Simple Summary
Milk is considered a staple and complete food that contains several essential nutrients for humans. For instance, it is an excellent natural source of vitamin B12 (B12) due to the presence in the bovine rumen of a myriad of bacteria and archaea capable of producing the vitamin. This vitamin is only produced by prokaryotic microorganisms; vegetal products do not naturally contain it. A 250-mL glass of milk contains about 46% of the daily recommended dietary allowance of B12 for individuals over 13 years old. However, B12 concentration is variable in milk; therefore, identifying factors contributing to its variation is critical to ensure a stable B12 supply for consumers. The aims of these experiments are to gather more knowledge on possible sources of variation in B12 concentrations in milk in order to optimize and stabilize its levels and thereby improve the perception of milk in terms of its health benefits. We observed that B12 concentration increases when the conditions of the rumen are optimal, such as with elevated pH. We also studied if bedding type—e.g., recycled manure solid bedding or straw, which has been reported to impact milk microbiota—could have an impact on milk B12 concentration. In this study, no such correlation was detected. This paper is one of a series seeking to elucidate factors responsible for variations in milk B12 concentration.
Abstract
Milk is an excellent source of vitamin B12 (B12) for humans. Therefore, being able to guarantee a high and consistent concentration of this vitamin would enhance consumer perception of milk as a health food. The aim of the paper was to gather additional knowledge on factors that could explain B12 variation in cow milk through two observational studies: (1) to explore the relationship between milk B12 and ruminal conditions, such as pH and volatile fatty acid concentrations; and (2) to examine the impact of bedding on B12 concentrations in bulk tank milk. For study 1, a total of 72 milk and ruminal liquid samples were obtained from 45 Holstein cows fitted with ruminal cannula between 10 and 392 days of lactation. For study 2, bulk tank milk samples were obtained from 83 commercial herds; 26 herds used recycled manure solid bedding and 57 used straw bedding. Milk samples were analyzed for B12 using radioassay. Using principal component regression analysis, we observed that ruminal pH and the acetate:propionate ratio for cows receiving the early lactation ration were positively correlated with milk B12. Bedding did not influence milk B12 in bulk tanks, which averaged 4276 pg/mL. In conclusion, as B12 is synthesized by ruminal bacteria, optimizing ruminal conditions had a positive effect on milk B12, while bedding management had no influence.
1. Introduction
As early as the 1980s, Canadian and American reports indicated that overall fluid milk consumption per capita was decreasing [1,2]. The same declining trend is continuing in many countries including Canada, USA, and Switzerland [3]. One explanation is the increasing consumption of nondairy, plant-based beverages which are available on the market. A survey conducted on consumers of plant-based beverages which sought to understand the decreasing consumption of dairy products indicated that drinkers of nondairy beverages believed that plant-based drinks helped to reduce the negative impacts of animal production on the environment [4]. Also, there was concern about the health benefits of milk given its saturated fat content [5]. Studies have shown differing effects of milk fat consumption on human health, even though no strong evidence of deleterious impact of moderate milk consumption has been shown [6]. Nevertheless, cow milk is an excellent source of essential nutrients. Based on amino acid profile, the protein quality of cow milk is superior to that of plant-based beverages for human consumption [7]. Another example is the natural content of cow milk vitamin B12, also known as cyanocobalamin. Indeed, vitamin B12 is solely synthesized by bacteria and archaea when cobalt is not limiting [8]. Bacteria and archaea living in the rumen are able to synthesize vitamin B12, which is ultimately secreted in milk [9]. Animal products, especially those from ruminants, are an excellent source of vitamin B12, whereas plant-based products, such as soy beverage do not contain it unless they are fortified with the synthetic form of the vitamin. The use of synthetic vitamin B12 to fortify soy beverages may not offer the perceived benefit to the consumer. Using a pig model, it was shown that the natural form of vitamin B12 in cow milk and cheddar cheese is more bioavailable than the synthetic form [10,11].
The major role of vitamin B12 in human health is to prevent megaloblastic anemia and neuropathy [12]. According to the United States Department of Agriculture [13], a 250-mL glass of milk should provide 46% of the recommended dietary allowance (RDA) for humans above 13 years old. Hence, milk is a staple food of major importance in human diet to fulfill the daily vitamin B12 requirement. McCarthy et al. [4] concluded that the dairy industry should make efforts to educate consumers on the excellent nutritional value of cow milk. The fact that cow milk naturally contains vitamin B12 in contrast with plant-based beverages is a good example of information of which consumers should be made aware.
During the last few years, our research group has focused on refining knowledge regarding vitamin B12 concentration in milk, in particular on factors explaining its large concentration variability therein. It has been shown that milk vitamin B12 concentrations vary greatly among herds and even among cows housed within the same herd [14,15,16]. Duplessis et al. [14] reported that a 250-mL glass of milk from 100 herds provided between 28 and 61% of the RDA for humans above 13 years old, and that 50% of herds did not reach the 46% threshold. Previous research has reported that this huge variability could be in part due to animal characteristics such as parity and breed [14,17], season [15], milk productivity [14], diet composition [14,15], animal genetics [15,16], and rumen microbiota [18]. These studies aimed to better define factors that cause significant vitamin B12 concentration variability in milk in order to be able to offer a product with constant and high vitamin B12 concentration, and hence, to increase consumer perception of the benefits of fluid milk consumption.
Although conflicting results exist in the literature, some authors reported that apparent ruminal synthesis (ARS) of vitamin B12 is correlated with some indicators of ruminal fermentation [19,20,21]. As the vitamin B12 secreted in milk is synthesized by the rumen bacteria, it could be hypothesized that indicators of ruminal fermentation could also be used to estimate milk vitamin B12 concentration. Bedding type has been shown to have an impact on milk microbiota [22], but, at present, there is no data demonstrating the impact of environmental factors affecting milk microbiota on vitamin B12 concentration in milk. As vitamin B12 is only synthesized by bacteria, we hypothesized that any environmental factor modifying milk microbiota could have an impact on vitamin B12 concentration in milk. In our attempt to refine knowledge regarding the factors influencing vitamin B12 concentrations in milk, the first objective of this study was to assess the impact of indicators of ruminal fermentation (Study 1). A second study was undertaken to assess the impact of recycled manure solid (RMS) bedding and straw bedding on the vitamin B12 concentrations of bulk tank milk (Study 2).
