New Evaluation of Postovulatory Follicle Degeneration at High-Temperature Regimes Refines Criteria for the Identification of Spawning Cohorts in the European Anchovy (Engraulis encrasicolus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
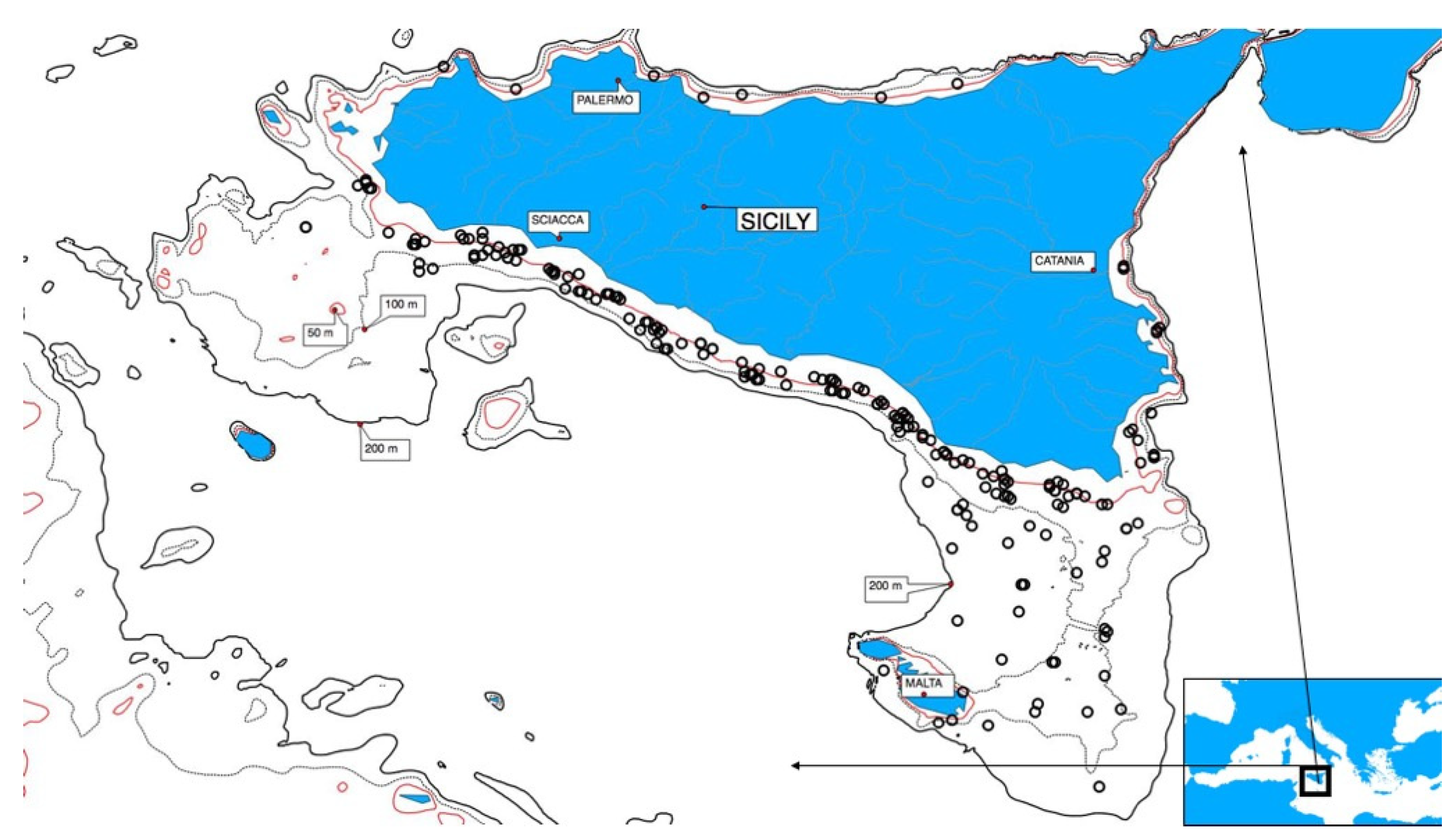
2.1. Field Sampling
2.2. Laboratory Analysis
2.3. Age Estimate
3. Results
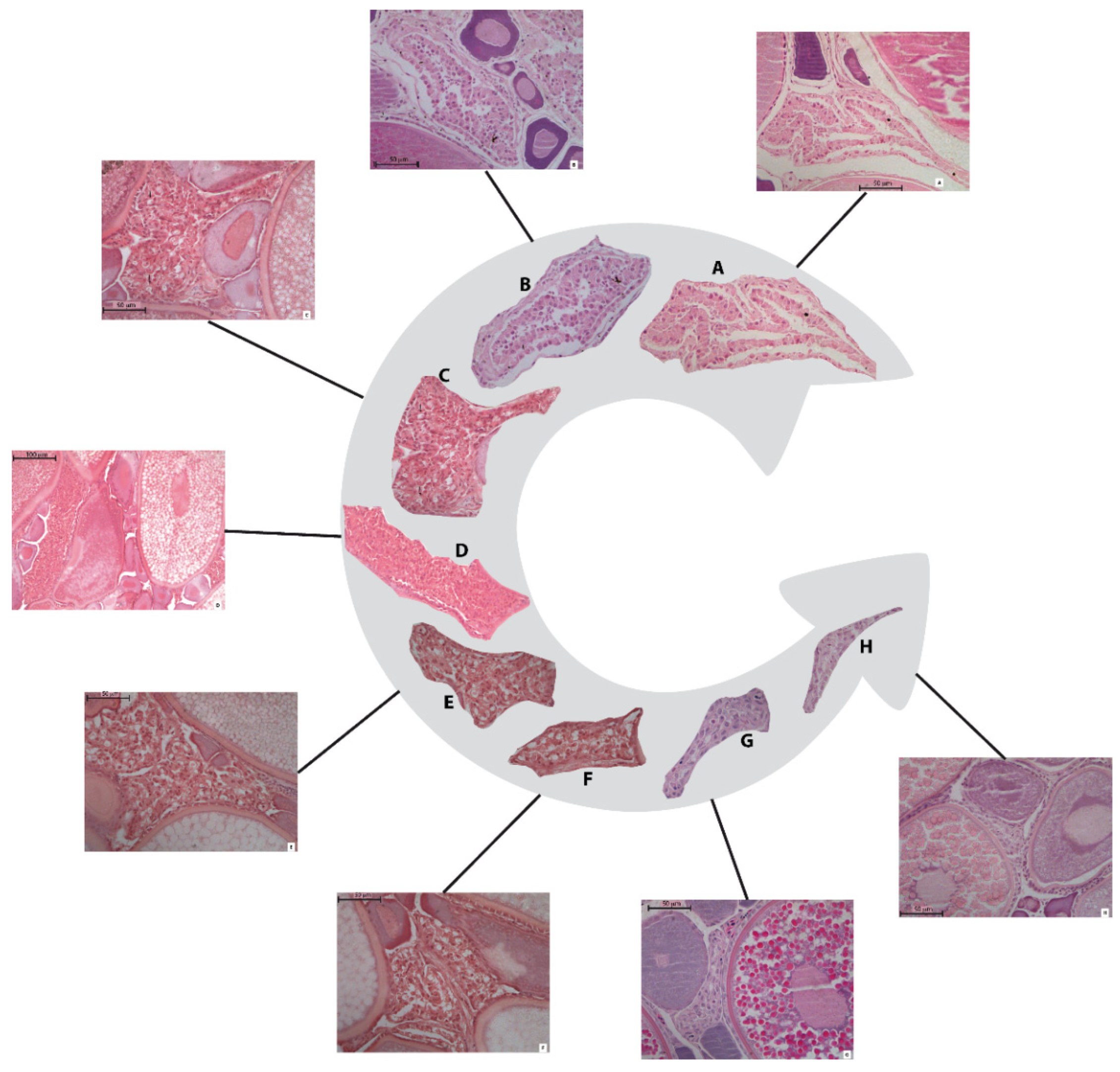
3.1. POF Stages Identification
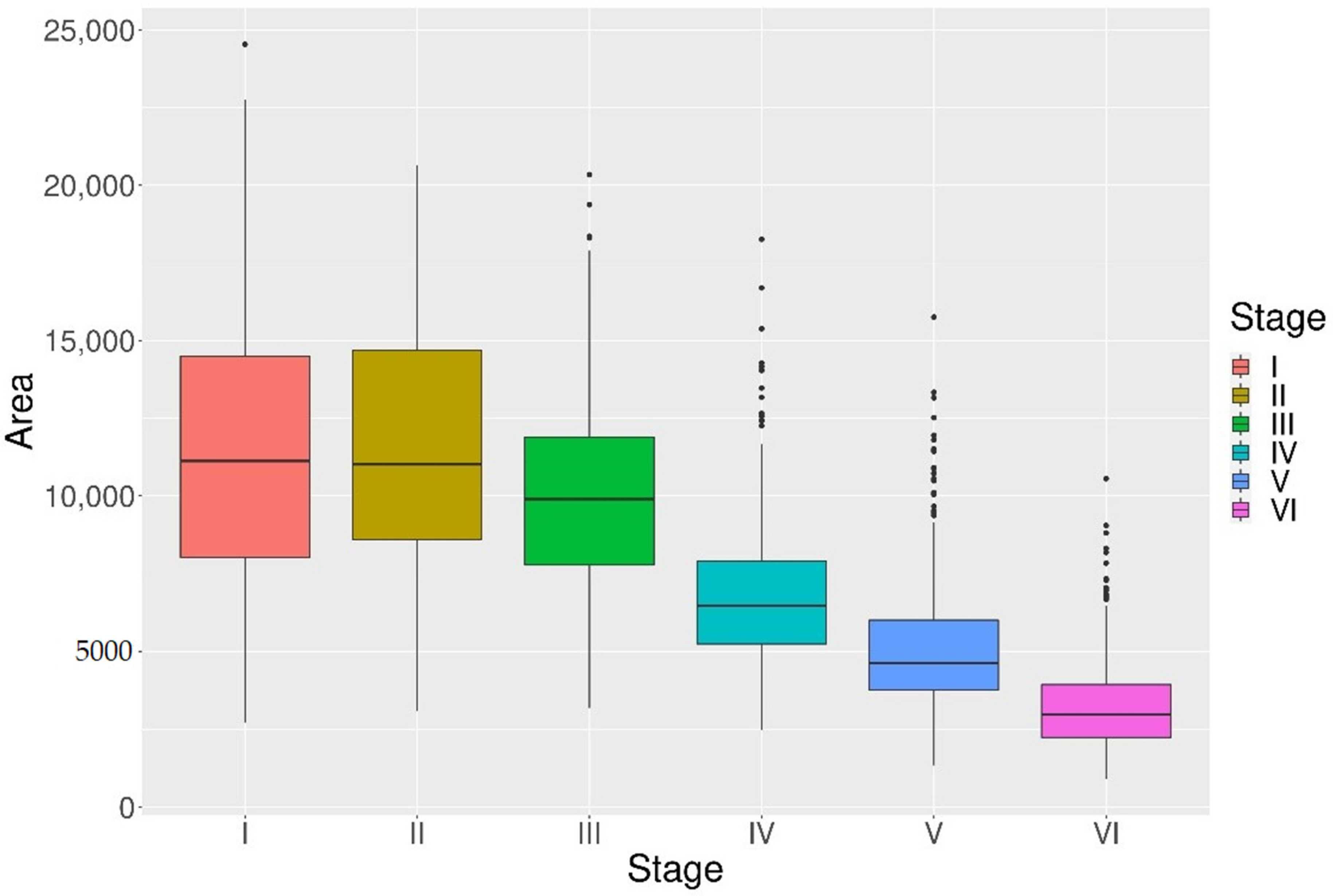
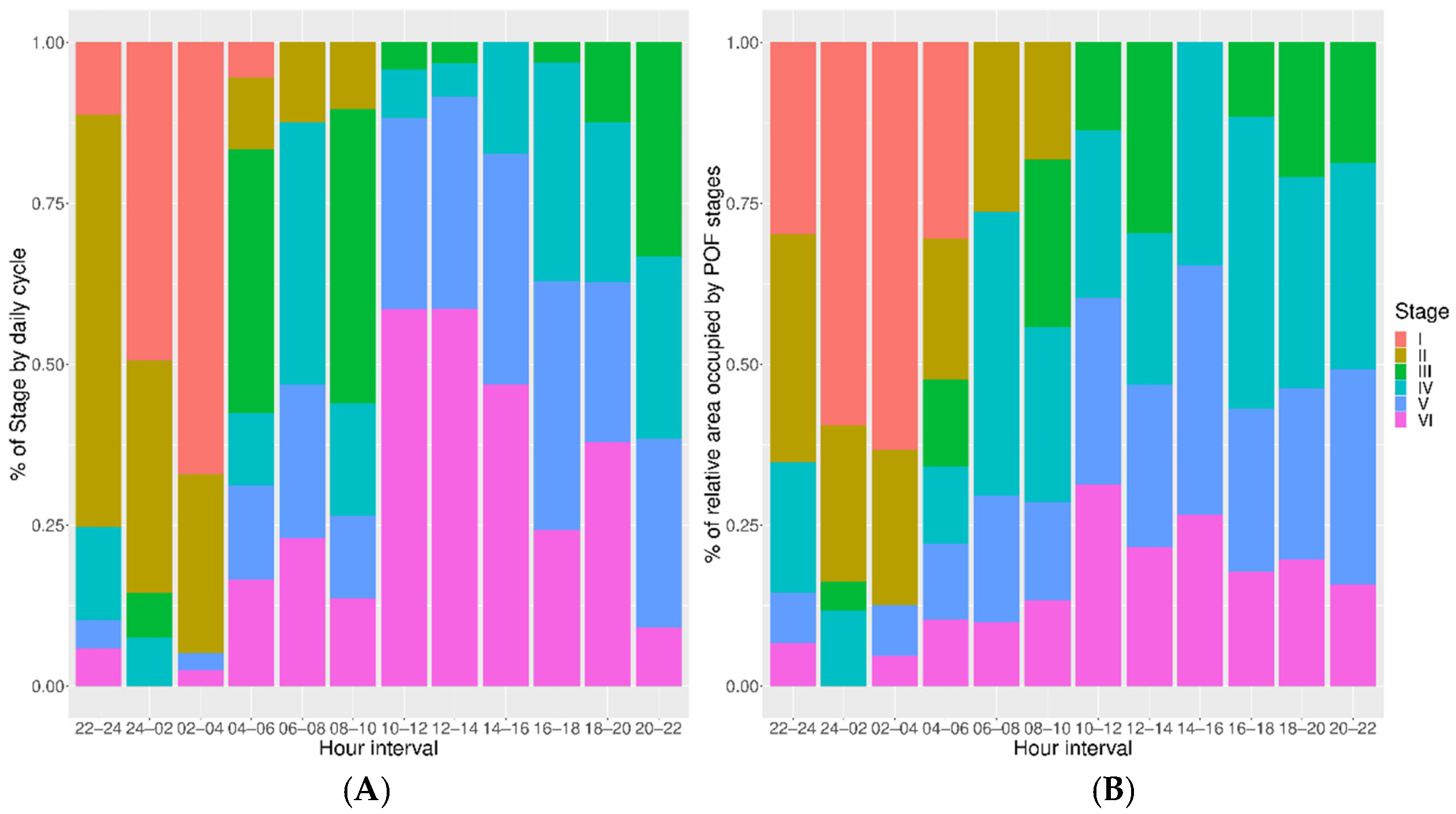
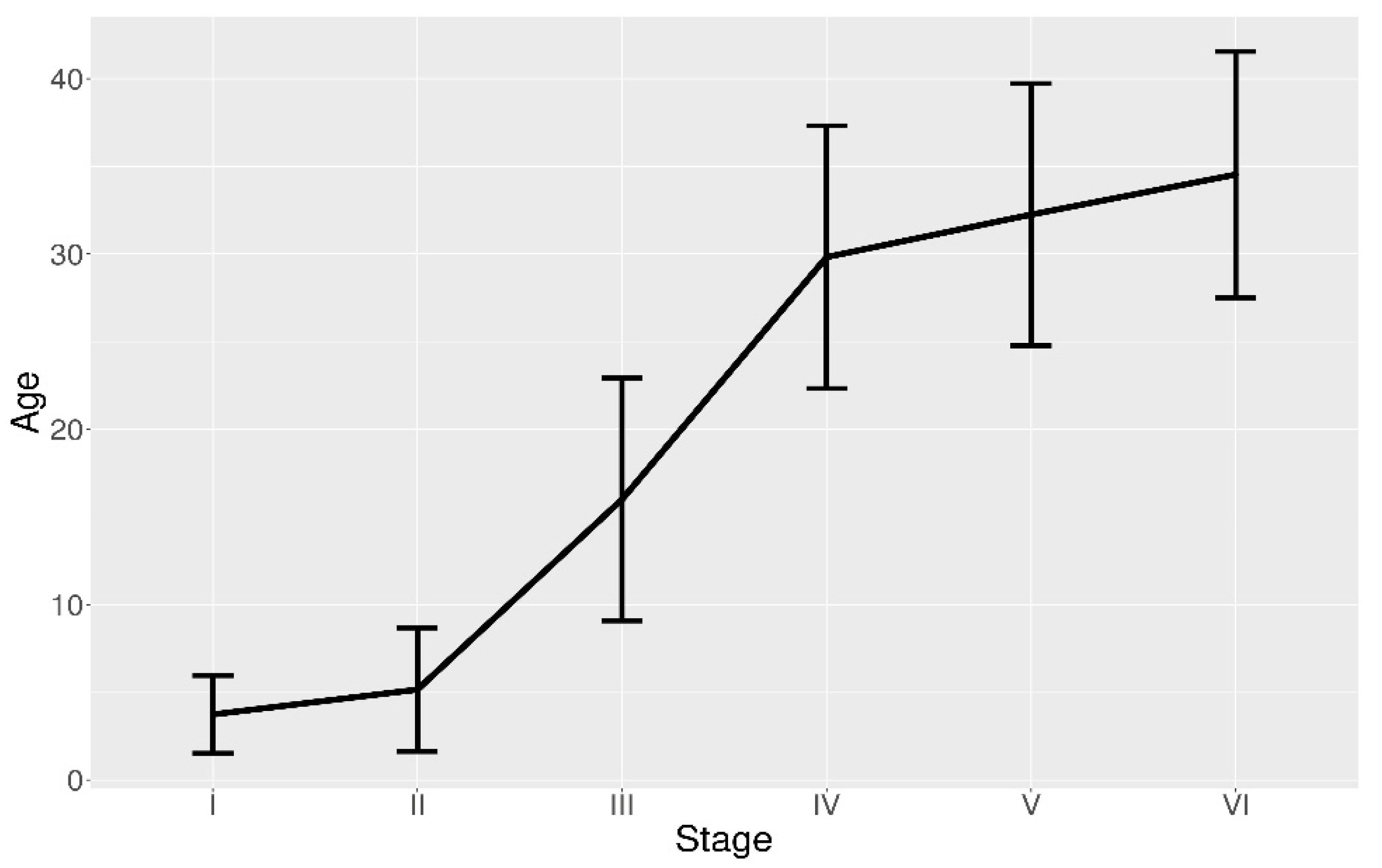
3.2. POF Size and Age
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palomera, I.; Olivar, M.P.; Salat, J.; Sabatés, A.; Coll, M.; García, A.; Morales-Nin, B. Small pelagic fish in the NW Mediterranean Sea: An ecological review. Prog. Oceanogr. 2007, 74, 377–396. [Google Scholar] [CrossRef]
- Giannoulaki, M.; Iglesias, M.; Tugores, M.P.; Bonanno, A.; Patti, B.; De Felice, A.; Leonori, I.; Bigot, J.L.; Tičina, V.; Pyrounaki, M.M.; et al. Characterizing the potential habitat of European anchovy Engraulis encrasicolus in the Mediterranean Sea, at different life stages. Fish. Oceanogr. 2013, 22, 69–89. [Google Scholar] [CrossRef]
- Bonanno, A.; Giannoulaki, M.; Barra, M.; Basilone, G.; Machias, A.; Genovese, S.; Goncharov, S.; Popov, S.; Rumolo, P.; Di Bitetto, M.; et al. Habitat selection response of small pelagic fish in different environments. Two examples from the oligotrophic Mediterranean Sea. PLoS ONE 2014, 9, e101498. [Google Scholar] [CrossRef]
- Morgan, M.J. Understanding biology to improve advice for fisheries management. ICES J. Mar. Sci. 2018, 75, 923–931. [Google Scholar] [CrossRef]
