Clinicopathological and Genomic Characterization of a Simmental Calf with Generalized Bovine Juvenile Angiomatosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Pathological Investigation
2.2. DNA Sample and Whole-Genome Sequencing
2.3. Evaluation of the Molecular Consequences of Amino Acid Substitutions
3. Results
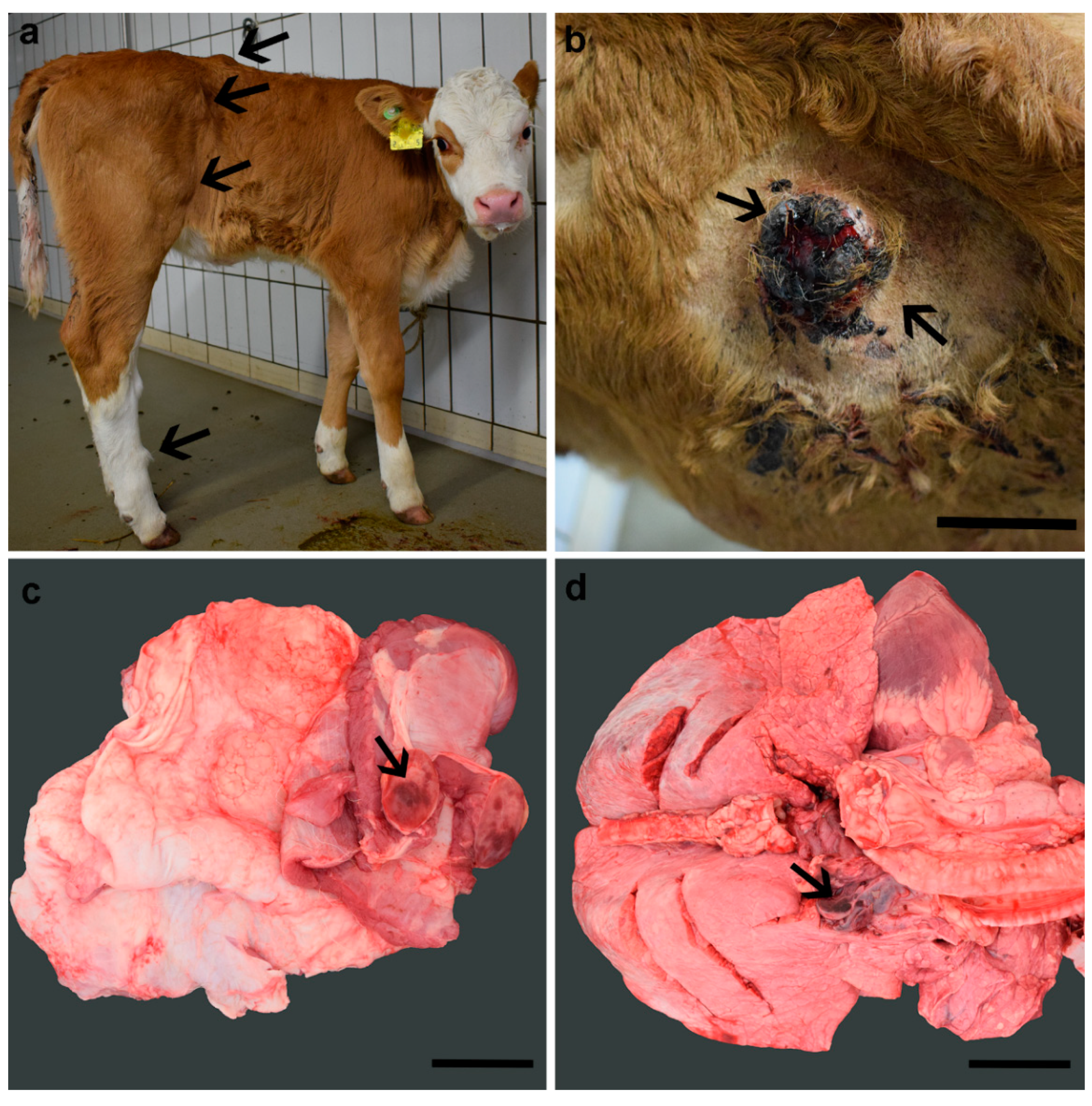
3.1. Clinical Phenotype
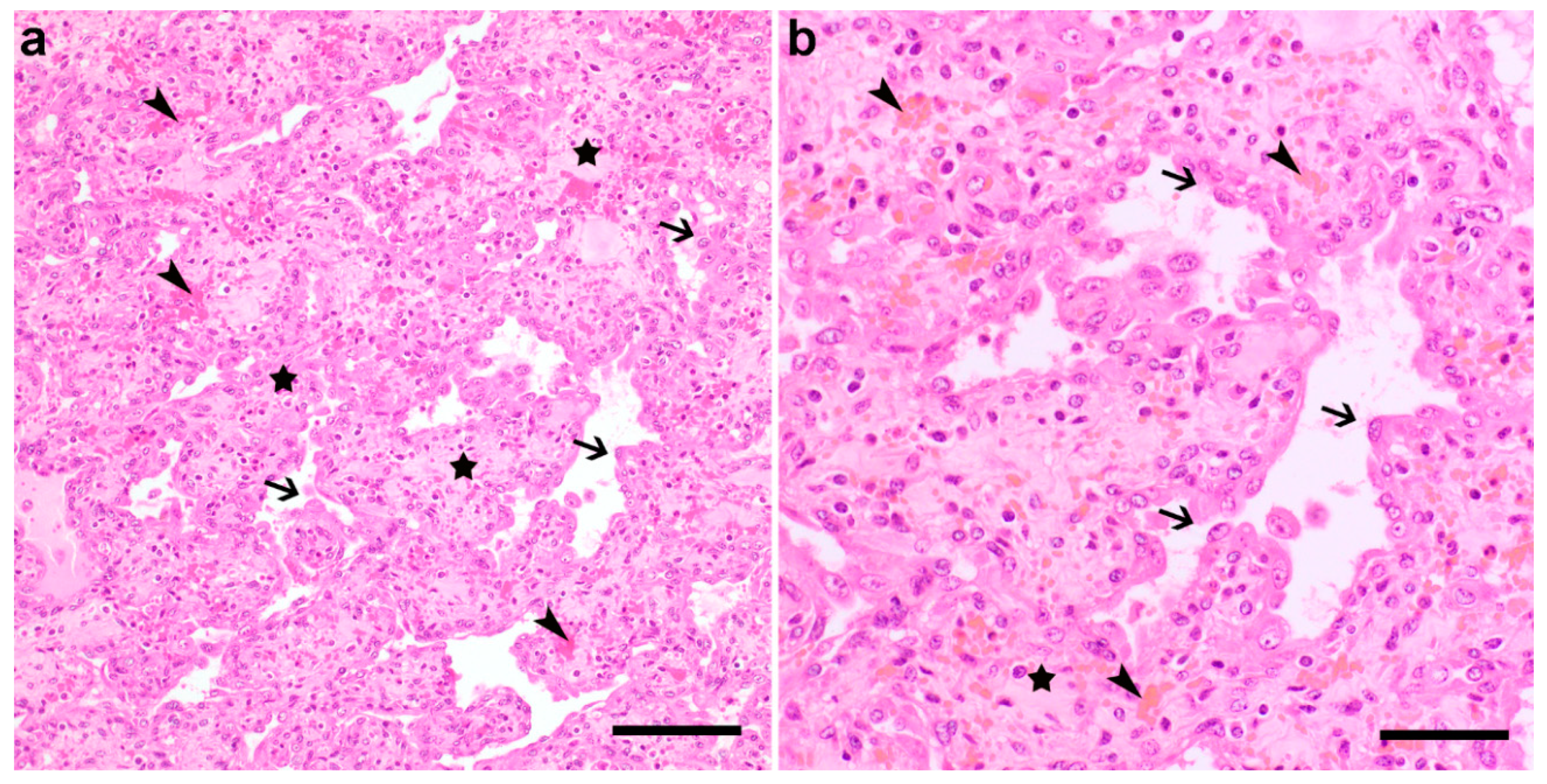
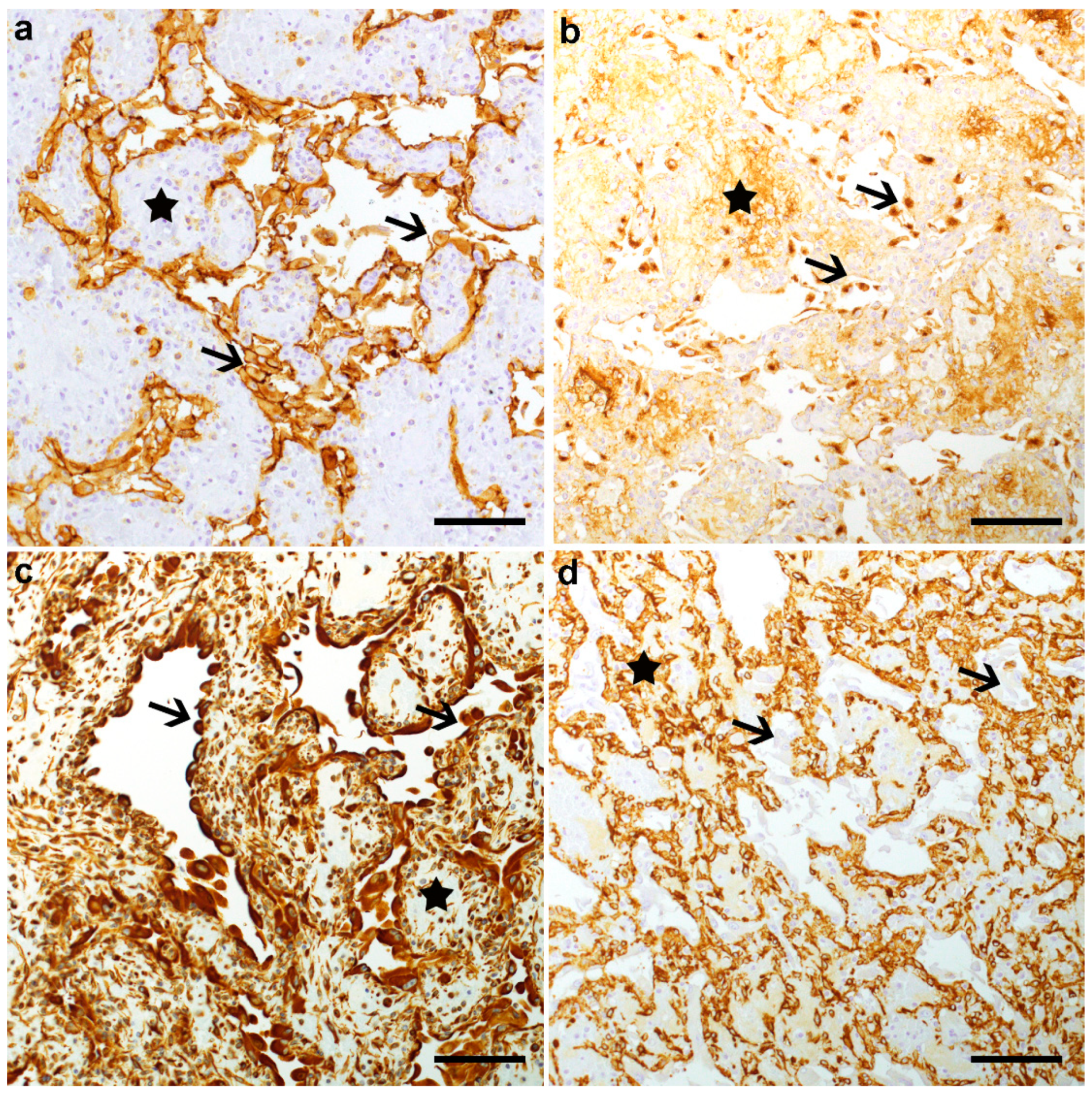
3.2. Pathological Phenotype
3.3. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamo, L.; Beck-Popovic, M.; Gudinchet, F.; Meuli, R. Congenital tumors: Imaging when life just begins. Insights Imaging 2011, 2, 297–308. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E. Vascular Anomalies Classi fi cation: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, 203–214. [Google Scholar] [CrossRef] [PubMed]
- ISSVA Classification of Vascular Anomalies. Available online: issva.org/classification (accessed on 23 November 2020).
