Comparison of Fecal Microbiota of Horses Suffering from Atypical Myopathy and Healthy Co-Grazers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Inclusion Criteria and Group Definition
- a tentative diagnosis of AM based on the algorithm proposed by van Galen et al. (2012) [7], i.e., a compatible history (non-exercise-induced acute rhabdomyolysis syndrome in a horse kept at pasture) and clinical signs highly suggestive of AM (i.e., acute onset of muscle weakness, stiffness and/or pigmenturia) during spring/autumn,
- grazing in a pasture where a case of AM had been diagnosed in the previous 24 h.
- having a normal clinical and dynamic examination at walk (no signs of AM or other obvious disease) at the time of sampling.
2.3. Bacterial DNA Extraction and High-Throughput Sequencing
2.4. Sequence Analysis and 16S rDNA Profiling
2.5. Data Analysis
3. Results
3.1. AnImals
Diet
3.2. Analysis of Microbial Population
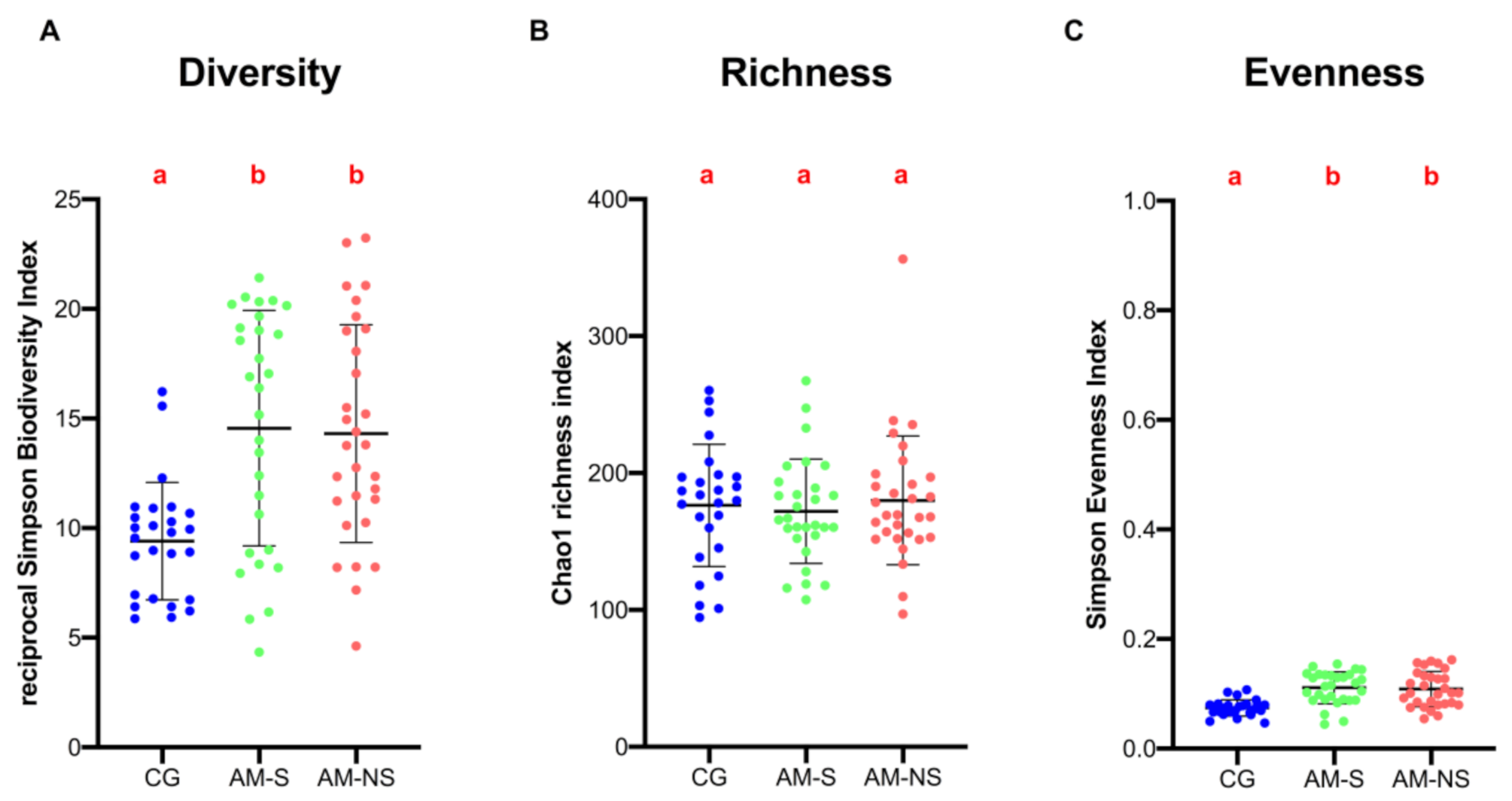
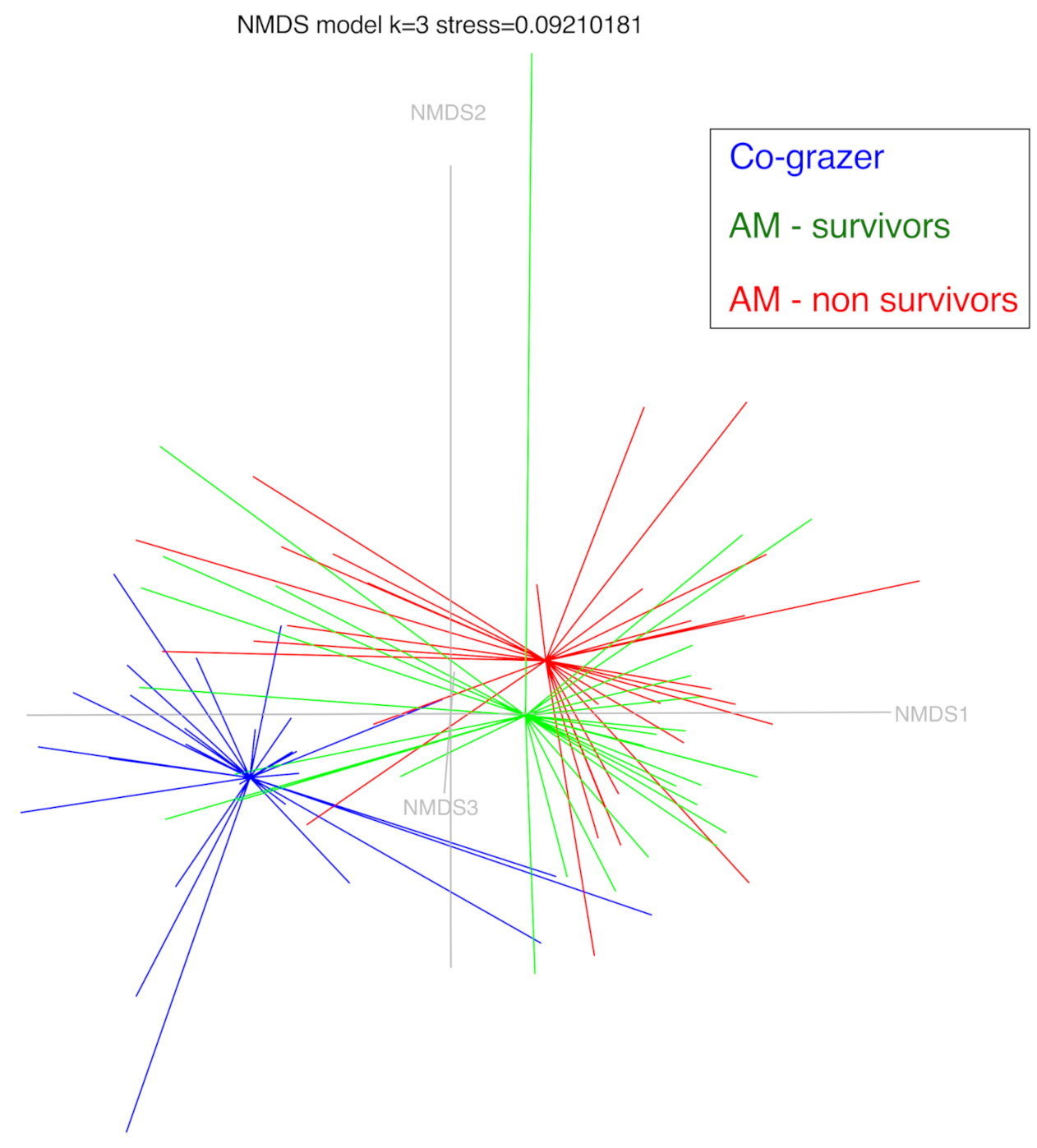
3.3. α-Diversity and β-Diversity Analysis
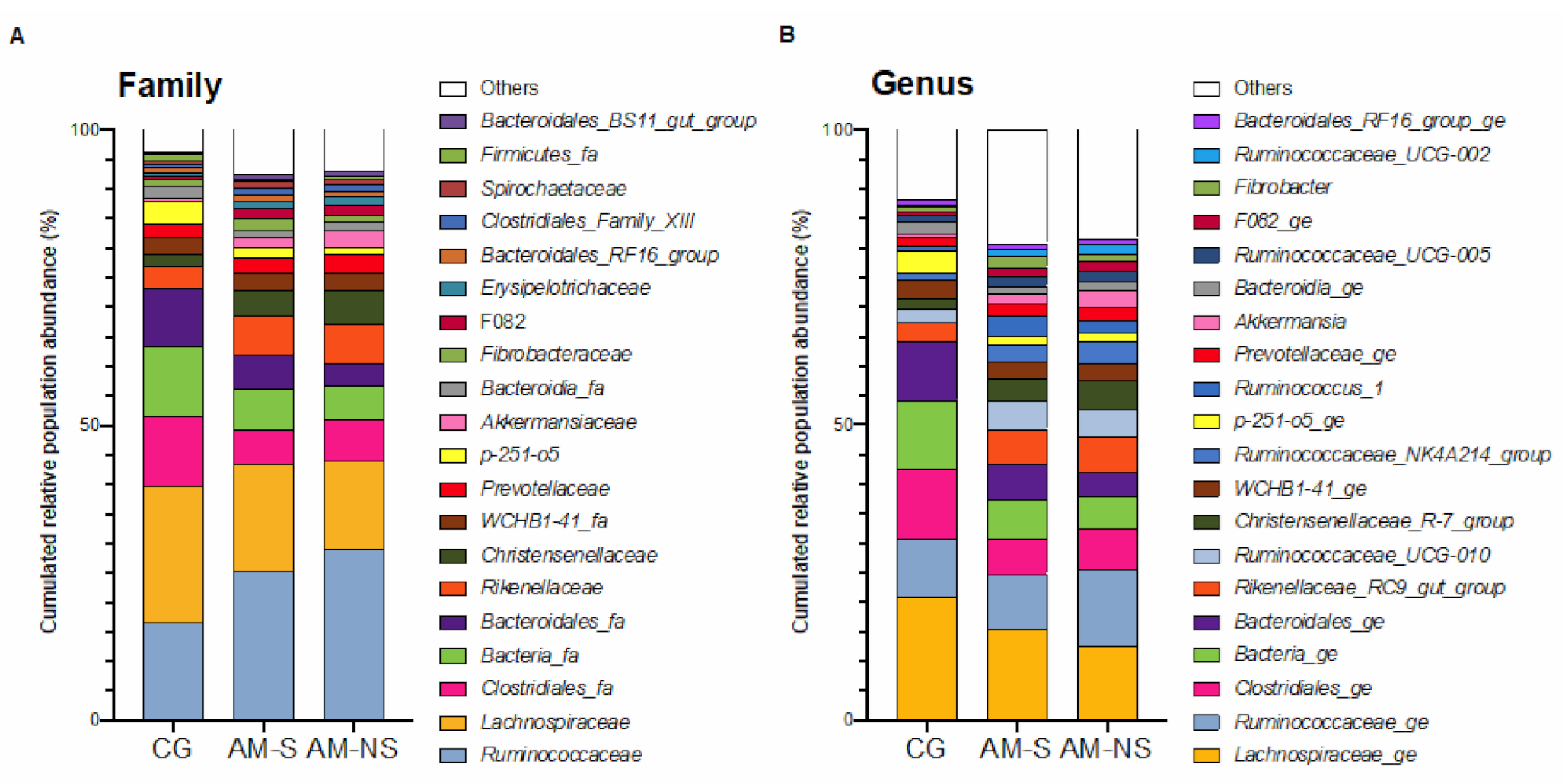
3.4. Composition of Fecal Microbiota
3.5. Statistical Differences in Fecal Microbiota Composition
4. Discussion
4.1. Differences in Community Structure between CG and Horses with AM
4.2. Differences in Community Composition Between CG and Horses with AM
4.3. Association with Outcome in Horses with AM
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Votion, D.-M.; François, A.-C.; Kruse, C.; Renaud, B.; Farinelle, A.; Bouquieaux, M.-C.; Marcillaud-Pitel, C.; Gustin, P. Answers to the Frequently Asked Questions Regarding Horse Feeding and Management Practices to Reduce the Risk of Atypical Myopathy. Animals 2020, 10, 365. [Google Scholar] [CrossRef]
- Votion, D.-M.; Serteyn, D. Equine Atypical Myopathy: A Review. Vet. J. 2008, 178, 185–190. [Google Scholar] [CrossRef]
- Votion, D.-M.; Linden, A.; Delguste, C.; Amory, H.; Thiry, E.; Engels, P.; van Galen, G.; Navet, R.; Sluse, F.; Serteyn, D.; et al. Atypical Myopathy in Grazing Horses: A First Exploratory Data Analysis. Vet. J. 2009, 180, 77–87. [Google Scholar] [CrossRef]