2. Materials and Methods
The first experiment protocol was approved by the Institutional Committee for Animal Care of the Sherbrooke Research and Development Centre (Agriculture & Agri-Food Canada, Sherbrooke, QC, Canada) and all procedures were conducted according to the code of practice of the National Farm Animal Care Council [23] and the guidelines of the Canadian Council on Animal Care [24]. The second trial was approved by the Animal Care and Use Committee for the Veterinary College of the Université de Montréal (Faculté de médecine vétérinaire, Saint-Hyacinthe, QC, Canada) following the same guidelines of the study 1. In each experiment, significance of results was considered when p ≤ 0.05 and a tendency when p was between 0.05 and 0.10.
2.1. Study 1
2.1.1. Animals and Management
The cows and management of this observational study have already been described by Franco-Lopez et al. [18]. Briefly, all lactating Holstein cows from parity 1 to 6, between 2.9 and 8.0 years of age, and 10 and 392 days in milk (DIM) from the dairy herd at the Sherbrooke Research and Development Centre equipped with rumen cannula (n = 45) were sampled from May to August 2018. Herd average milk yield was 10194 kg with 4.22% fat and 3.15% true protein. The Holstein breed was used as it makes up 93% of Canadian cows [25]. This study was first undertaken to evaluate the link between rumen microbiota and milk vitamin B12 concentration; the results have been presented elsewhere [18]. A total of 27 cows were sampled twice at 62 ± 5 d apart, while the remaining (n = 18) cows were sampled once because they were dry at one of the samplings. This yielded a total of 72 sets of samples. Cows were milked twice daily at 12-h intervals in a milking parlor and housed in a tie-stall barn under 17 h of light per day. Cows were fed once daily as total mixed ration (TMR) at 09:00 h according to one of two lactation rations (Table 1). Cows in group 1 averaged 125 (SD: 89) DIM, ranging from 10 to 346 DIM, while cows in group 2 averaged 307 (SD: 61) DIM, ranging from 230 to 392 DIM.

Table 1.
Ingredients and nutrient composition of lactation total mixed ration diets offered to dairy cows 1.
2.1.2. Sample Collection
The quantity of TMR served and orts were recorded on three consecutive days before the sampling day, and daily dry matter intake (DMI) was calculated (Figure 1). Total mixed ration samples were collected on the same three consecutive days. The body weight (BW) of cows was estimated using heart girth circumference and the equation of Yan et al. [26]. Milk samples were collected from the morning milking at 07:00 h using calibrated inline milk meters. One aliquot was preserved with bronopol and stored at 4 °C. Another aliquot was taken, without bronopol, for vitamin B12 analysis. Milk yield was recorded. Whole-rumen digesta samples were collected before the morning meal at five different rumen sites (cranial dorsal, cranial ventral, central, caudal dorsal, and caudal ventral) [27] and manually mixed to get a composite sample. Ruminal fluid was obtained with a 50-mL syringe screwed to a stainless tube with a fine-metal mesh probe (Bar Diamond Inc., Parma, ID, USA) at one end at 0, 1, 2, 4, and 6 h after morning meal distribution. Fifty milliliters of each of the following ruminal locations was collected and mixed to get one composite sample: anterior dorsal, anterior ventral, medium ventral, posterior ventral, and posterior ventral. Ruminal liquid pH was immediately taken after collection (Accumet pH meter; Fisher Scientific, Ottawa, ON, Canada) and aliquots for ammonia N (N-NH3) concentration were acidified to pH 2 with 50% H2SO4. Rectum grab samples of feces were taken 5 h after the morning meal. Blood samples were taken 5 h after the morning meal from the coccygeal vein by venipuncture using a Vacutainer system (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Tubes with EDTA were put on ice, centrifuged within 30 min for 15 min at 2400× g and 4 °C, and plasma was harvested. All samples, unless otherwise specified, were stored at −20 °C until processing.
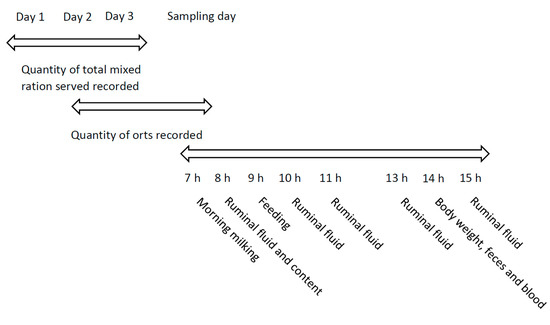
Figure 1.
Sampling scheme of study 1. Timeline is based on a 24-h scheme.
2.1.3. Laboratory Analyses
Total mixed ration samples were dried in an air-forced oven at 55 °C for 48 h and ground to a particle size of 1 mm (Wiley mill, A. H. Thomas Co., Philadelphia, PA, USA). A composite TMR sample from the three sampling days was then made and analyzed by wet chemistry (SGS Canada, Guelph, ON, Canada) for crude protein (method 990.03; AOAC International [28]), ADF (Ankom Technology, Macedon, NY, USA, Method 12; solutions as in method 973.18; AOAC International [28]), aNDF (Ankom Technology Method 13; solutions as in Van Soest et al. [29] with the inclusion of heat-stable α-amylase), crude fat (Ankom Technology Method 2; AOCS [30]), starch (method 996.11; AOAC International [28]), Ca, P, Mg, K, and Co (inductively coupled plasma; methods 985.01 and 965.09; AOAC International [28]). Net energy of lactation and nonfiber carbohydrates were calculated according to the National Research Council [31].
