Comprehensive Proteomics Analysis of In Vitro Canine Oviductal Cell-Derived Extracellular Vesicles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Collection of Conditioned Medium and Isolation of Canine In Vitro Oviductal Cell-Derived Extracellular Vesicles
2.3. Characterization of Canine In Vitro Oviductal Cell-Derived Extracellular Vesicles
2.4. Preparation of Canine In Vitro Oviductal Cell-Derived Extracellular Vesicles Protein Fraction by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and in-Gel Tryptic Digestion
2.5. Protein Identification with Liquid Chromatography with Tandem Mass Spectrometry Analysis
2.6. Bioinformatic Analysis for the Characterization of Identified Canine In Vitro Oviductal Cell-Derived Extracellular Vesicles Proteins by Proteomic Methods
3. Results
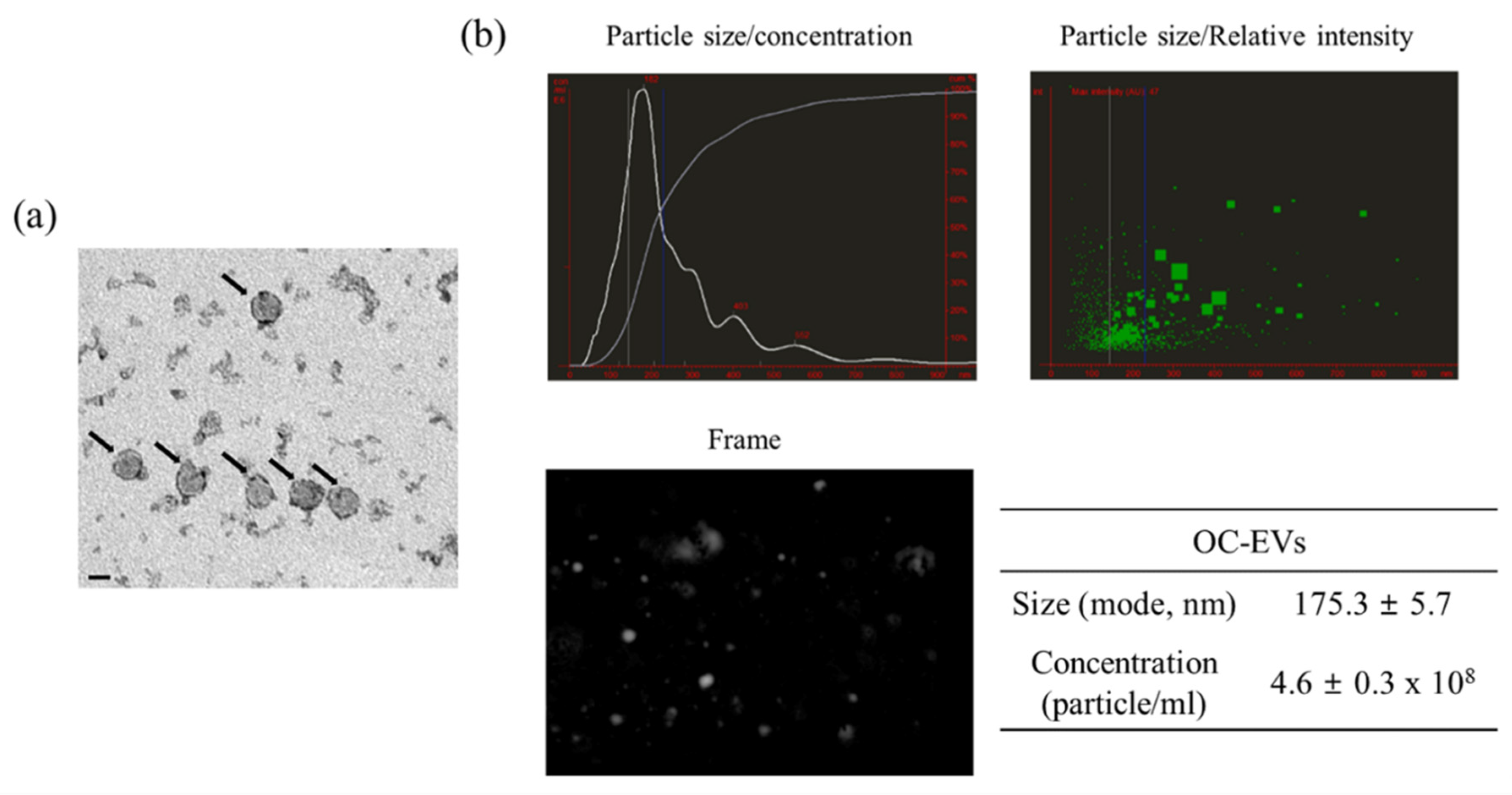
3.1. Characterization of In Vitro Oviductal Cell-Derived Extracellular Vesicles
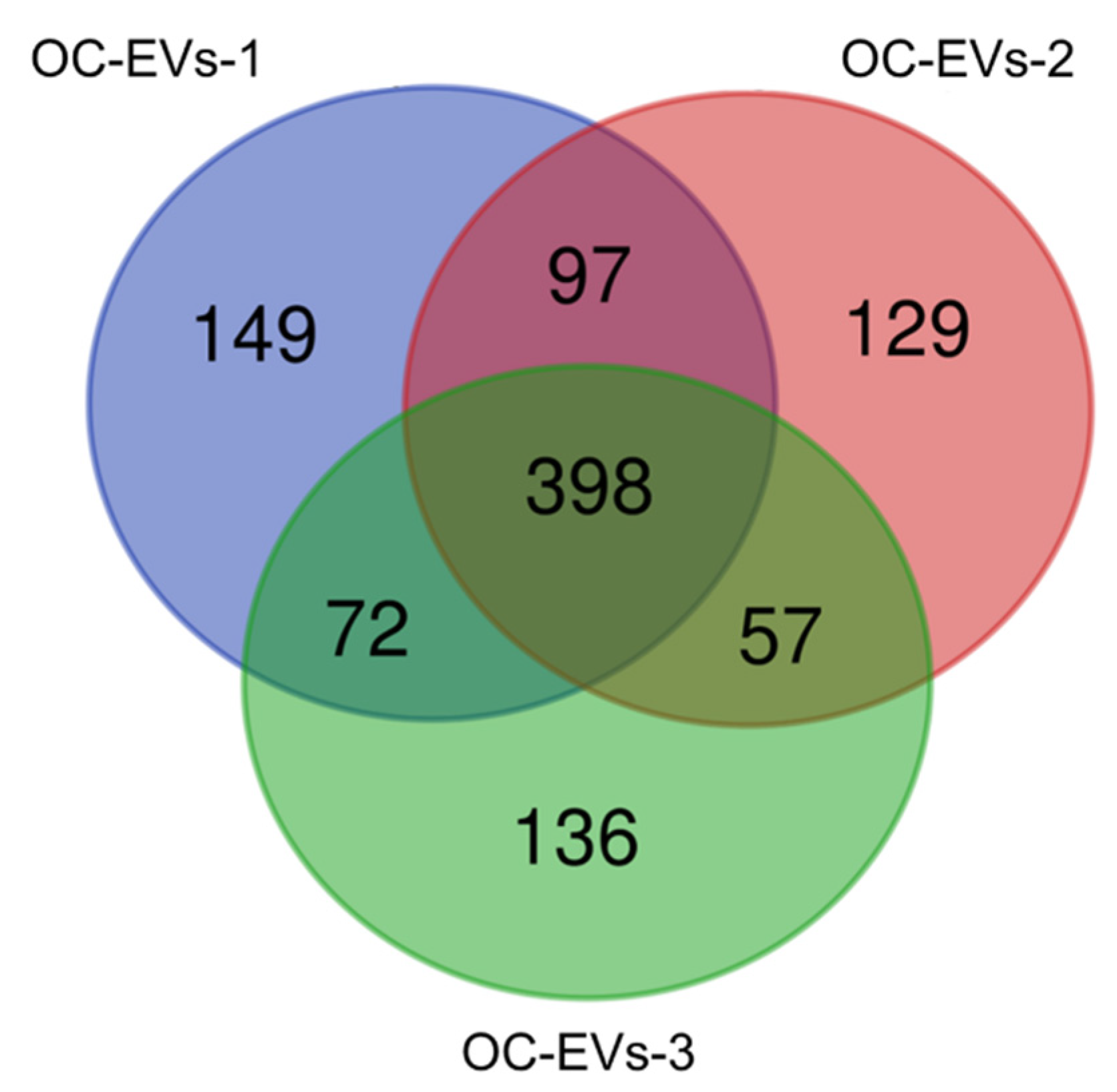
3.2. Functional Enrichment Analysis of Common Proteins Identified in Canine In Vitro Oviductal Cell-Derived Extracellular Vesicles
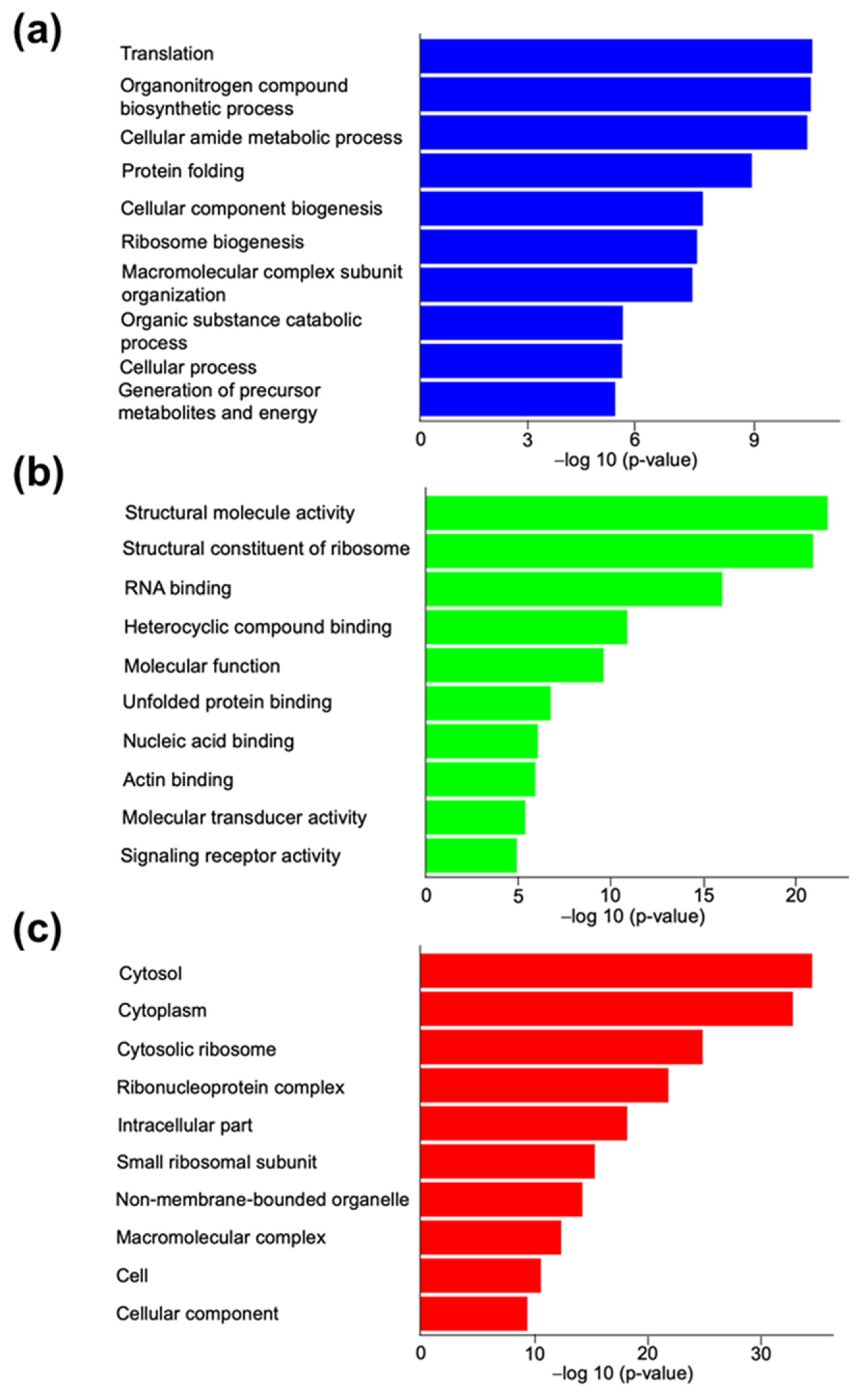
3.2.1. Gene Ontology Analysis for Canine In Vitro Oviductal Cell-Derived Extracellular Vesicle Proteomes
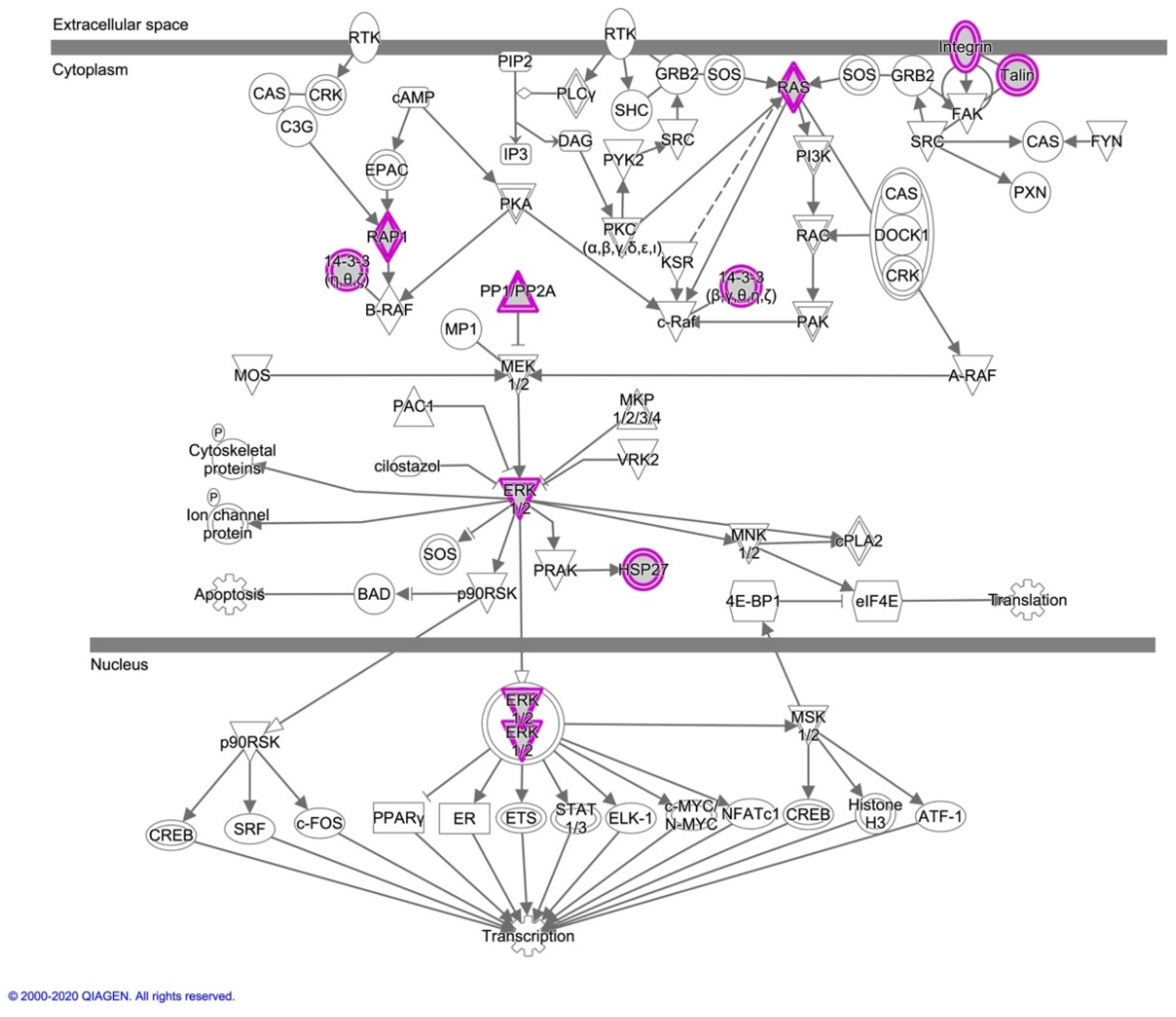
3.2.2. Ingenuity Pathway Analysis for Canine In Vitro Oviductal Cell-Derived Extracellular Vesicle Proteomes
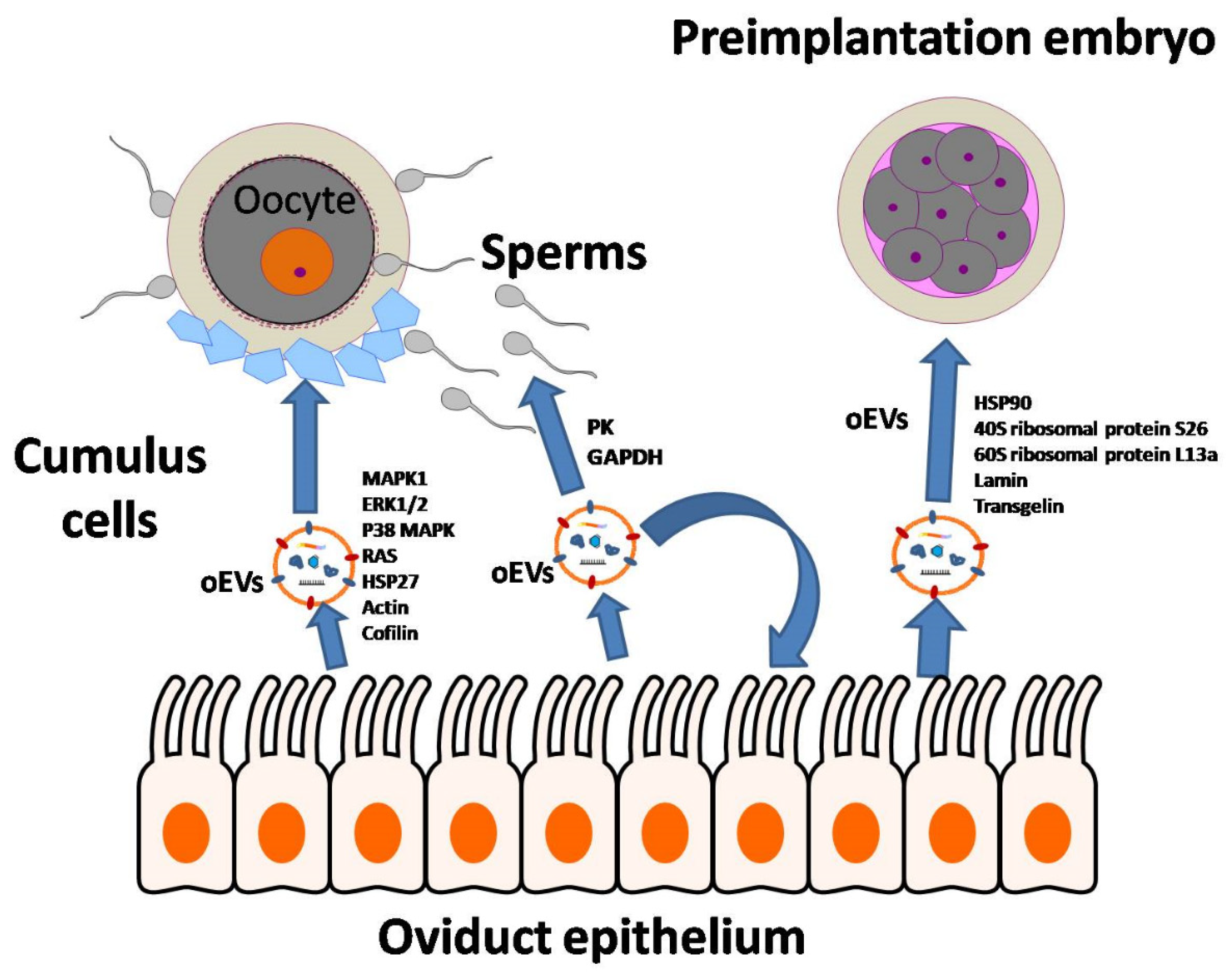
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| OC-EVs | In vitro oviductal cell-derived extracellular vesicles |
| COCs | Cumulus–oocyte complexes |
| EGFR | Epidermal growth factor receptor |
| MAPK | Mitogen-activated protein kinase |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| NTA | Nanoparticle tracking analysis |
| HSP | Heat shock protein |
| GO | Gene Ontology |
| BPs | Biological processes |
| MFs | Molecular functions |
| CCs | Cellular components |