- Bernal, M.; Somarakis, S.; Witthames, P.R.; van Damme, C.J.G.; Uriarte, A.; Lo, N.C.H.; Dickey-Collas, M. Egg production methods in marine fisheries: An introduction. Fish. Res. 2012, 117–118, 1–5. [Google Scholar] [CrossRef]
- Hunter, J.; Goldberg, S. Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish. Bull. 1980, 77, 641–652. [Google Scholar]
- Ganias, K. Thirty years of using the postovulatory follicles method: Overview, problems and alternatives. Fish. Res. 2012, 117–118, 63–74. [Google Scholar] [CrossRef]
- Alheit, J. Use of the Daily Egg Production Method for estimating biomass of clupeoid fishes: A review and evaluation. Bull. Mar. Sci. 1993, 53, 750–767. [Google Scholar]
- Stratoudakis, Y.; Bernal, M.; Ganias, K.; Uriarte, A. The daily egg production method: Recent advances, current applications and future challenges. Fish Fish. 2006, 7, 35–57. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J. Sexual maturity, batch fecundity, spawning frequency and temporal pattern of spawning for the northern anchovy, Engraulis mordax, during the 1976 spawning season. Calif. Coop. Ocean. Fish. Investig. Rep. 1980, 21, 139–149. [Google Scholar]
- Hunter, J.R.; Macewicz, B.J. Measurement of spawning frequency in multiple spawning fishes. NOAA Tech. Rep. NMFS 36 1985, 79–94. [Google Scholar]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A.J. Evaluation of spawning frequency in a Mediterranean sardine population (Sardina pilchardus sardina). Mar. Biol. 2003, 142, 1169–1179. [Google Scholar] [CrossRef]
- Alday, A.; Uriarte, A.; Santos, M.; Martín, I.; Martinez de Murguia, A.; Motos, L. Degeneration of postovulatory follicles of the Bay of Biscay anchovy (Engraulis encrasicolus L.). Sci. Mar. 2008, 72, 565–575. [Google Scholar] [CrossRef]
