrupts the BMP15 protein and causes either premature termination or produces an amino acid change in the mature coding region, which changes the protein function. These studies support that p.L252P is associated with reproductive effects in Cele black sheep. In this study, no EE genotype of p.L252P was observed. To date, it is not clear if ewes with the EE genotype are sterile or if the sample size was insufficient. In summary, this locus is a major gene which controls the multi-fetal performance of Cele black sheep.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/2076-2615/11/3/668/s1, Figure S1A: PCR products of the FecXH gene; Figure S1B: Digestion of PCR products of the FecXH gene. Figure S2A: PCR products of the FecXI gene; Figure S2B: Digestion of PCR products of the FecXI gene; Figure S3A: PCR products of the FecXG gene; Figure S3B: Digestion of PCR products of the FecXG gene; Figure S4A: PCR products of the FecXB gene; Figure S4B: Digestion of PCR products of the FecB gene.
Author Contributions
Conceptualization, Z.-g.N., Y.-g.D., and S.T.; design of the study and methodology, H.-c.S., Z.-g.N. and Y.-g.D.; data analysis and interpretation, Z.-g.N., S.T., and Y.J.; writing—original draft preparation, Z.-g.N. and J.Q.; critical revision, X.-D.D.; funding, H.-c.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Funds of China (No. 30760161), the Xinjiang Uygur Autonomous Region Regional Collaborative Innovation Special Project (Shanghai Cooperation Organization Technology Partnership Program, No. 2017E01017), and the Xinjiang Uygur Autonomous Region Duolang sheep genetic improvement plan project.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Xinjiang Academy of Animal Science.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study was mutation sites detect by using the restriction enzyme. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This study was supported by the College of Animal Science and Technology, China Agricultural University. We thank the staff of the Animal Husbandry and Veterinary Bureau of Cele County who recorded the lambing and helped to take samples. Particular thanks go to Quanyou Yang and Muniresha.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Abdoli, R.; Mirhoseini, S.Z.; Ghavi Hossein-Zadeh, N.; Zamani, P.; Gondro, C. Genome-wide association study to identify genomic regions affecting prolificacy in Lori-Bakhtiari sheep. Anim. Genet. 2018, 49, 488–491. [Google Scholar] [CrossRef]
- Narjess, L.; Zohra, B.; Florent, W.; Ahmed, R.; Mohamed, A.; Mourad, R.; Stephane, F.; Sonia, B.R. FecX a Novel BMP15 mutation responsible for prolificacy and female sterility in Tunisian Barbarine Sheep. BMC Genet. 2017, 18, 43. [Google Scholar]
- Morteza, M.; Shahram, N.; Davood, S.H. Mutation in BMPR-IB gene is associated with litter size in Iranian Kalehkoohi sheep. Anim. Reprod. Sci. 2014, 147, 93–98. [Google Scholar]
- McNatty, K.P.; Juengell, J.L.; Wilson, T.; Galloway, S.M.; Davis, G.H.; Hudson, N.L.; Moeller, C.L.; Cranfield, M.; Reade, K.L.; Laitinen, M.P.; et al. Oocyte-derived growth factors and ovulation rate in sheep. Reproduction 2003, 61, 339–351. [Google Scholar] [CrossRef]
- Davis, G.H. Major genes affecting ovulation rate in sheep. Genet. Sel. Evol. 2005, 37 (Suppl. 1), S11–S23. [Google Scholar] [CrossRef]
- Hiam, N.; Karima, G.M.M.; Mohamed, M.M.K.; Nermeen, A.H.; Shawky, S.I.; Mahmoud, F.N.; Othman, E. PCR-RFLP of bone morphogenetic protein 15 (BMP15/FecX) gene as a candidate for prolificacy in sheep. Int. J. Vet. Sci. Med. 2018, 6, S68–S72. [Google Scholar]