- Unger, L.; Nicholson, A.; Jewitt, E.M.; Gerber, V.; Hegeman, A.; Sweetman, L.; Valberg, S. Hypoglycin A Concentrations in Seeds of Acer Pseudoplatanus Trees Growing on Atypical Myopathy-Affected and Control Pastures. J. Vet. Intern. Med. 2014, 28, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Baise, E.; Habyarimana, J.A.; Amory, H.; Boemer, F.; Douny, C.; Gustin, P.; Marcillaud-Pitel, C.; Patarin, F.; Weber, M.; Votion, D.-M. Samaras and Seedlings of Acer Pseudoplatanus Are Potential Sources of Hypoglycin A Intoxication in Atypical Myopathy without Necessarily Inducing Clinical Signs. Equine Vet. J. 2016, 48, 414–417. [Google Scholar]
- Bochnia, M.; Sander, J.; Ziegler, J.; Terhardt, M.; Sander, S.; Janzen, N.; Cavalleri, J.-M.; Zuraw, A.; Wensch-Dorendorf, M.; Zeyner, A. Detection of MCPG Metabolites in Horses with Atypical Myopathy. PLoS ONE 2019, 14, e0211698. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, G.; Marcillaud Pitel, C.; Saegerman, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European Outbreaks of Atypical Myopathy in Grazing Equids (2006–2009): Spatiotemporal Distribution, History and Clinical Features: Outbreaks of Atypical Myopathy: Spatiotemporal Distribution, History and Clinical Features. Equine Vet. J. 2012, 44, 614–620. [Google Scholar] [CrossRef]
- González-Medina, S.; Ireland, J.L.; Piercy, R.J.; Newton, J.R.; Votion, D.M. Equine Atypical Myopathy in the UK: Epidemiological Characteristics of Cases Reported from 2011 to 2015 and Factors Associated with Survival. Equine Vet. J. 2017, 49, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Fabius, L.S.; Westermann, C.M. Evidence-Based Therapy for Atypical Myopathy in Horses. Equine Vet. Educ. 2018, 30, 616–622. [Google Scholar] [CrossRef]
- Dunkel, B.; Ryan, A.; Haggett, E.; Knowles, E.J. Atypical Myopathy in the South-East of England: Clinicopathological Data and Outcome in Hospitalised Horses. Equine Vet. Educ. 2020, 32, 90–95. [Google Scholar] [CrossRef]