One aliquot of TMR, whole-rumen digesta and feces samples was put in an air-forced oven at 100 °C for 72 h for dry matter (DM) content determination. Another aliquot of whole-rumen digesta and feces samples was lyophilized (Virtis, SP Scientific, Warminster, PA, USA) and ground to pass through a 1-mm sieve (Wiley mill, A. H. Thomas Co.). Vitamin B12 concentration in the latter samples was determined in duplicate as per the method described by Beaudet et al. [19] using a commercial kit (SimulTRAC-S Vitamin B12 [Co57]/Folate [I125], catalog number 06B−254932, MP Biomedicals, Solon, OH, USA). The interassay coefficients of variation for whole-rumen digesta and feces were 3.8 and 3.3%, respectively. Milk samples with bronopol were immediately sent to the Lactanet laboratory (Canadian Network for Dairy Excellence, Sainte-Anne-de-Bellevue, QC, Canada) for fat, protein, and lactose concentration analyses by midinfrared reflectance spectrometry (MilkoScan FT 6000, Foss, Hillerød, Denmark). Vitamin B12 concentration in milk and plasma was analyzed in duplicate according to the method described by Duplessis et al. [32], i.e., by radioassay using a commercial kit (SimulTRAC-S Vitamin B12 [Co57]/Folate [I125], MP Biomedicals). The interassay coefficients of variation for milk and plasma were 3.7 and 4.0%, respectively.
The ruminal fluid volatile fatty acid (VFA) profile and ammonia N concentration were analyzed as previously described [33]. Ammonia N concentration was determined using a Varioskan spectrophotometer (type 3001, cat. no 5250010, Thermo Electron Corporation, Vantaa, Finland) at 625 nm, and the VFA profile was obtained with a gas chromatograph (Agilent 6890N; Agilent Technologies Canada Inc., Mississauga, ON, Canada) and a flame detector.
2.1.4. Statistical Analyses
Proc UNIVARIATE of SAS [34] was used to obtain descriptive statistics about the vitamin B12 concentrations in different sample types. A first Proc MIXED of SAS was carried out to evaluate the effect of feeding groups as a fixed effect on whole-rumen digesta, feces, plasma, and milk vitamin B12 concentrations, and on variables such as DMI, DIM, BW, milk production and components, ruminal ammonia N and VFA concentrations in order to describe the cow population of this study. Cow was added as a random effect to overcome the fact that some cows were sampled twice.
To achieve the objective of the first study, we first had to determine the independent variables to be used in the model. Because liquid ruminal samples were taken several times relative to the meal, there were several possibilities per variable. For instance, propionate concentration at time 0 relative to the meal, minimal and maximal propionate concentrations and average propionate concentration throughout the sampling times could be used as independent variables. For each VFA, these different variables were highly correlated according to the Proc CORR of SAS (Spearman correlation coefficients, ρ > 0.6) and hence they could not be used in the same model. As the vitamin B12 concentration in morning milking samples is representative of the last 12 h, between two milkings, we hypothesized that the average of each individual VFA concentration throughout the sampling collection was the most relevant to our model. With the addition of some variables related to the cow, the continuous independent variables considered in our model were as follows: estimated BW, milk yield, milk fat and protein concentrations, DMI, DIM, whole-rumen digesta, feces, and plasma vitamin B12 concentrations, and average concentrations of ruminal pH, acetate: propionate ratio, acetate, propionate, butyrate, valerate, isobutyrate, isovalerate, and ammonia N throughout sampling times. To evaluate possible multicollinearity among variables, Proc CORR and Proc REG of SAS were used to obtain Spearman correlation coefficients and variance inflation factor, respectively. A correlation coefficient > 0.6 and a variance inflation factor > 10 suggested multicollinearity [35]. Average concentration of propionate was highly correlated with average concentrations of valerate and isovalerate (ρ > 0.7), and the variance inflation factor of average concentration of propionate was 25. To address this multicollinearity, the methodology described by Duplessis et al. [14] was used, i.e., principal component (PC) analysis was used to reduce the dimensionality of interdependent variables [36]. Proc PRINCOMP of SAS was first used to determine the number of PC to retain for further analysis. Principal components with an Eigenvalue less than 1 were not included for further assessment. Using the number of PC previously determined, Proc FACTOR of SAS was performed with the option VARIMAX rotation to facilitate the PC interpretation. Indeed, with this option, a variable could be associated with a PC when its absolute rotated factor pattern was the largest among the studied PC. The rotated factor pattern had to be higher than 0.5 for a variable to be included in a PC. Each PC was then considered as a continuous independent variable in the following PC regression analysis model:
where Yi is the vitamin B12 concentration in milk of the i-th cow, b0 is the fixed intercept; b1 is the regression coefficients for feeding group and x1i the category for feeding group (i.e., Group 1 and 2) of the i-th cow; b2 is the regression coefficient of PC1 and PC1 is the score of the first PC of the i-th cow and so forth; vi is the random intercept of the i-th cow; and Ɛi is the Gaussian error term. The interaction between feeding group and each PC was also examined. Only significant interactions were kept in the final model. Cook’s distance was computed with the INFLUENCE option of SAS, and four records were deleted according to the method of Kaps and Lamberson [35], leaving 68 data for the analysis. The linear relationship between dependent and independent variables was visually assessed with Proc GPLOT of SAS. Residuals were assessed for normality and homoscedasticity. Normality assumption was violated and milk vitamin B12 concentration data was then log transformed. Parity was not included in the model because there were only three cows in first parity, and they were at the end of their lactation. Proc REG of SAS was used to determine the pseudo-R2 between predicted and observed values of milk vitamin B12 concentration obtained from the MIXED model using OUTP option.
Yi = b0 + b1 × 1i + b2PC1i + b3PC2i + … + bnPCni + vi + Ɛi
2.2. Study 2
2.2.1. Herds
The herds involved in this cross-sectional study and cow enrollment have already been described in detail [37,38]. Briefly, herds in Quebec and Eastern Ontario, Canada using RMS bedding and straw bedding for at least six months before the beginning of the study were recruited and visited once from January to July 2018. The main breed of most herds was Holstein. Herds were located within 250 km of the Faculty of Veterinary Medicine of Université de Montréal (Saint-Hyacinthe, QC, Canada). A total of 26 RMS (herd size from 55 to 900 cows, median: 111; freestall, n = 20 and tiestall, n = 6) and 57 straw herds (herd size from 43 to 229 cows, median: 65; freestall, n = 2 and tiestall, n = 55) participated in this study. The majority of RMS herds (96%) used RMS bedding which had been composted 3 h to 3 d before its use. Management for straw herds consisted of replacing the bedding every 12 to 24 h. For RMS herds, management used was either shallow or deep bedding. For shallow RMS herds (n = 17), bedding was replaced every 12 h, whereas for deep-bedding RMS herds (n = 9), it was added from once every other day to once every 8 d.