| IPA | Ingenuity Pathway Analysis |
| FBS | Fetal bovine serum |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TFA | Trifluoroacetic acid |
| FDR | False Discovery Rate |
References
- Fahrendorff, M.; Oliveri, R.S.; Johansson, P.I. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products—A systematic review and meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Hyun, K.A.; Gwak, H.; Lee, J.; Kwak, B.; Jung, H.I. Salivary Exosome and Cell-Free DNA for Cancer Detection. Micromachines (Basel) 2018, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013, 13, 3354–3364. [Google Scholar] [CrossRef]
- Street, J.M.; Koritzinsky, E.H.; Glispie, D.M.; Yuen, P.S.T. Urine Exosome Isolation and Characterization. Methods Mol. Biol. 2017, 1641, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Kurian, N.K.; Modi, D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J. Assist. Reprod. Genet. 2019, 36, 189–198. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Vigano, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, Y.; Kanke, T.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS ONE 2019, 14, e0217760. [Google Scholar] [CrossRef]
- Alminana, C.; Corbin, E.; Tsikis, G.; Alcantara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.A.; Tuna, K.M.; Alli, A.A.; Tribulo, P.; Hansen, P.J.; Koh, J.; Paula-Lopes, F.F. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod. Fertil. Dev. 2019, 31, 888–897. [Google Scholar] [CrossRef]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Bathala, P.; Caplan, J.L.; Martin-DeLeon, P.A. Oviductosome-Sperm Membrane Interaction in Cargo Delivery: Detection of fusion and underlying molecular players using three-dimensional super-resolution structured illumination microscopy (SR-SIM). J. Biol. Chem. 2015, 290, 17710–17723. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltran-Brena, P.; Calle, A.; Redruello, A.; Lopez-Martin, S.; Gutierrez-Adan, A.; Yanez-Mo, M.