- Krägeloh, T.; Cavalleri, J.M.V.; Ziegler, J.; Sander, J.; Terhardt, M.; Breves, G.; Cehak, A. Identification of Hypoglycin A Binding Adsorbents as Potential Preventive Measures in Co-Grazers of Atypical Myopathy Affected Horses. Equine Vet. J. 2018, 50, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bochnia, M.; Ziegler, J.; Sander, J.; Uhlig, A.; Schaefer, S.; Vollstedt, S.; Glatter, M.; Abel, S.; Recknagel, S.; Schusser, G.F.; et al. Hypoglycin A Content in Blood and Urine Discriminates Horses with Atypical Myopathy from Clinically Normal Horses Grazing on the Same Pasture. PLoS ONE 2015, 10, e0136785. [Google Scholar] [CrossRef] [PubMed]
- Venable, E.B.; Bland, S.D.; McPherson, J.L.; Francis, J. Role of the Gut Microbiota in Equine Health and Disease. Anim. Front. 2016, 6, 43–49. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3-V5 Region of the 16S RRNA Gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef]
- Milinovich, G.J.; Klieve, A.V.; Pollitt, C.C.; Trott, D.J. Microbial Events in the Hindgut During Carbohydrate-Induced Equine Laminitis. Vet. Clin. North. Am. Equine Pract. 2010, 26, 79–94. [Google Scholar]
- Elzinga, S.E.; Weese, J.S.; Adams, A.A. Comparison of the Fecal Microbiota in Horses With Equine Metabolic Syndrome and Metabolically Normal Controls Fed a Similar All-Forage Diet. J. Equine Vet. Sci. 2016, 44, 9–16. [Google Scholar] [CrossRef]
- Jevit, M.J. Microflora of the Equine Gut and Its Ramifications on the Development of Laminitis; A Comparison of Fecal and Cecal Diversity and Illumina and Roche 454 Sequencers. Master’s Thesis, Duquesne University, Pittsburgh, PA, USA, 2016. [Google Scholar]
- Leng, J.; Proudman, C.; Darby, A.; Blow, F.; Townsend, N.; Miller, A.; Swann, J. Exploration of the Fecal Microbiota and Biomarker Discovery in Equine Grass Sickness. J. Proteome. Res. 2018, 17, 1120. [Google Scholar] [CrossRef]
- Stewart, H.L.; Southwood, L.L.; Indugu, N.; Vecchiarelli, B.; Engiles, J.B.; Pitta, D. Differences in the Equine Faecal Microbiota between Horses Presenting to a Tertiary Referral Hospital for Colic Compared with an Elective Surgical Procedure. Equine Vet. J. 2019, 51, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.-L.; Zhang, J.; Wu, G.; Zhu, W.-Y. Utilization of Amino Acids by Bacteria from the Pig Small Intestine. Amino acids 2010, 39, 1201–1215. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino Acid Metabolism in Intestinal Bacteria: Links between Gut Ecology and Host Health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Re-Print of “Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host. ” Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Melde, K.; Jackson, S.; Bartlett, K.; Sherratt, H.S.A.; Ghisla, S. Metabolic Consequences of Methylenecyclopropylglycine Poisoning in Rats. Biochem. J. 1991, 274, 395–400. [Google Scholar] [CrossRef]
- Stewart, H.L.; Pitta, D.; Indugu, N.; Vecchiarelli, B.; Engiles, J.B.; Southwood, L.L. Characterization of the Fecal Microbiota of Healthy Horses. Am. J. Vet. Res. 2018, 79, 811–819. [Google Scholar] [CrossRef]
- Westermann, C.M.; Dorland, L.; Votion, D.M.; de Sain-van der Velden, M.G.M.; Wijnberg, I.D.; Wanders, R.J.A.; Spliet, W.G.M.; Testerink, N.; Berger, R.; Ruiter, J.P.N.; et al. Acquired Multiple Acyl-CoA Dehydrogenase Deficiency in 10 Horses with Atypical Myopathy. Neuromuscul. Disord. 2008, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Detilleux, J.; Cello, C.; Amory, H.; Marcillaud-Pitel, C.; Richard, E.; van Galen, G.; van Loon, G.; Lefère, L.; Votion, D.-M. Acylcarnitines Profile Best Predicts Survival in Horses with Atypical Myopathy. PLoS ONE 2017, 12, e0182761. [Google Scholar]
- Cerri, S.; Taminiau, B.; Lusancay, A.H.; Lecoq, L.; Amory, H.; Daube, G.; Cesarini, C. Effect of Oral Administration of Omeprazole on the Microbiota of the Gastric Glandular Mucosa and Feces of Healthy Horses. J. Vet. Intern. Med. 2020, 34, 2727–2737. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. AEM 2009, 75, 7537–7541. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools | Nucleic Acids Research | Oxford Academic. Available online: https://academic.oup.com/nar/article/41/D1/D590/1069277, (accessed on 11 December 2020).
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D. Choosing and Using Diversity Indices: Insights for Ecological Applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef]