2.2.2. Sample Collection and Laboratory Analysis
Bulk tank milk samples were taken after 5 min agitation. Samples were then collected via the top of the tank using a sterile straw and syringe, and transferred into a sterile conical tube. If the level of milk in the tank was too low to perform the milk collection as described above, milk was collected in a sterile bag from the outlet valve after discarding a small amount. Milk was then transferred into the conical tube with a sterile syringe. Samples were stored at −20 °C until analysis. The vitamin B12 concentrations in those samples were determined as previously explained for study 1. The interassay coefficient of variation was 4.8%.
2.2.3. Statistical Analysis
Proc UNIVARIATE of SAS was used to compute descriptive statistics regarding bulk tank milk vitamin B12 concentration. Proc MIXED of SAS was used to evaluate the effect of bedding type as fixed effect on bulk tank milk vitamin B12 concentration.
3. Results
3.1. Study 1
3.1.1. Descriptive Statistics
Based on the 68 samples included for further analysis, vitamin B12 concentration in milk averaged 3643 pg/mL (SD: 1497; min to max: 1245 to 8437). Whole-rumen digesta, feces, and plasma vitamin B12 concentrations averaged 652 ng/g of DM (SD: 170; min to max: 300 to 1021), 612 ng/g of DM (SD: 125; min to max: 361 to 920), and 215 pg/mL (SD: 70; min to max: 116 to 415), respectively. The dry matter content of whole-rumen digesta and feces averaged 15.7% (SD: 1.5; min to max: 12.0 to 19.2) and 14.1% (SD: 1.6; min to max: 10.4 to 17.2), respectively.
Cows fed with the group 1 ration were associated with lower estimated BW and DIM than those fed the group 2 ration (p ≤ 0.0003; Table 2). Cows in group 1 were mainly in early lactation, with 25% having less than 31 DIM and 75% less than 194 DIM. The morning milk yield of cows fed group 1 was almost twice as much as that of group 2 (p < 0.0001), but all milk component concentrations were lower for the former (p ≤ 0.02). The whole-rumen digesta of cows fed group 1 had greater vitamin B12 concentration than that of cows receiving group 2; the opposite was observed for vitamin concentration in milk (p ≤ 0.02; Table 2). Regarding ruminal fermentation characteristics, average pH throughout the sampling times was greater by 0.29 for group 2-fed cows compared with those in the other group (p < 0.0001). Ruminal liquid concentration of butyrate was greater for cows receiving the ration of group 1 than group 2. There was a tendency for greater proportion of acetate and acetate: propionate ratio for group 2 cows compared with group 1 cows (p ≤ 0.08).

Table 2.
Cow characteristics, vitamin B12 concentrations of whole-rumen digesta, feces, plasma, and milk, and ruminal fermentation characteristics according to the feeding group of the cow 1.
3.1.2. Principal Component Analysis
Six PC having an Eigenvalue > 1 and explaining 75% of the total variation were retained for further analysis (Table 3). Indeed, the PC 6 had an Eigenvalue of 1.04 and the PC 7, 0.82. The first, second and third PC had a variance of 4.8, 2.8, and 2.3, explaining 27, 16, and 13% of the total variation, respectively. Two PC were solely related to ruminal fermentation characteristics (PC1 and PC3). The first PC was positively correlated with propionate and valerate and negatively with the acetate:propionate ratio, whereas the third PC was positively associated with acetate, butyrate, isobutyrate and isovalerate. The second PC pertained to milk production, correlating positively with DIM and morning milk protein concentration and negatively with morning milk yield. The fourth PC was positively associated with estimated cow BW and ruminal pH, and negatively with whole-ruminal digesta vitamin B12 concentration. The fifth PC correlated positively with plasma vitamin B12 concentration and morning milk fat concentration, and the sixth PC correlated positively with feces vitamin B12 concentration, DMI, and ruminal ammonia N concentration. These six PC, each representing a set of interrelated variables, could be considered as independent variables in the multivariable analysis. The rotated pattern coefficients depicted in Table 3 represent the relative contribution of each variable to a PC.

Table 3.
Rotated pattern coefficients 1 of the principal component (PC) analysis.
3.1.3. Relationship among Milk Vitamin B12 Concentration and Independent Variables
The interaction feeding group × PC1 as well as three other PC had a significant relationship with milk vitamin B12 concentration (p ≤ 0.05; Table 4). Cows fed group 2 had greater milk vitamin B12 concentration when ruminal concentrations of propionate and valerate increased and the acetate:propionate ratio decreased (PC1), whereas the opposite was observed for cows receiving group 1 (Feeding group × PC1 interaction, p = 0.002). The PC2 and PC5, related to milk productivity, were positively correlated with milk vitamin B12 concentration (p ≤ 0.02), meaning that this vitamin in milk increased along with morning milk fat and protein concentrations as DIM increased, even though there was an inverse relationship between milk vitamin B12 concentration and morning milk yield. Moreover, milk vitamin B12 concentration was positively associated with its plasma concentration (PC5). Finally, vitamin B12 concentration in milk was positively correlated with estimated cow BW and ruminal pH and negatively with whole-rumen digesta vitamin B12 concentration (PC4; p = 0.05). Pseudo-R2 was 93%, meaning that the current model explained 93% of the milk vitamin B12 variability. When considering only PC2 and PC5 in the model, i.e., PC related to milk productivity and plasma vitamin B12 concentration, the pseudo-R2 was 88%.

Table 4.
Results from the principal component (PC) regression analysis regarding the relationship between milk vitamin B12 concentration and ruminal fermentation characteristics, as well as milk productivity and cow characteristics 1.