- Shi, H.; Bai, J.; Niu, Z.; Esha, M.; Fen, L.; Jia, B. Study on Candidate Gene for Fecundity Traits in Xingjiang Cele Black Sheep. Afr. J. Biotechnol. 2010, 9, 8498–8505. [Google Scholar]
- Galloway, S.M.; McNatty, K.P.; Cambridge, L.M.; Laitinen, M.P.E.; Juengel, J.L.; Jokiranta, T.S.; McLaren, R.J.; Luiro, K.; Dodds, K.G.; Montgomery, G.W.; et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.H.; Mcewan, J.C.; Fennessy, P.; Dodds, K.G. Discovery of the Inverdale gene (FecX). In Proceedings of the New Zealand Society of Animal Production conference, Mosgiel, New Zealand, 27–30 August 1995. [Google Scholar]
- Davis, G.H.; Bruce, G.D.; Dodds, K.G. Ovulation rate and litter size of prolific Inverdale (FecX I) and Hanna (FecX H) sheep. Proc. Assoc. Adv. Anim. Breed Genet. 2001, 14, 175–178. [Google Scholar]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Bodin, L.; Di-Pasquale, E.; Fabre, S.; Bontoux, M.; Monget, P.; Persani, L.; Mulsant, P. A novel mutation in the bone morphogenetic protein 15 gene causing defective protein secretion is associated with both increased ovulation rate and sterility in Lacaune sheep. J. Endocrinol. 2007, 148, 393–400. [Google Scholar] [CrossRef]
- Martinez-Royo, A.; Jurado, J.J.; Smulders, J.P.; Marti, J.I.; Alabart, J.L.; Roche, A.; Fantova, E.; Bodin, L.; Mulsant, P.; Serrano, M.; et al. A deletion in the bone morphogenetic protein 15 gene causes sterility and increased prolificacy in Rasa Aragonesa sheep. Anim. Genet. 2008, 39, 294–297. [Google Scholar] [CrossRef]
- Monteagudo, L.V.; Ponz, R.; Tejedor, M.T.; Lavina, A.; Sierra, I. A 17 bp deletion in the Bone Morphogenetic Protein 15 (BMP15) gene is associated to increased prolificacy in the Rasa Aragonesa sheep breed. Anim. Reprod. Sci. 2009, 110, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.R.; Ajaki, A.; Farahi, M.; Heidari, M.; Pirali, A.; Forouzanfar, M.; Eghbalsaied, S. The novel t755c mutation in BMP15 is associated with the litter size of iranian afshari, ghezel, and shal breeds. Arch. Anim. Breed. 2013, 61, 153–160. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. Cold Spring Harb. Protoc. 2006, 2006. [Google Scholar] [CrossRef] [PubMed]
- Dube, J.L.; Wang, P.; Elvin, J.; Lyons, K.M.; Celeste, A.J.; Matzuk, M.M. The Bone Morphogenetic Protein 15 Gene Is X-Linked and ExPressed in Oocytes. Mol. Endocrinol. 1998, 12, 1809–1817. [Google Scholar] [CrossRef]
- Juengel, J.L.; Hudson, N.L.; Heath, D.A.; Smith, P.; Reader, K.L.; Lawrence, S.B.; O’Connell, A.R.; Laitinen, M.P.; Cranfield, M.; Groome, N.P.; et al. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol. Reprod. 2002, 67, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; Hudson, N.L.; Berg, M.; Hamel, K.; Smith, P.; Lawrence, S.B.; Whiting, L.; McNatty, K.P. Effects of active immunization against growth differentiation factor 9 and/or bone morphogenetic protein 15 on ovarian function in cattle. Reproduction 2009, 138, 107–114. [Google Scholar] [CrossRef]
- McNatty, K.P.; Hudson, N.L.; Whiting, L.; Reader, K.L.; Lun, S.; Western, A.; Heath, D.A.; Smith, P.; Moore, L.G.; Juengel, J.L. The effects of immunizing sheep with different BMP15 or GDF9 peptide sequences on ovarian follicular activity and ovulation rate. Biol. Reprod. 2007, 76, 552–560. [Google Scholar] [CrossRef]
- Juengel, J.L.; Hudson, N.L.; Whiting, L.; McNatty, K.P. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol. Reprod. 2004, 70, 557–561. [Google Scholar] [CrossRef]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).