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-Deleon, P.A. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Lee, B.C. Exosomes derived from oviduct cells mediate the EGFR/MAPK signaling pathway in cumulus cells. J. Cell. Physiol. 2020, 235, 1386–1404. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Lee, B.C. Canine oviductal exosomes improve oocyte development via EGFR/MAPK signaling pathway. Reproduction 2020, 160, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.C.; Mermillod, P.; Alminana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, S.; Mermillod, P.; Alminana, C. The Oviductal Extracellular Vesicles’ RNA Cargo Regulates the Bovine Embryonic Transcriptome. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef]
- Gegenfurtner, K.; Frohlich, T.; Kosters, M.; Mermillod, P.; Locatelli, Y.; Fritz, S.; Salvetti, P.; Forde, N.; Lonergan, P.; Wolf, E.; et al. Influence of metabolic status and genetic merit for fertility on proteomic composition of bovine oviduct fluiddagger. Biol. Reprod. 2019, 101, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hackenbroch, L.; Meyer, F.R.L.; Reiser, J.; Razzazi-Fazeli, E.; Nobauer, K.; Besenfelder, U.; Vogl, C.; Brem, G.; Mayrhofer, C. Identification of Rabbit Oviductal Fluid Proteins Involved in Pre-Fertilization Processes by Quantitative Proteomics. Proteomics 2019, 19, e1800319. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.; Nogues, P.; Combes-Soia, L.; Tsikis, G.; Labas, V.; Mermillod, P.; Druart, X.; Saint-Dizier, M. Identification by proteomics of oviductal sperm-interacting proteins. Reproduction 2018, 155, 457–466. [Google Scholar] [CrossRef]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef]