3.2. Study 2
Bulk tank milk vitamin B12 concentration did not differ between RMS and straw bedding herds (p = 0.99), and averaged 4276 (SD: 789) pg/mL. The lowest and highest concentrations were 1953 and 5946 pg/mL, respectively (Figure 2).
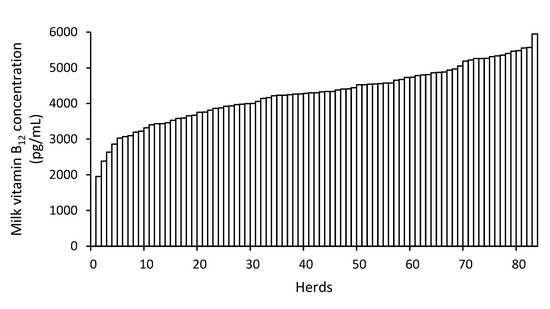
Figure 2.
Bulk tank milk vitamin B12 concentration among dairy herds.
4. Discussion
Milk is considered a staple and complete food. It has been reported that milk and dairy products are the most important sources of daily vitamin B12 requirement fulfilment in humans in several countries [39,40,41], and especially in those below the age of 18. According to the National Academy of Sciences [42], vitamin B12 RDA for humans above 13 years old is 2.4 µg/d, increasing to 2.6 and 2.8 µg/d for pregnant and lactating women, respectively. A 250-mL milk glass from cows of study 1 could provide between 13 and 88% of the RDA for nongestating and nonlactating humans above 13 years old. Regarding study 2, from 20 to 62% of the RDA could be fulfilled by a 250-mL glass of milk from the involved herds, which is similar to figures presented by Duplessis et al. [14], who reported a RDA fulfillment of between 28 and 61% from a 250-mL glass of milk from 100 commercial dairy herds. The average vitamin B12 concentrations in milk reported in study 1 seem low compared to what has been previously reported in the literature [14,16], whereas the average bulk tank milk vitamin B12 concentration of study 2 was similar [14]. The results from study 1 could be due to the time of year when the samples were collected. Indeed, it has been reported that milk vitamin B12 concentration was lower during the summer months [15,43], which is when experiment 1 was performed. Nevertheless, as commonly reported in the literature, milk vitamin B12 concentration was highly variable within cows in the same herd and among herds [14,15,16,17]. Plasma vitamin B12 concentrations were similar to those in previous trials [17,44]. To our knowledge, this is the first time that dairy cow whole-rumen digesta and feces vitamin B12 concentrations have been reported in the literature.
Cows receiving the group 1 ration in study 1 were typically in early lactation, producing more milk and requiring a diet containing more energy and less fiber. This is supported by lower ruminal pH and acetate:propionate ratio for those cows compared with cows receiving the ration of group 2. Moreover, it has been previously reported on those cows that ruminal microbiota differed between cows fed groups 1 and 2 [18]. Franco-Lopez et al. [18] also found that ruminal microbiota was different between cows with high and low vitamin B12 concentrations in whole-rumen digesta. That could explain the significant difference in whole-rumen digesta vitamin B12 concentrations between feeding groups. The milk vitamin B12 concentration was about 18% greater for cows receiving the ration of group 2 compared with group 1.
The objectives of this paper were to gather more information about factors driving variations in milk vitamin B12 concentration. Few studies exist pertaining to the causes of this variation in milk. As milk is an excellent source of vitamin B12 for humans who rely on exogenous sources to get this required nutrient, acquiring more knowledge in this regard would help in the development of methods to optimize the secretion of this vitamin in milk. The premise behind study 1 was as follows: as vitamin B12 is synthesized by ruminal microbiota, a part of the synthesized vitamin would be used by the cow cells and another part would be secreted in milk; therefore, any factors modifying the vitamin synthesis in the rumen would also modify its secretion in milk. Using diet printouts of 13 commercial herds, we previously observed a positive relationship between milk vitamin B12 concentration and dietary percentage of ADF [15]. Then, a study to assess the impact of the actual ration given to dairy cows on milk vitamin B12 concentration was conducted on 100 commercial Holstein herds [14]. Using the actual nutrient composition of the diet, we showed that vitamin B12 in milk was positively correlated with dietary fiber concentration and negatively correlated with dietary starch and energy concentrations. Moreover, previous results suggested that vitamin B12 ARS was correlated with dietary NDF [19,21,45]. This is in line with our results as, in the PC regression analysis, we noted a positive relationship between this vitamin concentration in milk and ruminal pH. It is indeed well recognized that ruminal pH generally increases as dietary forage NDF or physically effective NDF also increases [46,47]. Nevertheless, there is no consensus in the literature about the correlation between vitamin B12 ARS and ruminal pH; some authors reported none [19,48], while others observed a negative [21,45] or positive [20] association. This could be explained by the variety of diets offered to dairy cows in those studies, combined with various pH ranges.
In the PC regression analysis, there was a significant feeding group x PC1 interaction effect on milk vitamin B12 concentration. PC1 included variables related to ruminal fermentation such as concentrations of propionate and valerate, and the propionate:acetate ratio. With the difference in ration nutrient composition between feeding groups and its subsequent impact on ruminal fermentation, it was not surprising to observe an interaction with feeding group and PC1. However, feeding group might also have a confounding effect with DIM. Duplessis et al. [14] observed a positive relationship between milk vitamin B12 concentration and percentage of chopped mixed silage, and a negative relationship with percentage of corn silage in the ration. These results are in accordance with our findings, as cows fed group 2 had greater milk vitamin B12 concentration coupled with greater dietary percentage of chopped mixed silage and lower percentage of corn silage than cows fed group 1. These changes in the ration could explain the ruminal fermentation characteristic differences between feeding groups. Franco-Lopez et al. [18] observed, for cows in study 1, that a high concentration of whole-rumen digesta vitamin B12 was associated with an increased abundance of the genus Prevotella in the rumen. This microorganism is recognized to have a role in propionate synthesis [49]. In the same line, in the current study, cows fed group 1 had greater whole-rumen digesta vitamin B12 concentration and a lower acetate:propionate ratio. It is surprising that vitamin B12 concentration in milk was negatively correlated with whole-rumen digesta vitamin B12 concentration according to PC4. It is noteworthy that whole-rumen digesta vitamin B12 concentration is far from being comparable to vitamin B12 ARS. Indeed, vitamin ARS is calculated as the difference of vitamin duodenal flow minus vitamin intake [21]. Whole-rumen digesta concentration could be influenced by rumen fill, passage rate and dietary fiber concentration. This could explain the opposite relationship between whole-rumen digesta and milk vitamin B12 concentrations.