- Chao, A. A New Statistical Approach for Assessing Compositional Similarity Based on Incidence and Abundance Data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Schoster, A.; Staempfli, H.R.; Guardabassi, L.G.; Jalali, M.; Weese, J.S. Comparison of the Fecal Bacterial Microbiota of Healthy and Diarrheic Foals at Two and Four Weeks of Life. BMC Vet. Res. 2017, 13, 144. [Google Scholar] [CrossRef]
- Rodriguez, C.; Taminiau, B.; Brévers, B.; Avesani, V.; Van Broeck, J.; Leroux, A.; Gallot, M.; Bruwier, A.; Amory, H.; Delmée, M. Faecal Microbiota Characterisation of Horses Using 16 Rdna Barcoded Pyrosequencing, and Carriage Rate of Clostridium difficile at Hospital Admission. BMC Microbiol. 2015, 15, 181. [Google Scholar]
- Steelman, S.M.; Chowdhary, B.P.; Dowd, S.; Suchodolski, J.; Janečka, J.E. Pyrosequencing of 16S RRNA Genes in Fecal Samples Reveals High Diversity of Hindgut Microflora in Horses and Potential Links to Chronic Laminitis. BMC Vet. Res. 2012, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Adair, H.S.; Reinemeyer, C.R.; Morgan, S.J.; Brooks, A.C.; Longhofer, S.L.; Elliott, J. Plasma Concentrations of Endotoxin and Platelet Activation in the Developmental Stage of Oligofructose-Induced Laminitis. Vet. Immunol. and Immunopathol. 2009, 129, 167–173. [Google Scholar] [CrossRef]
- Tuniyazi, M.; He, J.; Guo, J.; Li, S.; Zhang, N.; Hu, X.; Fu, Y. Changes of Microbial and Metabolome of the Equine Hindgut during Oligofructose-Induced Laminitis. BMC Vet. Res. 2021, 17, 11. [Google Scholar] [CrossRef]
- Milinovich, G.J.; Trott, D.J.; Burrell, P.C.; Croser, E.L.; Al Jassim, R.A.; Morton, J.M.; Van Eps, A.W.; Pollitt, C.C. Fluorescence in Situ Hybridization Analysis of Hindgut Bacteria Associated with the Development of Equine Laminitis. Environ. Microbiol. 2007, 9, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Woodmansey, E.J. Intestinal Bacteria and Ageing. J. Appl. Microbiol. 2007, 102, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; de la Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Geor, R.J.; Nielsen, B.D.; Schott, H.C.; Elzinga, S.; Newbold, C.J. Characterisation of the Faecal Bacterial Community in Adult and Elderly Horses Fed a High Fibre, High Oil or High Starch Diet Using 454 Pyrosequencing. PLoS ONE 2014, 9, e87424. [Google Scholar] [CrossRef]
