X-Linked Hypohidrotic Ectodermal Dysplasia in Crossbred Beef Cattle Due to a Large Deletion in EDA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd history and Clinical Investigation
2.2. Pathological Investigation
2.3. Genetic Investigation
3. Results
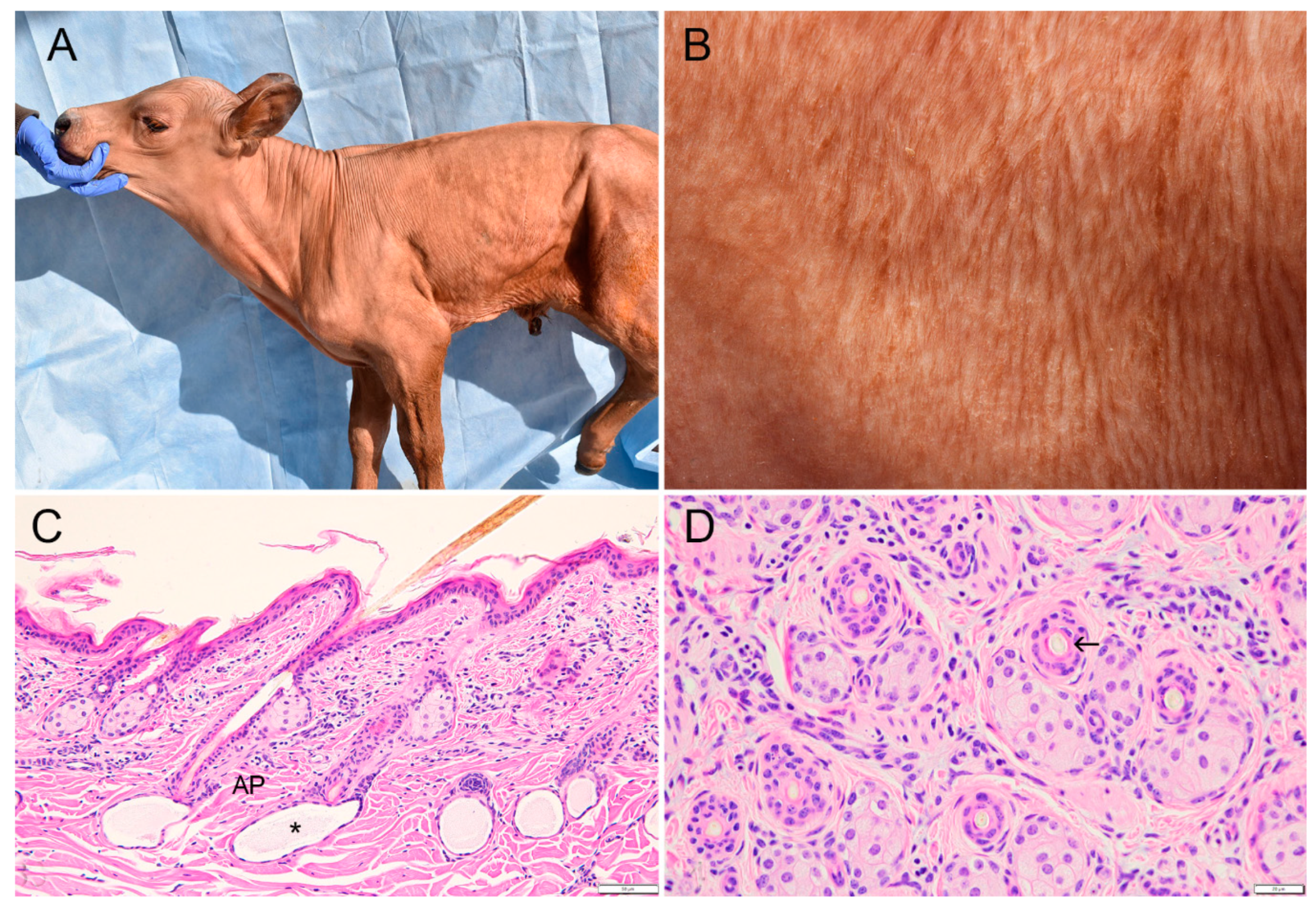
3.1. Clinicopathological Phenotype
3.2. Genetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cluzeau, C.; Hadj-Rabia, S.; Jambou, M.; Mansour, S.; Guigue, P.; Masmoudi, S.; Bal, E.; Chassaing, N.; Vincent, M.-C.; Viot, G.; et al. Only four genes (EDA1, EDAR, EDARADD, and WNT10A) account for 90% of hypohidrotic/anhidrotic ectodermal dysplasia cases. Hum. Mutat. 2010, 32, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T.; Fete, M.; Schneider, H.; Zinser, M.; Koster, M.I.; Clarke, A.J.; Hadj-Rabia, S.; Tadini, G.; Pagnan, N.; Visinoni, A.F.; et al. Ectodermal dysplasias: Classification and organization by phenotype, genotype and molecular pathway. Am. J. Med. Genet. Part A 2019, 179, 442–447. [Google Scholar] [CrossRef]
- Sadier, A.; Viriot, L.; Pantalacci, S.; Laudet, V. The ectodysplasin pathway: From diseases to adaptations. Trends Genet. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Darwin, C. The Variation of Animals and Plants under Domestication, 2nd ed.; John Murray: London, UK, 1875; Volume 2, p. 319. [Google Scholar]
- Beahrs, J.O.; Lillington, G.A.; Rosan, R.C.; Russin, L.; Lindgren, J.A.; Rowley, P.T. Anhidrotic ectodermal dysplasia: Predisposition to bronchial disease. Ann. Intern. Med. 1971, 74, 92. [Google Scholar] [CrossRef] [PubMed]
- Casal, M.L.; Jezyk, P.F.; Greek, J.M.; Goldschmidt, M.H.; Patterson, D.F. X-Linked ectodermal dysplasia in the dog. J. Hered. 1997, 88, 513–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del-Pozo, J.; MacIntyre, N.; Azar, A.; Headon, D.; Schneider, P.; Cheeseman, M. Role of ectodysplasin signalling in middle ear and nasal pathology in rat and mouse models of hypohidrotic ectodermal dysplasia. Dis. Model. Mech. 2019, 12, dmm037804. [Google Scholar] [CrossRef]
- Drogemuller, C.; Distl, O.; Leeb, T. Partial deletion of the bovine ED1 gene causes anhidrotic ectodermal dysplasia in cattle. Genome Res. 2001, 11, 1699–1705. [Google Scholar] [CrossRef][Green Version]
- Drögemüller, C.; Distl, O.; Leeb, T. X-linked anhidrotic ectodermal dysplasia (ED1) in men, mice, and cattle. Genet. Sel. Evol. 2003, 35, S137–S145. [Google Scholar] [CrossRef]
- Escouflaire, C.; Rebours, E.; Charles, M.; Orellana, S.; Cano, M.; Rivière, J.; Grohs, C.; Hayes, H.; Capitan, A. A de novo 3.8-Mb inversion affecting the EDA and XIST genes in a heterozygous female calf with generalized hypohidrotic ectodermal dysplasia. BMC Genom. 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pispa, J.; Hartung, A.J.; Du, Y.; Ezer, S.; Jenks, T.; Shimada, T.; Pekkanen, M.; Mikkola, M.L.; Ko, M.S.H.; et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc. Natl. Acad. Sci. USA 1997, 94, 13069–13074. [Google Scholar] [CrossRef]