Vitamin B12 concentration in milk was positively associated with estimated cow BW, which is in line with the results of Duplessis et al. [14]. In the current experiment, it could not be determined if the effect of estimated BW was linked to parity. Duplessis et al. [14] observed that milk vitamin B12 concentration increased with parity and BW. They concluded that this could be due to the fact that first parity cows, who usually have a lower BW than older cows, use the vitamin for their growth, and hence, less of the vitamin ends up in the milk [14]. Comparable results to those of the present study regarding milk yield and milk fat and protein concentrations were obtained by Duplessis et al. [14], who included over 4300 cows in their model. These authors also reported that milk vitamin B12 concentration followed the same typical curve as milk fat and protein concentrations, meaning a concentration nadir of around 50–55 DIM followed by a gradual increase. In the current trial, we did not observe a milk vitamin B12 concentration nadir in early lactation, and expDIM, as per Wilmink [50], was not significant in the model and was not included. This could be due to the small number of cows in the current experiment. Nevertheless, the current data suggest that this vitamin concentration in milk increased as lactation progressed, along with milk fat and protein concentrations. Vitamin B12 concentration in milk was positively correlated with plasma vitamin B12 concentration. It has been reported elsewhere, however, that the Spearman rank correlation coefficient between milk and plasma vitamin B12 concentrations of Holstein cows is weak, although significant [17].
The model explained 93% of the variability of milk vitamin B12 concentration of study 1, which is excellent. A pseudo-R2 of 52% was obtained in a previous study by Duplessis et al. [14] assessing the impact of diet management, milk productivity and cow characteristics on milk vitamin B12 concentration variation. However, it is noteworthy that the major difference between study 1 and that of Duplessis et al. [14] was the number of herds involved, i.e., 1 vs. 100 herds, respectively. Involving more herds in a study increases variability due to, among other factors, different management approaches. This is likely the explanation of the higher pseudo-R2 in the current experiment. Interestingly, both the current study and that of Duplessis et al. [14] concluded that milk productivity was the main driver of milk vitamin B12 concentration variation. Limitations of study 1 include the fact that some cows had to be sampled twice, as there were not sufficient rumen-cannulated cows in the herd. Moreover, the need of rumen-cannulated cows limited the sampling collection to one herd and a relatively small number of animals. Hence, the current results might not be representative of the population. This should be kept in mind when interpreting the results of study 1. Dietary Co concentration was not limiting in the current study as it was above the recommendation of 0.11 mg/kg of DM [31].
Most previous studies evaluating milk vitamin B12 concentration variation were directed at factors affecting vitamin synthesis in the rumen, for instance, diet nutrient composition that would first modify vitamin B12 synthesis by ruminal bacteria and then its secretion into milk. Recently, evidence has emerged that the udder has a microbiota, although this is still controversial [51]. Nonetheless, it is clear that the teat canal and apex do have a microbiota [51]. Environmental factors such as milking equipment, flies and bedding material could be potential factors affecting mammary microbiota [52]. To the best of our knowledge, there is no information in the literature about potential syntheses or the use of vitamin B12 by the mammary microbiota. Using the participating herds of study 2, Gagnon et al. [38] found a higher abundance of Streptococcus spp. and Enterococcus faecalis in bulk milk samples of herds using RMS bedding compared to straw bedding, suggesting a slightly different milk microbiota between these two bedding systems. These species are known to produce vitamin B12 [8,53]. However, we did not observe an influence of bedding type on vitamin B12 concentration of milk bulk tank samples.
5. Conclusions
This paper showed that ruminal fermentation characteristics such as ruminal pH could have an impact on milk vitamin B12 concentration. However, the impact of ruminal concentrations of propionate, valerate and the acetate:propionate ratio on milk vitamin B12 concentration was different according to the rations given to cows. This suggests that, as expected, the rations modified the ruminal fermentation characteristics and cannot be excluded as a factor influencing milk vitamin B12 concentration variability when ruminal fermentation characteristics are also considered. Milk fat and protein concentrations, DIM, estimated cow BW and plasma vitamin B12 concentration were all positively associated, and milk yield and whole-rumen digesta vitamin B12 concentration were negatively related with milk vitamin B12 concentration. The most important factors explaining this were related to milk productivity and plasma vitamin B12 concentration; the impact of other studied factors was relatively small. Bedding type, such as RMS and straw beddings, did not have any impact on vitamin B12 concentration in bulk tank milk. This study obtained further knowledge regarding factors explaining the huge variation of vitamin B12 concentrations in milk.
Author Contributions
Conceptualization, M.D., A.F. and J.R.; methodology, M.D., A.F., W.P., L.B. and J.R.; validation M.D., A.F. and J.R.; formal analysis, M.D.; investigation, M.D., A.F., W.P., L.B. and J.R.; resources, M.D.; data curation, M.D.; writing—original draft preparation, M.D.; writing—review and editing, M.D., A.F., W.P., L.B. and J.R.; supervision, M.D., A.F. and J.R.; project administration, M.D. and J.R.; funding acquisition, J.R. and A.F. team. All authors have read and agreed to the published version of the manuscript.
Funding
Study 1 was supported by an Op+Lait Subventions Nouvelles Initiatives grant. Study 2 was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), Novalait, Consortium de recherche et innovations en bioprocédés industriels au Québec, and the Fonds de recherche du Québec-Nature et technologies (FRQ-NT).