- Guraya, S.S. The cell and molecular biology of fish oogenesis. Monogr. Dev. Biol. 1986, 18, 1–223. [Google Scholar] [CrossRef]
- Witthames, P.R.; Thorsen, A.; Kjesbu, O.S. The fate of vitellogenic follicles in experimentally monitored Atlantic cod Gadus morhua (L.): Application to stock assessment. Fish. Res. 2010, 104, 27–37. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Alarcon, V.; Alheit, J. Postovulatory follicle histology of the pacific sardine, Sardinops sagax, from Peru. Fish. Bull. 1984, 82, 443–445. [Google Scholar]
- Basilone, G.; Ganias, K.; Ferreri, R.; D’Elia, M.; Quinci, E.M.; Mazzola, S.; Bonanno, A. Application of GAMs and multinomial models to assess the spawning pattern of fishes with daily spawning synchronicity: A case study in the European anchovy (Engraulis encrasicolus) in the central Mediterranean Sea. Fish. Res. 2015, 167, 92–100. [Google Scholar] [CrossRef]
- Somarakis, S.; Palomera, I.; Garcia, A.; Quintanilla, L.; Koutsikopoulos, C.; Uriarte, A.; Motos, L. Daily egg production of anchovy in European waters. ICES J. Mar. Sci. 2004, 61, 944–958. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2020. In General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2020; ISBN 9789251337240. [Google Scholar] [CrossRef]
- Gascuel, D.; Doring, R.; Druon, J.N. Report of the SGMOS-10-03 Working Group. In Development of the Ecosystem Approach to Fisheries Management (EAFM) in European Seas; Publications Office of the European Union: Luxembourg, 2010; ISBN 9789279187490. [Google Scholar] [CrossRef]
- FAO. Fisheries management. 2. The ecosystem approach to fisheries. 2.1 Best practices in ecosystem modelling for informing an ecosystem approach to fisheries. In FAO Fisheries Technical Guidelines for Responsible Fisheries; FAO: Rome, Italy, 2008; 78p, ISBN 9780470015902.