- Waluk, D.P.; Zur, G.; Kaufmann, R.; Welle, M.M.; Jagannathan, V.; Drögemüller, C.; Müller, E.J.; Leeb, T.; Galichet, A. A Splice Defect in the EDA Gene in dogs with an X-Linked Hypohidrotic Ectodermal Dysplasia (XLHED) Phenotype. G3 Genes|Genomes|Genet. 2016, 6, 2949–2954. [Google Scholar] [CrossRef]
- Falconer, D.S. A Totally sex-linked gene in the house mouse. Nat. Cell Biol. 1952, 169, 664–665. [Google Scholar] [CrossRef]
- Grüneberg, H. Genes and genotypes affecting the teeth of the mouse. J. Embryol. Exp. Morphol. 1965, 14, 137–159. [Google Scholar]
- Grüneberg, H. The glandular aspects of the tabby syndrome in the mouse. J. Embryol. Exp. Morphol. 1971, 25, 1–19. [Google Scholar] [PubMed]
- Casal, M.L.; Lewis, J.R.; Mauldin, E.A.; Tardivel, A.; Ingold, K.; Favre, M.; Schneider, P. Significant correction of disease after postnatal administration of recombinant ecto-dysplasin A in canine X-linked ectodermal dysplasia. Am. J. Hum. Genet. 2007, 81, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Gaide, O.; Schneider, P. Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nat. Med. 2003, 9, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Margolis, C.A.; Schneider, P.; Huttner, K.; Kirby, N.; Houser, T.P.; Wildman, L.; Grove, G.L.; Schneider, H.; Casal, M.L. Prenatal treatment of X-Linked hypohidrotic ectodermal dysplasia using recombinant ectodysplasin in a canine model. J. Pharmacol. Exp. Ther. 2019, 370, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Faschingbauer, F.; Schuepbach-Mallepell, S.; Körber, I.; Wohlfart, S.; Dick, A.; Wahlbuhl, M.; Kowalczyk-Quintas, C.; Vigolo, M.; Kirby, N.; et al. Prenatal correction of X-Linked hypohidrotic ectodermal dysplasia. N. Engl. J. Med. 2018, 378, 1604–1610. [Google Scholar] [CrossRef]
- Barlund, C.S.; Clark, E.G.; Leeb, T.; Drögemüller, C.; Palmer, C.W. Congenital hypotrichosis and partial anodontia in a crossbred beef calf. Can. Vet. J. 2007, 48, 612–614. [Google Scholar] [PubMed]
- Braun, U.; Ansari, A.H.; Hediger, R.; Süss, U.; Ehrensperger, F. Hypotrichosis and oligodontia, combined with an Xq-deletion, in a calf of the Swiss Holstein breed. Tierarztl. Prax. 1988, 16, 39–44. [Google Scholar] [PubMed]
- Drögemüller, C.; Peters, M.; Pohlenz, J.; Distl, O.; Leeb, T. A single point mutation within the ED1 gene disrupts correct splicing at two different splice sites and leads to anhidrotic ectodermal dysplasia in cattle. J. Mol. Med. 2002, 80, 319–323. [Google Scholar] [CrossRef]
- Gargani, M.; Valentini, A.; Pariset, L. A novel point mutation within the EDA gene causes an exon dropping in mature RNA in Holstein Friesian cattle breed affected by X-linked anhidrotic ectodermal dysplasia. BMC Vet. Res. 2011, 7, 35. [Google Scholar] [CrossRef]
- Karlskov-Mortensen, P.; Cirera, S.; Nielsen, O.L.; Arnbjerg, J.; Reibel, J.; Fredholm, M.; Agerholm, J.S. Exonization of a LINE1 fragment implicated in X-linked hypohidrotic ectodermal dysplasia in cattle. Anim. Genet. 2011, 42, 578–584. [Google Scholar] [CrossRef]