- Theelen, M.; Wagenaar, M.; van Oldruitenborgh-Oosterbaan, M.M.S.; Rossen, J.W.A.; Schaafstra, F.J.; Van Doorn, D.A.; Zomer, A.L. Short- and Long-Term Effect of Hospitalization and Oral Trimethoprim-Sulfadiazine Administration on the Equine Faecal Microbiome. In Proceedings of the ECEIM Online Congress 2020, 20 November 2020. [Google Scholar]
- Schoster, A.; Mosing, M.; Jalali, M.; Staempfli, H.R.; Weese, J.S. Effects of Transport, Fasting and Anaesthesia on the Faecal Microbiota of Healthy Adult Horses. Equine Vet. J. 2016, 48, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Hinrichs, U.; Glitz, F.; Landes, E.; Schulze, C.; Deegen, E.; Pohlenz, J.; Coenen, M. Atypische Myoglobinurie Der Weidepferde. Pferdeheilkunde 1997, 13, 27–34. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. HORSE SPECIES SYMPOSIUM: The Microbiome of the Horse Hindgut: History and Current Knowledge1. J. Anim. Sci. 2016, 94, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, K.; Liu, Y.; Li, K.; Hu, D.; Wronski, T. Community Composition and Diversity of Intestinal Microbiota in Captive and Reintroduced Przewalski’s Horse (Equus Ferus Przewalskii). Front. Microbiol. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Edwards, J.E.; Shetty, S.A.; van den Berg, P.; Burden, F.; van Doorn, D.A.; Pellikaan, W.F.; Dijkstra, J.; Smidt, H. Multi-Kingdom Characterization of the Core Equine Fecal Microbiota Based on Multiple Equine (Sub) Species. Anim. Microbiome 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.-M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lübke-Becker, A.; Günther, S.; Wieler, L.H. The Gut Microbiome of Horses: Current Research on Equine Enteral Microbiota and Future Perspectives. Anim. Microbiome 2019, 1, 14. [Google Scholar] [CrossRef]
- Peris-Bondia, F.; Latorre, A.; Artacho, A.; Moya, A.; D’Auria, G. The Active Human Gut Microbiota Differs from the Total Microbiota. PLoS ONE 2011, 6, e22448. [Google Scholar] [CrossRef]