- Fogarty, M.J. The art of ecosystem-based fishery management. Can. J. Fish. Aquat. Sci. 2014, 73, 479–490. [Google Scholar] [CrossRef]
- Bonanno, A.; Barra, M.; Basilone, G.; Genovese, S.; Rumolo, P.; Goncharov, S.; Popov, S.; Buongiorno Nardelli, B.; Iudicone, D.; Procaccini, G.; et al. Environmental processes driving anchovy and sardine distribution in a highly variable environment: The role of the coastal structure and riverine input. Fish. Oceanogr. 2016, 25, 471–490. [Google Scholar] [CrossRef]
- Basilone, G.; Ferreri, R.; Barra, M.; Bonanno, A.; Pulizzi, M.; Gargano, A.; Fontana, I.; Giacalone, G.; Rumolo, P.; Mazzola, S.; et al. Spawning ecology of the European anchovy (Engraulis encrasicolus) in the Strait of Sicily: Linking variations of zooplankton prey, fish density, growth, and reproduction in an upwelling system. Prog. Oceanogr. 2020, 184, 102330. [Google Scholar] [CrossRef]
- Alheit, J.; Alarcon, V.H.; Macewicz, B.J. Spawning frequency and sex ratio in the Peruvian anchovy, Engraulis ringens. Calif. Coop. Ocean. Fish. Investig. Rep. 1984, 25, 43–52. [Google Scholar]
- Funamoto, T.; Aoki, I. Reproductive ecology of Japanese anchovy off the Pacific coast of eastern Honshu, Japan. J. Fish Biol. 2002, 60, 154–169. [Google Scholar] [CrossRef]
- Alday, A.; Santos, M.; Uriarte, A.; Martín, I.; Martínez, U.; Motos, L. Revision of criteria for the classification of postovulatory follicles degeneration, for the Bay of Biscay anchovy (Engraulis encrasicolus L.). Rev. Investig. Mar. 2010, 17, 165–171. [Google Scholar]