- Mansfield, S.A.; Williams, R.F.; Iacobas, I. Vascular tumors. Semin. Pediatr. Surg. 2020, 29. [Google Scholar] [CrossRef]
- Priestnall, S.L.; De Bellis, F.; Bond, R.; Alony-Gilboa, Y.; Summers, B.A. Spontaneous regression of congenital cutaneous hemangiomas in a calf. Vet. Pathol. 2010, 47, 343–345. [Google Scholar] [CrossRef]
- Watson, T.D.; Thompson, H. Juvenile bovine angiomatosis: A syndrome of young cattle. Vet. Rec. 1990, 127, 279–282. [Google Scholar]
- Rösti, L.; Lauper, J.; Merhof, K.; Gorgas, D.; Ross, S.; Grest, P.; Welle, M. Angeborene Gefässanomalien in der Maulhöhle bei zwei Kälbern. Schweiz Arch Tierheilkd 2013, 627–632. [Google Scholar] [CrossRef][Green Version]
- Sheahan, B.J.; Donnelly, W. Vascular hamartoma in the gingiva of two neonatal calves. J. Am. Vet. Med. Assoc. 1981, 184, 205–206. [Google Scholar]
- Yeruham, I.; Abramovitch, I.; Perl, S. Gingival vascular hamartoma in two calves. Aust. Vet. J. 2004, 82, 152–153. [Google Scholar] [CrossRef]
- Stanton, M.; Meunier, P.; Smith, D. Vascular hamartoma in the gingiva of two neonatal calves. J. Am. Vet. Med. Assoc. 1984, 184, 205–206. [Google Scholar]
- Wilson, R.B. Gingival vascular hamartoma in three calves. J. Vet. Diagnostic Investig. 1990, 2, 338–339. [Google Scholar] [CrossRef]
- Yeruham, I.; Perl, S.; Orgad, U. Congenital skin neoplasia in cattle. Vet. Dermatol. 1999, 10, 149–156. [Google Scholar] [CrossRef]
- Brisville, A.C.; Buczinski, S.; Chénier, S.; Francoz, D. A cardiac vascular hamartoma in a calf: Ultrasonographic and pathologic images. J. Vet. Cardiol. 2012, 14, 377–380. [Google Scholar] [CrossRef]
- Roth, L.; Bradley, G.A. Pulmonary hamartoma in a calf. J. Comp. Pathol. 1991, 105, 471–474. [Google Scholar] [CrossRef]
- Misdorp, W. Tumours in calves: Comparative aspects. J. Comp. Pathol. 2002, 127, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tontis, A. Kongenitales kavernöses Hämangiom beim Brown-Swiss-Kalb, ein seletenes orales Blastom. Tierarztl. Prax. 1994, 22, 137–139. [Google Scholar]
- Baker, J.C.; Hultgren, B.D.; Larson, V.L. Disseminated cavernous hemangioma in a calf. J. Am. Vet. Med. Assoc. 1982, 181, 172–173. [Google Scholar]
- Poulsen, K.; McSloy, A.; Perrier, M.; Prichard, M.; Steinberg, H.; Semrad, S. Primary mandibular hemangiosarcoma in a bull. Can. Vet. J. 2008, 49, 901–903. [Google Scholar] [PubMed]
- Stock, M.L.; Smith, B.I.; Engiles, J.B. Disseminated hemangiosarcoma in a cow. Can. Vet. J. 2011, 52, 409–413. [Google Scholar]
- Badylak, S.F. Congenital Multifocal Hemangiosarcoma in a Stillborn Calf. Vet. Pathol. 1983, 20, 245–247. [Google Scholar] [CrossRef]
- Hendrick, M.J. Mesenchymal Tumors of the Skin and Soft Tissues. Tumors Domest. Anim. 2016, 142–175. [Google Scholar] [CrossRef]
- Cotchin, E.; Swarbrick, O. Bovine cutaneous angiomatosis: A lesion resembling human pyogenic granuloma (granuloma telangiecticum). Vet. Rec. 1963, 75, 437–444. [Google Scholar]
- Lombard, C.; Levesque, L. H’emangiomatose hyperplasique cutan’ee de vaches Normandes. Ann. Anat. Pathol. (Paris) 1964, 9, 453–462. [Google Scholar] [PubMed]
- Moulton, J.E. Tumors in Domestic Animals, 2nd ed.; University of California Press: Berkeley, CA, USA, 1978. [Google Scholar]
- Waldvogel, A.; Hauser, B. “Bovine kutane Angiomatose” in der Schweiz. Schweiz. Arch. Tierheilkd. 1983, 125, 329–333. [Google Scholar] [PubMed]