- Costa, M.C.; Silva, G.; Ramos, R.V.; Staempfli, H.R.; Arroyo, L.G.; Kim, P.; Weese, J.S. Characterization and Comparison of the Bacterial Microbiota in Different Gastrointestinal Tract Compartments in Horses. The Vet. J. 2015, 205, 74–80. [Google Scholar] [CrossRef]
- Roediger, W.E.W. The Colonic Epithelium in Ulcerative Colitis: An Energy-Deficiency Disease? Lancet 1980, 316, 712–715. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The Type and Quantity of Dietary Fat and Carbohydrate Alter Faecal Microbiome and Short-Chain Fatty Acid Excretion in a Metabolic Syndrome ‘at-Risk’ Population. Int J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Jimenez, B.; Lodge, S.; et al. How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Proudman, C.J.; Duncan, S.H.; Flint, H.J.; Dyer, J.; Shirazi-Beechey, S.P. Alterations in Microbiota and Fermentation Products in Equine Large Intestine in Response to Dietary Variation and Intestinal Disease. Br. J. Nutr. 2012, 107, 989–995. [Google Scholar] [CrossRef]
- Britton, R.A.; Young, V.B. Interaction between the Intestinal Microbiota and Host in Clostridium Difficile Colonization Resistance. Trends Microbiol. 2012, 20, 313–319. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar]
- Mandal, M.; Olson, D.J.; Sharma, T.; Vadlamudi, R.K.; Kumar, R. Butyric Acid Induces Apoptosis by Up-Regulating Bax Expression via Stimulation of the c-Jun N-Terminal Kinase/Activation Protein-1 Pathway in Human Colon Cancer Cells. Gastroenterology 2001, 120, 71–78. [Google Scholar] [CrossRef]
- Nepelska, M.; Cultrone, A.; Béguet-Crespel, F.; Le Roux, K.; Doré, J.; Arulampalam, V.; Blottière, H.M. Butyrate Produced by Commensal Bacteria Potentiates Phorbol Esters Induced AP-1 Response in Human Intestinal Epithelial Cells. PLoS ONE 2012, 7, e52869. [Google Scholar] [CrossRef] [PubMed]
- Hess, J. AP-1 Subunits: Quarrel and Harmony among Siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The Human Gut Bacteria Christensenellaceae Are Widespread, Heritable, and Associated with Health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal Microbiota and Bile Acid Interactions with Systemic and Adipose Tissue Metabolism in