- Somarakis, S.; Schismenou, E.; Siapatis, A.; Giannoulaki, M.; Kallianiotis, A.; Machias, A. High variability in the Daily Egg Production Method parameters of an eastern Mediterranean anchovy stock: Influence of environmental factors, fish condition and population density. Fish. Res. 2012, 117–118, 12–21. [Google Scholar] [CrossRef]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Vergara, A.R.; Maneiro, I. Effect of habitat conditions on reproduction of the European anchovy (Engraulis encrasicolus) in the Strait of Sicily. Fish. Oceanogr. 2006, 15, 271–280. [Google Scholar] [CrossRef]
- Parker, K. A direct method for estimating northern anchovy. Fish. Bullettin U.S 1980, 78, 541–544. [Google Scholar]
- Barra, M.; Petitgas, P.; Bonanno, A.; Somarakis, S.; Woillez, M.; Machias, A.; Mazzola, S.; Basilone, G.; Giannoulaki, M. Interannual changes in biomass affect the spatial aggregations of anchovy and sardine as evidenced by Geostatistical and spatial indicators. PLoS ONE 2015, 10, e0135808. [Google Scholar] [CrossRef]
- Uriarte, A.; Alday, A.; Santos, M.; Motos, L. A re-evaluation of the spawning fraction estimation procedures for Bay of Biscay anchovy, a species with short interspawning intervals. Fish. Res. 2012, 117–118, 96–111. [Google Scholar] [CrossRef]
- Basilone, G.; Bonanno, A.; Patti, B.; Mazzola, S.; Barra, M.; Cuttitta, A.; Mcbride, R. Spawning site selection by European anchovy (Engraulis encrasicolus) in relation to oceanographic conditions in the Strait of Sicily. Fish. Oceanogr. 2013, 22, 309–323. [Google Scholar] [CrossRef]
- Ferreri, R.; Basilone, G.; D’Elia, M.; Traina, A.; Saborido-Rey, F.; Mazzola, S. Validation of macroscopic maturity stages according to microscopic histological examination for European anchovy. Mar. Ecol. 2009, 30, 181–187. [Google Scholar] [CrossRef]