- Schilcher, F.; Gasteiner, J.; Palmetshofer, G.; Baumgartnerm, W. Generalisierte juvenile Angiomatose bei einem Kalb. Wien. Tierarztl. Monatsschr. 2000, 87, 10–13. [Google Scholar]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Hayes, B.J.; Daetwyler, H.D. 1000 Bull Genomes Project to Map Simple and Complex Genetic Traits in Cattle: Applications and Outcomes. Annu. Rev. Anim. Biosci. 2019, 7, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Häfliger, I.M.; Wiedemar, N.; Švara, T.; Starič, J.; Cociancich, V.; Šest, K.; Gombač, M.; Paller, T.; Agerholm, J.S.; Drögemüller, C. Identification of small and large genomic candidate variants in bovine pulmonary hypoplasia and anasarca syndrome. Anim. Genet. 2020, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant review with the integrative genomics viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.; Lin, G.N.; Nam, H.-J.; Mort, M.; Cooper, D.; Sebat, J.; Iakoucheva, L.; et al. MutPred2: Inferring the molecular and phenotypic impact of amino acid variants. Nat. Commun. 2017, 134981. [Google Scholar] [CrossRef]
- Bendl, J.; Stourac, J.; Salanda, O.; Pavelka, A.; Wieben, E.D.; Zendulka, J.; Brezovsky, J.; Damborsky, J. PredictSNP: Robust and Accurate Consensus Classifier for Prediction of Disease-Related Mutations. PLoS Comput. Biol. 2014, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Welch, H.C.E.; Coadwell, W.J.; Ellson, C.D.; Ferguson, G.J.; Andrews, S.R.; Erdjument-Bromage, H.; Tempst, P.; Hawkins, P.T.; Stephens, L.R. P-Rex1, a PtdIns(3,4,5)P3- and gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 2002, 108, 809–821. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.B.; Finn, A.J.; Pedone, K.H.; Thomas, N.E.; Der, C.J.; Cox, A.D. ERK/MAPK signaling drives overexpression of the rac-GEF, PREX1, in BRAF-and NRAS-mutant melanoma. Mol. Cancer Res. 2016, 14, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.S.; Lopez-Haber, C.; Yang, C.; Wang, H.B.; Lemmon, M.A.; Busillo, J.M.; Luo, J.; Benovic, J.L.; Klein-Szanto, A.; Yagi, H.; et al. Identification of the Rac-GEF P-Rex1 as an Essential Mediator of ErbB Signaling in Breast Cancer. Mol. Cell 2010, 40, 877–892. [Google Scholar] [CrossRef]
- Goel, H.L.; Pursell, B.; Shultz, L.D.; Greiner, D.L.; Brekken, R.A.; Vander Kooi, C.W.; Mercurio, A.M. P-Rex1 Promotes Resistance to VEGF/VEGFR-Targeted Therapy in Prostate Cancer. Cell Rep. 2016, 14, 2193–2208. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.C.; Wu, H.; Li, H.; Deng, W.W.; Xiao, Y.; Wu, C.C.; Yang, L.L.; Zhang, W.F.; Sun, Z.J. Overexpression of PREX1 in oral squamous cell carcinoma indicates poor prognosis. J. Mol. Histol. 2020, 51, 531–540. [Google Scholar] [CrossRef]
- Venhoranta, H.; Pausch, H.; Flisikowski, K.; Wurmser, C.; Taponen, J.; Rautala, H.; Kind, A.; Schnieke, A.; Fries, R.; Lohi, H.; et al. In frame exon skipping in UBE3B is associated with developmental disorders and increased mortality in cattle. BMC Genom. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- Flex, E.; Ciolfi, A.; Caputo, V.; Fodale, V.; Leoni, C.; Melis, D.; Bedeschi, M.F.; Mazzanti, L.; Pizzuti, A.; Tartaglia, M.; et al. Loss of function of the E3 ubiquitin-protein ligase UBE3B causes kaufman oculocerebrofacial syndrome. J. Med. Genet. 2013, 50, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, F.; Yang, Z.N.; Zhang, T.T.; Yuan, Y.F.; Zhao, C.X.; Yeerjiang, Z.; Cui, B.; Hua, F.; Lv, X.X.; et al. TRIB3 promotes MYC-associated lymphoma development through suppression of UBE3B-mediated MYC degradation. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Uniprot UniProtKB - Q9Y5H1. Available online: https://www.uniprot.org/uniprot/Q9Y5H1 (accessed on 16 December 2020).