Institutional Review Board Statement
The first experiment protocol was approved by the Institutional Committee for Animal Care of the Sherbrooke Research and Development Centre (Agriculture & Agri-Food Canada, Sherbrooke, QC, Canada) The second trial was approved by the Animal Care and Use Committee for the Veterinary College of the Université de Montréal following the same guidelines of the study 1.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We express our gratitude to Christiane Girard (Agriculture and Agri-Food Canada, Sherbrooke, QC, Canada), Rachel Gervais (Université Laval, Québec, QC, Canada), and Simon Dufour (Université de Montréal, Saint-Hyacinthe, QC, Canada) for their invaluable contribution. We thank the technical help of Valérie Beaudet, Jasmin Brochu, Véronique Roy, Liette Veilleux (Agriculture and Agri-Food Canada, Sherbrooke, QC, Canada) and Yolaine Lebeuf (Université Laval, Québec, QC, Canada). We thank Marie-Ève Bouchard, Émélie Bell, Matthew Suitor, Étienne Viens, and the barn staff (Agriculture and Agri-Food Canada, Sherbrooke, QC, Canada) for their help during sample collection and care of cows throughout the study 1. We are grateful to dairy producers who participated in study 2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crane, F.G.; Clarke, T.K. Attitudes towards milk: A canadian view. Br. Food J. 1989, 91, 6–9. [Google Scholar] [CrossRef]
- Smith, L.M. Trends in per capita consumption of dairy foods. J. Dairy Sci. 1982, 65, 469–475. [Google Scholar] [CrossRef]
- Canadian Dairy Information Centre. Global Consumption of Dairy Products-Total Fluid Milk Consumption for Selected Countries. Available online: https://aimis-simia-cdic-ccil.agr.gc.ca/rp/index-eng.cfm?action=gR&r=264&signature=41A9A7137CF7257BDA88B83E8B108724&pdctc=&pTpl=1#wb-cont (accessed on 9 April 2020).
- McCarthy, K.S.; Parker, M.; Ameerally, A.; Drake, S.L.; Drake, M.A. Drivers of choice for fluid milk versus plant-based alternatives: What are consumer perceptions of fluid milk? J. Dairy Sci. 2017, 100, 6125–6138. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Faber, I.; Osorio, J.S.; Stergiadis, S. Consumer knowledge and perceptions of milk fat in denmark, the united kingdom, and the united states. J. Dairy Sci. 2020, 103, 4151–4163. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.; Jahn, D. Microbial production of vitamin b12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- González-Montaña, J.-R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between vitamin B12 and cobalt metabolism in domestic ruminant: An update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef]
- Dalto, D.B.; Audet, I.; Girard, C.L.; Matte, J.J. Bioavailability of vitamin b12 from dairy products using a pig model. Nutrients 2018, 10, 1134. [Google Scholar] [CrossRef]
- Matte, J.J.; Guay, F.; Girard, C.L. Bioavailability of vitamin b12 in cows’ milk. Br. J. Nutr. 2012, 107, 61–66. [Google Scholar] [CrossRef]
- Truswell, A.S. Vitamin b12. Nutr. Diet. 2007, 64, S120–S125. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Milk, Whole. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/781084/nutrients (accessed on 5 May 2020).
- Duplessis, M.; Pellerin, D.; Robichaud, R.; Fadul-Pacheco, L.; Girard, C.L. Impact of diet management and composition on vitamin b12 concentration in milk of holstein cows. Animal 2019, 13, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, M.; Pellerin, D.; Cue, R.I.; Girard, C.L. Short communication: Factors affecting vitamin b12 concentration in milk of commercial dairy herds: An exploratory study. J. Dairy Sci. 2016, 99, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.J.M.; Bouwman, A.C.; Sprong, R.C.; van Arendonk, J.A.M.; Visker, M.H.P.W. Genetic variation in vitamin B12 content of bovine milk and its association with snp along the bovine genome. PLoS ONE 2013, 8, e62382. [Google Scholar] [CrossRef]
- Duplessis, M.; Cue, R.I.; Santschi, D.E.; Lefebvre, D.M.; Girard, C.L. Short communication: Relationships among plasma and milk vitamin B12, plasma free fatty acids, and blood β-hydroxybutyrate concentrations in early lactation dairy cows. J. Dairy Sci. 2018, 101, 8559–8565. [Google Scholar] [CrossRef]
- Franco-Lopez, J.; Duplessis, M.; Bui, A.; Reymond, C.; Poisson, W.; Blais, L.; Chong, J.; Gervais, R.; Rico, D.E.; Cue, R.I.; et al. Correlations between the composition of the bovine microbiota and vitamin b12 abundance. mSystems 2020, 5, e00107–e00120. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, V.; Gervais, R.; Graulet, B.; Nozière, P.; Doreau, M.; Fanchone, A.; Castagnino, D.S.; Girard, C.L. Effects of dietary nitrogen levels and carbohydrate sources on apparent ruminal synthesis of some b vitamins in dairy cows. J. Dairy Sci. 2016, 99, 2730–2739. [Google Scholar] [CrossRef]
- Castagnino, D.S.; Kammes, K.L.; Allen, M.S.; Gervais, R.; Chouinard, P.Y.; Girard, C.L. High-concentrate diets based on forages harvested at different maturity stages affect ruminal synthesis of b vitamins in lactating dairy cows. Animal 2017, 11, 608–615. [Google Scholar] [CrossRef][Green Version]
- Seck, M.; Voelker Linton, J.A.; Allen, M.S.; Castagnino, D.S.; Chouinard, P.Y.; Girard, C.L. Apparent ruminal synthesis of b vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J. Dairy Sci. 2017, 100, 1914–1922. [Google Scholar] [CrossRef]
- Metzger, S.A.; Hernandez, L.L.; Skarlupka, J.H.; Suen, G.; Walker, T.M.; Ruegg, P.L. Influence of sampling technique and bedding type on the milk microbiota: Results of a pilot study. J. Dairy Sci. 2018, 101, 6346–6356. [Google Scholar] [CrossRef]
- National Farm Animal Care Council. Code of Practice for the Care and Handling of Dairy Cattle; Dairy Farmers of Canada and National Farm Animals Care Council: Ottawa, ON, Canada, 2009. [Google Scholar]
- Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals, 2nd ed.; Olfert, E.D., Cross, B.M., McWilliam, A.A., Eds.; Canadian Council on Animal Care: Ottawa, ON, Canada, 2009; Volume 1, p. 201. [Google Scholar]
- Canadian Dairy Information Centre. Average Milk Production by Breed Based on Milk Recording. Available online: https://dairyinfo.gc.ca/eng/dairy-statistics-and-market-information/dairy-animal-genetics/average-milk-production-by-breed/?id=1502476121954 (accessed on 10 February 2021).