- Korta, M.; Murua, H.; Quincoces, I.; Thorsen, A.; Witthames, P. Three-dimensional reconstruction of postovulatory follicles from histological sections. Fish. Res. 2010, 104, 38–44. [Google Scholar] [CrossRef]
- Clarke, T.A. Fecundity and spawning frequency of the Hawaiian anchovy or nehu, Encrasicholina purpurea. Fish. Bull. 1987, 85, 127–138. [Google Scholar]
- Ganias, K.; Nunes, C.; Stratoudakis, Y. Degeneration of postovulatory follicles in the Iberian sardine Sardina pilchardus: Structural changes and factors affecting resorption. Fish. Bull. 2007, 105, 131–139. [Google Scholar]
- Dickerson, T.L.; Macewicz, B.J.; Hunter, J.R. Spawning Frequency and Batch Fecundity of Chub Mackerel, Scomber Japonicus, During 1985. CalCOFI Rep. 1992, 33, 130–140. [Google Scholar]
- Fitzhugh, G.R.; Carolina, R.N.; Hettler, W.F. Temperature influence on postovulatory follicle degeneration in Atlantic menhaden, Brevoortia tyrannus. Atlantic 1995, 93, 568–572. [Google Scholar]
- Murua, H.; Motos, L.; Lucio, P. Reproductive modality and batch fecundity of the European hake (Merluccius merluccius L.) in the Bay of Biscay. Calif. Coop. Ocean. Fish. Investig. Rep. 1998, 39, 196–203. [Google Scholar]
- Lowerre-Barbieri, S.K.; Ganias, K.; Saborido-Rey, F.; Murua, H.; Hunter, J.R. Reproductive timing in marine fishes: Variability, temporal scales, and methods. Mar. Coast. Fish. 2011, 3, 71–91. [Google Scholar] [CrossRef]
- Haslob, H.; Kraus, G.; Saborido-Rey, F. The dynamics of postovulatory follicle degeneration and oocyte growth in Baltic sprat. J. Sea Res. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Ganias, K.; Nunes, C. Bathymetric segregation of spawning stages in the Atlantic sardine Sardina pilchardus. Mar. Ecol. Prog. Ser. 2011, 428, 235–244. [Google Scholar] [CrossRef]