- Song, J.; Wu, S.; Xia, X.; Wang, Y.; Fan, Y.; Yang, Z. Cell adhesion-related gene somatic mutations are enriched in aggressive papillary thyroid microcarcinomas 06 Biological Sciences 0604 Genetics. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Tischfield, D.J.; Saraswat, D.K.; Furash, A.; Fowler, S.C.; Fuccillo, M.V.; Anderson, S.A. Loss of the neurodevelopmental gene Zswim6 alters striatal morphology and motor regulation. Neurobiol. Dis. 2017, 103, 174–183. [Google Scholar] [CrossRef]
- InterPro Zinc finger, SWIM-type (IPR007527). Available online: http://www.ebi.ac.uk/interpro/entry/InterPro/IPR007527/ (accessed on 24 November 2020).
- Twigg, S.R.F.; Ousager, L.B.; Miller, K.A.; Zhou, Y.; Elalaoui, S.C.; Sefiani, A.; Bak, G.S.; Hove, H.; Hansen, L.K.; Fagerberg, C.R.; et al. Acromelic frontonasal dysostosis and ZSWIM6 mutation: Phenotypic spectrum and mosaicism. Clin. Genet. 2016, 90, 270–275. [Google Scholar] [CrossRef]
- Palmer, E.E.; Kumar, R.; Gordon, C.T.; Shaw, M.; Hubert, L.; Carroll, R.; Rio, M.; Murray, L.; Leffler, M.; Dudding-Byth, T.; et al. A Recurrent De Novo Nonsense Variant in ZSWIM6 Results in Severe Intellectual Disability without Frontonasal or Limb Malformations. Am. J. Hum. Genet. 2017, 101, 995–1005. [Google Scholar] [CrossRef]
- Scott, D.C.; Rhee, D.Y.; Duda, D.M.; Kelsall, I.R.; Olszewski, J.L.; Paulo, J.A.; de Jong, A.; Ovaa, H.; Alpi, A.F.; Harper, J.W.; et al. Two Distinct Types of E3 Ligases Work in Unison to Regulate Substrate Ubiquitylation. Cell 2016, 166, 1198–1214. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, S.; Wei, R.; Qiu, X.; Lu, K.; Fu, Y.; Li, H.; Xing, G.; Li, D.; Peng, R.; et al. The HECT type ubiquitin ligase NEDL2 is degraded by anaphase-promoting complex/cyclosome (APC/C)-Cdh1, and its tight regulation maintains the metaphase to anaphase transition. J. Biol. Chem. 2013, 288, 35637–35650. [Google Scholar] [CrossRef]
- Uren, P.J.; Bahrami-Samani, E.; de Araujo, P.R.; Vogel, C.; Qiao, M.; Burns, S.C.; Smith, A.D.; Penalva, L.O.F. High-throughput analyses of hnRNP H1 dissects its multi-functional aspect. RNA Biol. 2016, 13, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Fei, T.; Chen, Y.; Xiao, T.; Li, W.; Cato, L.; Zhang, P.; Cotter, M.B.; Bowden, M.; Lis, R.T.; Zhao, S.G.; et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl. Acad. Sci. USA 2017, 114, E5207–E5215. [Google Scholar] [CrossRef]
- Tron, A.E.; Arai, T.; Duda, D.M.; Kuwabara, H.; Olszewski, J.L.; Fujiwara, Y.; Bahamon, B.N.; Signoretti, S.; Schulman, B.A.; DeCaprio, J.A. The Glomuvenous Malformation Protein Glomulin Binds Rbx1 and Regulates Cullin RING Ligase-Mediated Turnover of Fbw7. Mol. Cell 2012, 46, 67–78. [Google Scholar] [CrossRef]