- Smits, K.; Nelis, H.; Van Steendam, K.; Govaere, J.; Roels, K.; Ververs, C.; Leemans, B.; Wydooghe, E.; Deforce, D.; Van Soom, A. Proteome of equine oviducal fluid: Effects of ovulation and pregnancy. Reprod. Fertil. Dev. 2017, 29, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.S.; Miller, K.A.; Galileo, D.S.; Martin-DeLeon, P.A. Murine SPAM1 is secreted by the estrous uterus and oviduct in a form that can bind to sperm during capacitation: Acquisition enhances hyaluronic acid-binding ability and cumulus dispersal efficiency. Reproduction 2008, 135, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.A.; Watson, P.F.; England, G.C. Nuclear staining and culture requirements for in vitro maturation of domestic bitch oocytes. Theriogenology 1998, 49, 1083–1101. [Google Scholar] [CrossRef]
- Hewitt, D.A.; England, G.C. The effect of oocyte size and bitch age upon oocyte nuclear maturation in vitro. Theriogenology 1998, 49, 957–966. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Setyawan, E.M.N.; Lee, B.C. Interaction of the EGFR signaling pathway with porcine cumulus oocyte complexes and oviduct cells in a coculture system. J. Cell. Physiol. 2019, 234, 4030–4043. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Choi, Y.B.; Jo, Y.K.; Setyawan, E.M.N.; Lee, B.C. Effect of co-culture canine cumulus and oviduct cells with porcine oocytes during maturation and subsequent embryo development of parthenotes in vitro. Theriogenology 2018, 106, 108–116. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Choi, Y.B.; Jo, Y.K.; Setyawan, E.M.N.; Lee, B.C. Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J. Assist. Reprod. Genet. 2017, 34, 929–938. [Google Scholar] [CrossRef]
- Choi, C.W.; Park, E.C.; Yun, S.H.; Lee, S.Y.; Lee, Y.G.; Hong, Y.; Park, K.R.; Kim, S.H.; Kim, G.H.; Kim, S.I. Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. J. Proteome Res. 2014, 13, 4298–4309. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Supek, F.; Bosnjak, M.; Skunca, N.; Smuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Kalous, J.; Tetkova, A.; Kubelka, M.; Susor, A. Importance of ERK1/2 in Regulation of Protein Translation during Oocyte Meiosis. Int. J. Mol. Sci. 2018, 19, 698. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK During Porcine Oocyte Maturation1. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Intawicha, P.; Tsai, L.-K.; Yen, S.-Y.; Lo, N.-W.; Ju, J.-C. Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase p38 Dynamics during In Vitro Maturation of Porcine Oocytes. Animals 2019, 9, 163. [Google Scholar] [CrossRef]
- Alminana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Tabb, D.L.; Vega-Montoto, L.; Rudnick, P.A.; Variyath, A.M.; Ham, A.J.; Bunk, D.M.; Kilpatrick, L.E.; Billheimer, D.D.; Blackman, R.K.; Cardasis, H.L.; et al. Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J. Proteome Res. 2010, 9, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Tiruvayipati, S.; Wolfgeher, D.; Yue, M.; Duan, F.; Andrade, J.; Jiang, H.; Schuger, L. Variability in protein cargo detection in technical and biological replicates of exosome-enriched extracellular vesicles. PLoS ONE 2020, 15, e0228871. [Google Scholar] [CrossRef]
- Nagaraj, N.; Mann, M. Quantitative analysis of the intra- and inter-individual variability of the normal urinary proteome. J. Proteome Res. 2011, 10, 637–645. [Google Scholar] [CrossRef]
- Bittremieux, W.; Tabb, D.L.; Impens, F.; Staes, A.; Timmerman, E.; Martens, L.; Laukens, K. Quality control in mass spectrometry-based proteomics. Mass Spectrom. Rev. 2018, 37, 697–711. [Google Scholar] [CrossRef]
- Pillai, V.V.; Weber, D.M.; Phinney, B.S.; Selvaraj, V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE 2017, 12, e0188105. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Bauersachs, S. Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success. Bioengineering (Basel) 2019, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Perrini, C.; Albini, G.; Modina, S.; Lodde, V.; Orsini, E.; Esposti, P.; Cremonesi, F. Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction 2017, 154, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019, 31, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Yamamoto, S.; Okada, A.; Nakajima, T.; Sato, M.; Takagi, T.; Tomooka, Y. Role of extracellular vesicles in the interaction between epithelial and mesenchymal cells during oviductal ciliogenesis. Biochem. Biophys. Res. Commun. 2017, 483, 245–251. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Martin-Deleon, P.A. Role of exosomes in the reproductive tract Oviductosomes mediate interactions of oviductal secretion with gametes/early embryo. Front. Biosci. (Landmark Ed.) 2016, 21, 1278–1285. [Google Scholar] [CrossRef]
- de Almeida Monteiro Melo Ferraz, M.; Nagashima, J.B.; Noonan, M.J.; Crosier, A.E.; Songsasen, N. Oviductal Extracellular Vesicles Improve Post-Thaw Sperm Function in Red Wolves and Cheetahs. Int. J. Mol. Sci. 2020, 21, 3733. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.; Dai, X.X.; Liu, H.L.; Cui, X.S.; Kim, N.H.; Wang, Z.B.; Wang, Q.; Sun, S.C. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol. Reprod. 2014, 90, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Xu, Y.N.; Jo, Y.J.; Namgoong, S.; Kim, N.H. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol. Reprod. Dev. 2015, 82, 849–858. [Google Scholar] [CrossRef]
- de Pablo, Y.; Marasek, P.; Pozo-Rodrigalvarez, A.; Wilhelmsson, U.; Inagaki, M.; Pekna, M.; Pekny, M. Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes. Cells 2019, 8, 1016. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Tanaka, H.; Inoko, A.; Goto, H.; Yonemura, S.; Kobori, K.; Hayashi, Y.; Kondo, E.; Itohara, S.; Izawa, I.; et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem. 2013, 288, 35626–35635. [Google Scholar] [CrossRef]