- Motos, L.; Uriarte, A.; Prouzet, P.; Santos, M.; Sagarminaga, Y. A Ssessing the Bay of Biscay Anchovy Population by depm: A Review 1989–2001. In Proceedings of the SPACC Meeting, Concepción, Chile, 14–16 January 2004; pp. 14–16. [Google Scholar]
- Hunter, J.; Macewicz, B.; Sibert, J. The spawning frequency of Skipjack tuna, Katsuwonus pelamis, from the South Pacific. Fish. Bull. 1986, 84, 895–903. [Google Scholar]
- Schaefer, K.M. Reproductive biology of yellowfin tuna (Thunnus albacares) in the eastern Pacific Ocean. Inter-Am. Trop. Tuna Comm. 1998, 21, 205–272. [Google Scholar]
- Farley, J.H.; Williams, A.J.; Hoyle, S.D.; Davies, C.R.; Nicol, S.J. Reproductive Dynamics and Potential Annual Fecundity of South Pacific Albacore Tuna (Thunnus alalunga). PLoS ONE 2013, 8, e60577. [Google Scholar] [CrossRef] [PubMed]


| Year | Sampling Period | No. F | No. Ovaries |
|---|---|---|---|
| 2008 | August | 152 | 11 |
| 2009 | August | 446 | 29 |
| 2012 | June–July | 474 | 46 |
| 2013 | June | 298 | 41 |
| 2014 | July | 222 | 44 |
| 2015 | July | 516 | 74 |
| Time | No. Catches | No. POF |
|---|---|---|
| 00:00–01:59 | 4 | 37 |
| 02:00–03:59 | 4 | 34 |
| 04:00–05:59 | 33 | 369 |
| 06:00–07:59 | 8 | 47 |
| 08:00–09:59 | 50 | 471 |
| 10:00–11:59 | 25 | 158 |
| 12:00–13:59 | 32 | 208 |
| 14:00–15:59 | 38 | 262 |
| 16:00–17:59 | 27 | 197 |
| 18:00–19:59 | 9 | 47 |
| 20:00–21:59 | 8 | 54 |
| 22:00–23:59 | 8 | 54 |
| POF Stage | POF I | POF II | POF III | POF IV | POF V | POF VI |
|---|---|---|---|---|---|---|
| POF I | 1.00 | 1.00 | 0.000459 | 0.00 | 0.00 | |