- Brouillard, P.; Boon, L.M.; Mulliken, J.B.; Enjolras, O.; Ghassibé, M.; Warman, M.L.; Tan, O.T.; Olsen, B.R.; Vikkula, M. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am. J. Hum. Genet. 2002, 70, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Irrthum, A.; Brouillard, P.; Enjolras, O.; Gibbs, N.F.; Eichenfield, L.F.; Olsen, B.R.; Mulliken, J.B.; Boon, L.M.; Vikkula, M. Linkage disequilibrium narrows locus for venous malformation with glomus cells (VMGLOM) to a single 1.48 Mbp YAC. Eur. J. Hum. Genet. 2001, 9, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Qin, Z.; Wu, K.; Zhu, Y.; Hu, L.; Kong, X. Rare Germline GLMN Variants Identified from Blue Rubber Bleb Nevus Syndrome Might Impact mTOR Signaling. Comb. Chem. High Throughput Screen. 2019, 22, 675–682. [Google Scholar] [CrossRef]
| Gene | Effect | Protein-Changing | pLI 1 | PROVEAN Score | PROVEAN Impact | MutPred2 Score | MutPred2 Impact | PredictSNP1 Score | PredictSNP1 Impact |
|---|---|---|---|---|---|---|---|---|---|
| PREX1 | missense | p.Arg401Cys | 1 | −5.149 | deleterious | 0.387 | neutral | 0.719 | deleterious |
| UBE3B | missense | p.Ala32Val | 0 | −1.946 | neutral | 0.583 | neutral | 0.510 | deleterious |
| PCDHGA2 | disruptive in-frame deletion | p.Lys141_Val142del | 0 | −11.366 | deleterious | 0.338 | neutral | NA | NA |
| ZSWIM6 | disruptive in-frame deletion | p.Ala146_Gly148del | 1 | 1.280 | neutral | 0.366 | neutral | NA | NA |
| NR1H3 | missense | p.Thr46Met | 0.9 | −0.371 | neutral | 0.086 | neutral | 0.653 | neutral |
| C23H6orf132 | missense | p.Gly692Glu | NA | −0.907 | neutral | 0.035 | neutral | 0.826 | neutral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacinto, J.G.P.; Häfliger, I.M.; Borel, N.; Zanolari, P.; Drögemüller, C.; Veiga, I.M.B. Clinicopathological and Genomic Characterization of a Simmental Calf with Generalized Bovine Juvenile Angiomatosis. Animals 2021, 11, 624. https://doi.org/10.3390/ani11030624
Jacinto JGP, Häfliger IM, Borel N, Zanolari P, Drögemüller C, Veiga IMB. Clinicopathological and Genomic Characterization of a Simmental Calf with Generalized Bovine Juvenile Angiomatosis. Animals. 2021; 11(3):624. https://doi.org/10.3390/ani11030624
Chicago/Turabian StyleJacinto, Joana G. P., Irene M. Häfliger, Nicole Borel, Patrik Zanolari, Cord Drögemüller, and Inês M. B. Veiga. 2021. "Clinicopathological and Genomic Characterization of a Simmental Calf with Generalized Bovine Juvenile Angiomatosis" Animals 11, no. 3: 624. https://doi.org/10.3390/ani11030624
APA StyleJacinto, J. G. P., Häfliger, I. M., Borel, N., Zanolari, P., Drögemüller, C., & Veiga, I. M. B. (2021). Clinicopathological and Genomic Characterization of a Simmental Calf with Generalized Bovine Juvenile Angiomatosis. Animals, 11(3), 624. https://doi.org/10.3390/ani11030624