- Ogino, A.; Kohama, N.; Ishikawa, S.; Tomita, K.; Nonaka, S.; Shimizu, K.; Tanabe, Y.; Okawa, H.; Morita, M. A novel mutation of the bovine EDA gene associated with anhidrotic ectodermal dysplasia in Holstein cattle. Hereditas 2011, 148, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ogino, A.; Shimizu, K.; Tanabe, Y.; Morita, M. De novo mutation of the bovine EDA gene associated with anhidrotic ectodermal dysplasia in Japanese Black cattle. Anim. Genet. 2011, 43, 646. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, F.; Drögemüller, C.; Tegtmeier, P.; Baumgartner, W.; Distl, O.; Leeb, T. Ectodysplasin-1 Deficiency in a german holstein bull associated with loss of respiratory mucous glands and chronic rhinotracheitis. J. Comp. Pathol. 2005, 132, 346–349. [Google Scholar] [CrossRef]
- Bourneuf, E.; Otz, P.; Pausch, H.; Jagannathan, V.; Michot, P.; Grohs, C.; Piton, G.; Ammermüller, S.; Deloche, M.C.; Fritz, S.; et al. Rapid discovery of de novo deleterious mutations in cattle enhances the value of livestock as model species. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Cole, L.J. A Defect of hair and teeth in cattle—Probably hereditary1. J. Hered. 1919, 10, 303–306. [Google Scholar] [CrossRef]
- Denis, B.; Théret, M.; Blin, P.; Bernard, C.; Lauvergne, J. Hypotrichose congénitale en race bovine normande. I.—Étude descriptive. Ann. Génétique Sélection Anim. 1975, 7, 251–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drieux, H.; Priouzeau, M.L.; Thiery, G. Congenital hypotrichosis with absence of teeth and horns, and macroglossia in a calf. Recl. Med. Vet. 1950, 126, 385–399. [Google Scholar]
- Wijeratne, W.V.; O’Toole, D.; Wood, L.; Harkness, J.W. A genetic, pathological and virological study of congenital hypotrichosis and incisor anodontia in cattle. Vet. Rec. 1988, 122, 149–152. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Smith, J.A.; Schlessinger, D.; Chan, C.-C. X-Linked anhidrotic ectodermal dysplasia disruption yields a mouse model for ocular surface disease and resultant blindness. Am. J. Pathol. 2005, 167, 89–95. [Google Scholar] [CrossRef]
- Mehta, U.; Brunworth, J.; Fete, T.J.; Sindwani, R. Head and neck manifestations and quality of life of patients with ectodermal dysplasia. Otolaryngol. Head Neck Surg. 2007, 136, 843–847. [Google Scholar] [CrossRef]
- Wohlfart, S.; Meiller, R.; Hammersen, J.; Park, J.; Menzel-Severing, J.; Melichar, V.O.; Huttner, K.; Johnson, R.; Porte, F.; Schneider, H. Natural history of X-linked hypohidrotic ectodermal dysplasia: A 5-year follow-up study. Orphanet J. Rare Dis. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Anbouba, G.M.; Carmany, E.P.; Natoli, J.L. The characterization of hypodontia, hypohidrosis, and hypotrichosis associated with X-linked hypohidrotic ectodermal dysplasia: A systematic review. Am. J. Med. Genet. Part A 2020, 182, 831–841. [Google Scholar] [CrossRef]