| POF II | 1.00 | 0.007490 | 0.00 | 0.00 | ||
| POF III | 0.000001 | 0.00 | 0.00 | |||
| POF IV | 0.00 | 0.00 | ||||
| POF V | 0.00 | |||||
| POF VI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreri, R.; Barra, M.; Gargano, A.; Aronica, S.; Bonanno, A.; Genovese, S.; Rumolo, P.; Basilone, G. New Evaluation of Postovulatory Follicle Degeneration at High-Temperature Regimes Refines Criteria for the Identification of Spawning Cohorts in the European Anchovy (Engraulis encrasicolus). Animals 2021, 11, 529. https://doi.org/10.3390/ani11020529
Ferreri R, Barra M, Gargano A, Aronica S, Bonanno A, Genovese S, Rumolo P, Basilone G. New Evaluation of Postovulatory Follicle Degeneration at High-Temperature Regimes Refines Criteria for the Identification of Spawning Cohorts in the European Anchovy (Engraulis encrasicolus). Animals. 2021; 11(2):529. https://doi.org/10.3390/ani11020529
Chicago/Turabian StyleFerreri, Rosalia, Marco Barra, Antonella Gargano, Salvatore Aronica, Angelo Bonanno, Simona Genovese, Paola Rumolo, and Gualtiero Basilone. 2021. "New Evaluation of Postovulatory Follicle Degeneration at High-Temperature Regimes Refines Criteria for the Identification of Spawning Cohorts in the European Anchovy (Engraulis encrasicolus)" Animals 11, no. 2: 529. https://doi.org/10.3390/ani11020529
APA StyleFerreri, R., Barra, M., Gargano, A., Aronica, S., Bonanno, A., Genovese, S., Rumolo, P., & Basilone, G. (2021). New Evaluation of Postovulatory Follicle Degeneration at High-Temperature Regimes Refines Criteria for the Identification of Spawning Cohorts in the European Anchovy (Engraulis encrasicolus). Animals, 11(2), 529. https://doi.org/10.3390/ani11020529