- Dhar-Chowdhury, P.; Harrell, M.D.; Han, S.Y.; Jankowska, D.; Parachuru, L.; Morrissey, A.; Srivastava, S.; Liu, W.; Malester, B.; Yoshida, H.; et al. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J. Biol. Chem. 2005, 280, 38464–38470. [Google Scholar] [CrossRef]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Lemmers, B.; Blanchard, J.M. Cell cycle, cytoskeleton dynamics and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015, 14, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.; Perecin, F.; Silveira, J.C.D. Extracellular Vesicles Mediated Early Embryo-Maternal Interactions. Int. J. Mol. Sci. 2020, 21, 1163. [Google Scholar] [CrossRef] [PubMed]
- Maddox-Hyttel, P.; Svarcova, O.; Laurincik, J. Ribosomal RNA and nucleolar proteins from the oocyte are to some degree used for embryonic nucleolar formation in cattle and pig. Theriogenology 2007, 68 (Suppl. 1), S63–S70. [Google Scholar] [CrossRef]
- Jansova, D.; Tetkova, A.; Koncicka, M.; Kubelka, M.; Susor, A. Localization of RNA and translation in the mammalian oocyte and embryo. PLoS ONE 2018, 13, e0192544. [Google Scholar] [CrossRef]
- Fu, B.; Ma, H.; Liu, D. Extracellular Vesicles Function as Bioactive Molecular Transmitters in the Mammalian Oviduct: An Inspiration for Optimizing in Vitro Culture Systems and Improving Delivery of Exogenous Nucleic Acids during Preimplantation Embryonic Development. Int. J. Mol. Sci. 2020, 21, 2189. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramirez, M.A.; Yanez-Mo, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 461–470. [Google Scholar] [CrossRef]
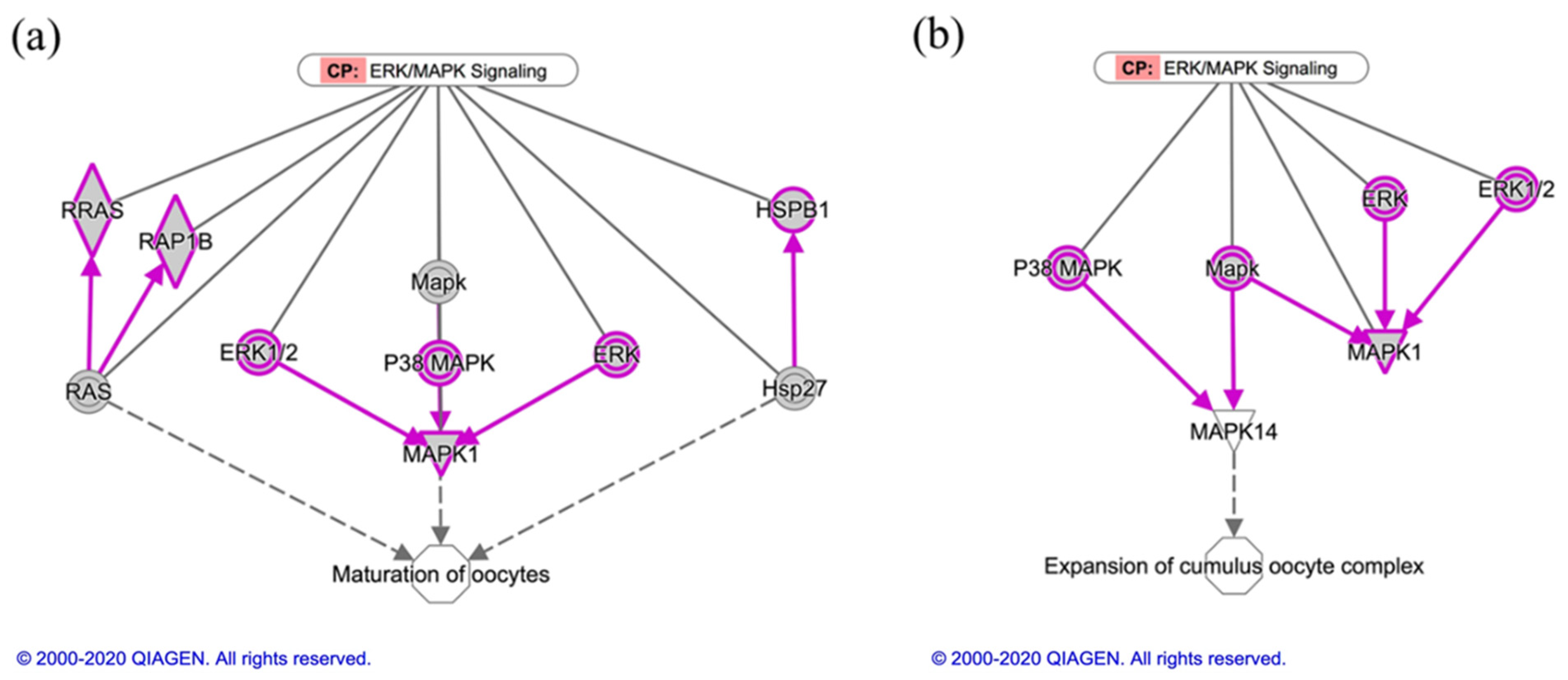
| Diseases or Functions Annotation Defined by Ingenuity Knowledge Base | Categories | p-Value | # Molecules |
|---|---|---|---|
| Initiation of translation of protein | Protein synthesis | 2.81 × 10−52 | 49 |
| Decay of mRNA | RNA damage and repair | 8.95 × 10−50 | 44 |
| Nonsense-mediated mRNA decay | RNA damage and repair | 7.67 × 10−48 | 42 |
| Translation | Protein synthesis | 1.39 × 10−42 | 66 |
| Metabolism of protein | Protein synthesis | 7.94 × 10−40 | 112 |