- Yan, T.; Mayne, C.S.; Patterson, D.C.; Agnew, R.E. Prediction of body weight and empty body composition using body size measurements in lactating dairy cows. Livest. Sci. 2009, 124, 233–241. [Google Scholar] [CrossRef]
- Rico, D.E.; Preston, S.H.; Risser, J.M.; Harvatine, K.J. Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows. Br. J. Nutr. 2015, 114, 358–367. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2017. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th revised ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Duplessis, M.; Mann, S.; Nydam, D.V.; Girard, C.L.; Pellerin, D.; Overton, T.R. Short communication: Folates and vitamin B12 in colostrum and milk from dairy cows fed different energy levels during the dry period. J. Dairy Sci. 2015, 98, 5454–5459. [Google Scholar] [CrossRef]
- Leduc, M.; Gervais, R.; Tremblay, G.F.; Chiquette, J.; Chouinard, P.Y. Milk fatty acid profile in cows fed red clover- or alfalfa-silage based diets differing in rumen-degradable protein supply. Anim. Feed Sci. Technol. 2017, 223, 59–72. [Google Scholar] [CrossRef]
- SAS Institute. User’s Guide: Statistics. Version 9.4; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Kaps, M.; Lamberson, W.R. Biostatistics for Animal Science, 3rd ed.; CAB International: London, UK, 2017; p. 547. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; p. 234. [Google Scholar]
- Lasprilla-Mantilla, M.I.; Wagner, V.; Pena, J.; Fréchette, A.; Thivierge, K.; Dufour, S.; Fernandez-Prada, C. Effects of recycled manure solids bedding on the spread of gastrointestinal parasites in the environment of dairies and milk. J. Dairy Sci. 2019, 102, 11308–11316. [Google Scholar] [CrossRef]
- Gagnon, M.; Hamelin, L.; Fréchette, A.; Dufour, S.; Roy, D. Effect of recycled manure solids as bedding on bulk tank milk and implications for cheese microbiological quality. J. Dairy Sci. 2020, 103, 128–140. [Google Scholar] [CrossRef]
- Auclair, O.; Han, Y.; Burgos, S.A. Consumption of milk and alternatives and their contribution to nutrient intakes among canadian adults: Evidence from the 2015 canadian community health survey-nutrition. Nutrients 2019, 11, 1948. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. The contribution of milk and milk products to micronutrient density and affordability of the us diet. J. Am. Coll. Nutr. 2011, 30, 422S–428S. [Google Scholar] [CrossRef]
- National Academy of Sciences. Vitamin B12. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin b6, Folate, Vitamin b12, Pantothenic Acid, Biotin and Choline; National Academy Press: Washington, DC, USA, 1998; pp. 306–356. [Google Scholar]
- Scott, K.J.; Bishop, D.R.; Zechalko, A.; Edwards-Webb, J.D. Nutrient content of liquid milk i. Vitamins a, d3, c and of the b complex in pasteurized bulk liquid milk. J. Dairy Res. 1984, 51, 37–50. [Google Scholar] [CrossRef]
- Weerathilake, W.A.D.V.; Brassington, A.H.; Williams, S.J.; Kwong, W.Y.; Sinclair, L.A.; Sinclair, K.D. Added dietary cobalt or vitamin b12, or injecting vitamin b12 does not improve performance or indicators of ketosis in pre- and post-partum holstein-friesian dairy cows. Animal 2019, 13, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Schwab, E.C.; Schwab, C.G.; Shaver, R.D.; Girard, C.L.; Putnam, D.E.; Whitehouse, N.L. Dietary forage and nonfiber carbohydrate contents influence b-vitamin intake, duodenal flow, and apparent ruminal synthesis in lactating dairy cows. J. Dairy Sci. 2006, 89, 174–187. [Google Scholar] [CrossRef]
- Li, C.; Beauchemin, K.A.; Yang, W. Feeding diets varying in forage proportion and particle length to lactating dairy cows: I. Effects on ruminal ph and fermentation, microbial protein synthesis, digestibility, and milk production. J. Dairy Sci. 2020, 103, 4340–4354. [Google Scholar] [CrossRef]
- Ranathunga, S.D.; Kalscheur, K.F.; Herrick, K.J. Ruminal fermentation, kinetics, and total-tract digestibility of lactating dairy cows fed distillers dried grains with solubles in low- and high-forage diets. J. Dairy Sci. 2019, 102, 7980–7996. [Google Scholar] [CrossRef] [PubMed]
- Castagnino, D.S.; Seck, M.; Beaudet, V.; Kammes, K.L.; Voelker Linton, J.A.; Allen, M.S.; Gervais, R.; Chouinard, P.Y.; Girard, C.L. Effects of forage family on apparent ruminal synthesis of b vitamins in lactating dairy cows. J. Dairy Sci. 2016, 99, 1884–1894. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in vfa production in cow rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Wilmink, J.B.M. Adjustment of test-day milk, fat and protein yield for age, season and stage of lactation. Livest. Prod. Sci. 1987, 16, 335–348. [Google Scholar] [CrossRef]
- Parente, E.; Ricciardi, A.; Zotta, T. The microbiota of dairy milk: A review. Int. Dairy J. 2020, 107, 104714. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Li, P.; Gu, Q.; Wang, Y.; Yu, Y.; Yang, L.; Chen, J.V. Novel vitamin B12-producing enterococcus spp. And preliminary in vitro evaluation of probiotic potentials. Appl. Microbiol. Biotechnol. 2017, 101, 6155–6164. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© Her Majesty the Queen in Right of Canada as represented by the Minister of Agriculture and Agri-Food, 2021 and the authors; Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).