- Moura, E.; Cirio, S.M. Clinical and genetic aspects of X-linked ectodermal dysplasia in the dog—A review including three new spontaneous cases. Vet. Dermatol. 2004, 15, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Reiter, A.M.; Mauldin, E.A.; Casal, M.L. Dental abnormalities associated with X-linked hypohidrotic ectodermal dysplasia in dogs. Orthod. Craniofacial Res. 2010, 13, 40–47. [Google Scholar] [CrossRef]
- Moura, E.; Rotenberg, I.S.; Pimpão, C.T. X-Linked hypohidrotic ectodermal dysplasia—General features and dental abnormalities in affected dogs compared with human dental abnormalities. Top. Companion Anim. Med. 2019, 35, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cornish, T.E.; Van Olphen, A.L.; Cavender, J.L.; Edwards, J.M.; Jaeger, P.T.; Vieyra, L.L.; Woodard, L.F.; Miller, D.R.; O’Toole, D. Comparison of ear notch immunohistochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhea virus. J. Vet. Diagn. Investig. 2005, 17, 110–117. [Google Scholar] [CrossRef]
- Häfliger, I.M.; Wiedemar, N.; Švara, T.; Starič, J.; Cociancich, V.; Šest, K.; Gombač, M.; Paller, T.; Agerholm, J.S.; Drögemüller, C. Identification of small and large genomic candidate variants in bovine pulmonary hypoplasia and anasarca syndrome. Anim. Genet. 2020, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Brown, W.A.; Christofferson, P.V.; Massler, M.; Weiss, M.B. Postnatal tooth development in cattle. Am. J. Vet. Res. 1960, 21, 7–34. [Google Scholar]
- Lyne, A.; Heideman, M.J. The Pre-Natal Development of Skin and hair in Cattle (Bos Taurus L.). Aust. J. Biol. Sci. 1959, 12, 72–95. [Google Scholar] [CrossRef]
- Cui, C.Y.; Kunisada, M.; Childress, V.; Michel, M.; Schlessinger, D. Shh is required for Tabby hair follicle development. Cell Cycle. 2011, 10, 3379–3386. [Google Scholar] [CrossRef]
- McEwan Jenkinson, D.; Nay, T. The sweat glands and hair follicles of European cattle. Aust. J. Biol. Sci. 1972, 25, 585–595. Available online: https://www.publish.csiro.au/bi/pdf/bi9720585 (accessed on 3 January 2021). [CrossRef] [PubMed][Green Version]
- Rouse, C.; Siegfried, E.; Breer, W.; Nahass, G. Hair and sweat glands in families with hypohidrotic ectodermal dysplasia: Further characterization. Arch. Dermatol. 2004, 140, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Blecher, S.R. Anhidrosis and absence of sweat glands in mice hemizygous for the tabby gene; supportive evidence for the hypothesis of homology between tabby and human anhidrotic (hypohidrotic) ectodermal dysplasia (Christ-Siemens-Touraine Syndrome). J. Investig. Dermatol. 1986, 87, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Donegan, S.; Hayman, R. Sweating rate at different body regions in cattle and its correlation with some quantitative components of sweat gland volume for a given area of skin. Aust. J. Agric. Res. 1969, 20, 395–403. [Google Scholar] [CrossRef]