| Synthesis of protein | Protein synthesis | 1.19 × 10−39 | 76 |
| Translation of protein | Protein synthesis | 4.66 × 10−39 | 62 |
| Necrosis | Cell death and survival | 5.92 × 10−39 | 171 |
| Expression of protein | Protein synthesis | 6.81 × 10−37 | 65 |
| Cell death of osteosarcoma cells | Cell death and survival, and organismal injury | 5.45 × 10−32 | 34 |
| Canonical Pathway Defined by Ingenuity Knowledge Base | p-Value | # Ratio |
|---|---|---|
| EIF2 Signaling | 5.01 × 10−51 | 0.25 |
| Regulation of eIF4 and p70S6K Signaling | 3.98 × 10−27 | 0.20 |
| mTOR Signaling | 1.26 × 10−18 | 0.13 |
| Coronavirus Pathogenesis Pathway | 7.94 × 10−16 | 0.15 |
| Remodeling of Epithelial Adherens Junctions | 3.16 × 10−15 | 0.24 |
| Actin Cytoskeleton Signaling | 3.16 × 10−14 | 0.11 |
| Epithelial Adherens Junction Signaling | 1.58 × 10−13 | 0.13 |
| Integrin Signaling | 1.00 × 10−11 | 0.10 |
| Protein Ubiquitination Pathway | 2.51 × 10−11 | 0.08 |
| Glycolysis I | 7.94 × 10−11 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lira-Albarrán, S.; Saadeldin, I.M. Comprehensive Proteomics Analysis of In Vitro Canine Oviductal Cell-Derived Extracellular Vesicles. Animals 2021, 11, 573. https://doi.org/10.3390/ani11020573
Lee SH, Lira-Albarrán S, Saadeldin IM. Comprehensive Proteomics Analysis of In Vitro Canine Oviductal Cell-Derived Extracellular Vesicles. Animals. 2021; 11(2):573. https://doi.org/10.3390/ani11020573
Chicago/Turabian StyleLee, Seok Hee, Saúl Lira-Albarrán, and Islam M Saadeldin. 2021. "Comprehensive Proteomics Analysis of In Vitro Canine Oviductal Cell-Derived Extracellular Vesicles" Animals 11, no. 2: 573. https://doi.org/10.3390/ani11020573
APA StyleLee, S. H., Lira-Albarrán, S., & Saadeldin, I. M. (2021). Comprehensive Proteomics Analysis of In Vitro Canine Oviductal Cell-Derived Extracellular Vesicles. Animals, 11(2), 573. https://doi.org/10.3390/ani11020573