- Casal, M.L.; Mauldin, E.A.; Ryan, S.; Scheidt, J.L.; Kennedy, J.; Moore, P.F.; Felsburg, P.J. Frequent respiratory tract infections in the canine model of X-linked ectodermal dysplasia are not caused by an immune deficiency. Vet. Immunol. Immunopathol. 2005, 107, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, D.; Hewicker-Trautwein, M.; Klotz, D.; Fehr, M.; Ruseva, S.; Arndt, J.; Metzger, J.; Distl, O. A de novo EDA-Variant in a litter of shorthaired standard dachshunds with X-Linked hypohidrotic ectodermal dysplasia. G3 Genes|Genomes|Genet. 2019, 9, 95–104. [Google Scholar] [CrossRef]
- Boran, T.; Lesot, H.; Peterka, M.; Peterkova, R. Increased apoptosis during morphogenesis of the lower cheek teeth in Tabby/EDA Mice. J. Dent. Res. 2005, 84, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sofaer, J.A. Aspects of the tabby-crinkled-downless syndrome. I. The development of tabby teeth. J. Embryol. Exp. Morphol. 1969, 22, 181–205. [Google Scholar]
- Yen, C.L.E.; Brown, C.H., IV; Monetti, M.; Farese, R.V., Jr. A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005, 46, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Sankar, V.; Bashyam, M.D.; Bashyam, D. A novel large deletion that encompasses EDA and the downstream gene AWAT2 causes X-linked hypohidrotic/anhidrotic ectodermal dysplasia. J. Dermatol. Sci. 2016, 84, 105–107. [Google Scholar] [CrossRef] [PubMed]



| Calves | Number of Hair Follicles/Area (153,137 µm2) a | Mean Follicle Density/Area (153,137 µm2) | Mean Density Follicles/mm2 | |||
|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | ||||
| Affected | No. 1 | 30 | 31 | 30 | 30.3 | 164.50 |
| Affected b | No. 3 | 22 | 18 | 23 | 21.0 | |
| Unaffected c | No. 1 | 20 | 23 | 22 | 21.7 | 133.9 |
| Unaffected d | No. 2 | 18 | 21 | 19 | 19.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Toole, D.; Häfliger, I.M.; Leuthard, F.; Schumaker, B.; Steadman, L.; Murphy, B.; Drögemüller, C.; Leeb, T. X-Linked Hypohidrotic Ectodermal Dysplasia in Crossbred Beef Cattle Due to a Large Deletion in EDA. Animals 2021, 11, 657. https://doi.org/10.3390/ani11030657
O’Toole D, Häfliger IM, Leuthard F, Schumaker B, Steadman L, Murphy B, Drögemüller C, Leeb T. X-Linked Hypohidrotic Ectodermal Dysplasia in Crossbred Beef Cattle Due to a Large Deletion in EDA. Animals. 2021; 11(3):657. https://doi.org/10.3390/ani11030657
Chicago/Turabian StyleO’Toole, Donal, Irene M. Häfliger, Fabienne Leuthard, Brant Schumaker, Lynn Steadman, Brian Murphy, Cord Drögemüller, and Tosso Leeb. 2021. "X-Linked Hypohidrotic Ectodermal Dysplasia in Crossbred Beef Cattle Due to a Large Deletion in EDA" Animals 11, no. 3: 657. https://doi.org/10.3390/ani11030657
APA StyleO’Toole, D., Häfliger, I. M., Leuthard, F., Schumaker, B., Steadman, L., Murphy, B., Drögemüller, C., & Leeb, T. (2021). X-Linked Hypohidrotic Ectodermal Dysplasia in Crossbred Beef Cattle Due to a Large Deletion in EDA. Animals, 11(3), 657. https://doi.org/10.3390/ani11030657