Diet-Induced Weight Loss of Obese Postmenopausal Women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; Harris, P.A.; Girdwood, S.E.; Creevey, C.J.; Curtis, G.C.; Barfoot, C.F.; Argo, C.M.; Newbold, C.J. Changes in the Total Fecal Bacterial Population in Individual Horses Maintained on a Restricted Diet Over 6 Weeks. Front. Microbiol. 2017, 8. [Google Scholar]
- Biddle, A.S.; Tomb, J.-F.; Fan, Z. Microbiome and Blood Analyte Differences Point to Community and Metabolic Signatures in Lean and Obese Horses. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; Vos, W.M. de Microbes inside—from Diversity to Function: The Case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Theissig, F.; Ruckert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kuhn, S.; et al. Acute Appendicitis Is Characterised by Local Invasion with Fusobacterium Nucleatum/Necrophorum. Gut 2011, 60, 34–40. [Google Scholar]
- Swidsinski, A.; Loening-Baucke, V.; Herber, A. Mucosal Flora in Crohn’s Disease and Ulcerative Colitis—an Overview. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 61–71. [Google Scholar]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. in Feces of Children with Autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Sonoyama, K.; Fujiwara, R.; Takemura, N.; Ogasawara, T.; Watanabe, J.; Ito, H.; Morita, T. Response of Gut Microbiota to Fasting and Hibernation in Syrian Hamsters. AEM 2009, 75, 6451–6456. [Google Scholar]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Votion, D.-M. The Story of Equine Atypical Myopathy: A Review from the Beginning to a Possible End. Int. Sch. Res. Not. 2012, 2012, 1–14. [Google Scholar]
- Dai, Z.-L.; Li, X.-L.; Xi, P.-B.; Zhang, J.; Wu, G.; Zhu, W.-Y. Metabolism of Select Amino Acids in Bacteria from the Pig Small Intestine. Amino Acids 2012, 42, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; Harris, P.A.; Edwards, A.; Pachebat, J.A.; Blackmore, T.M.; Worgan, H.J.; Newbold, C.J. A Comparison of the Microbiome and the Metabolome of Different Regions of the Equine Hindgut. FEMS Microbiol. Ecol. 2012, 82, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
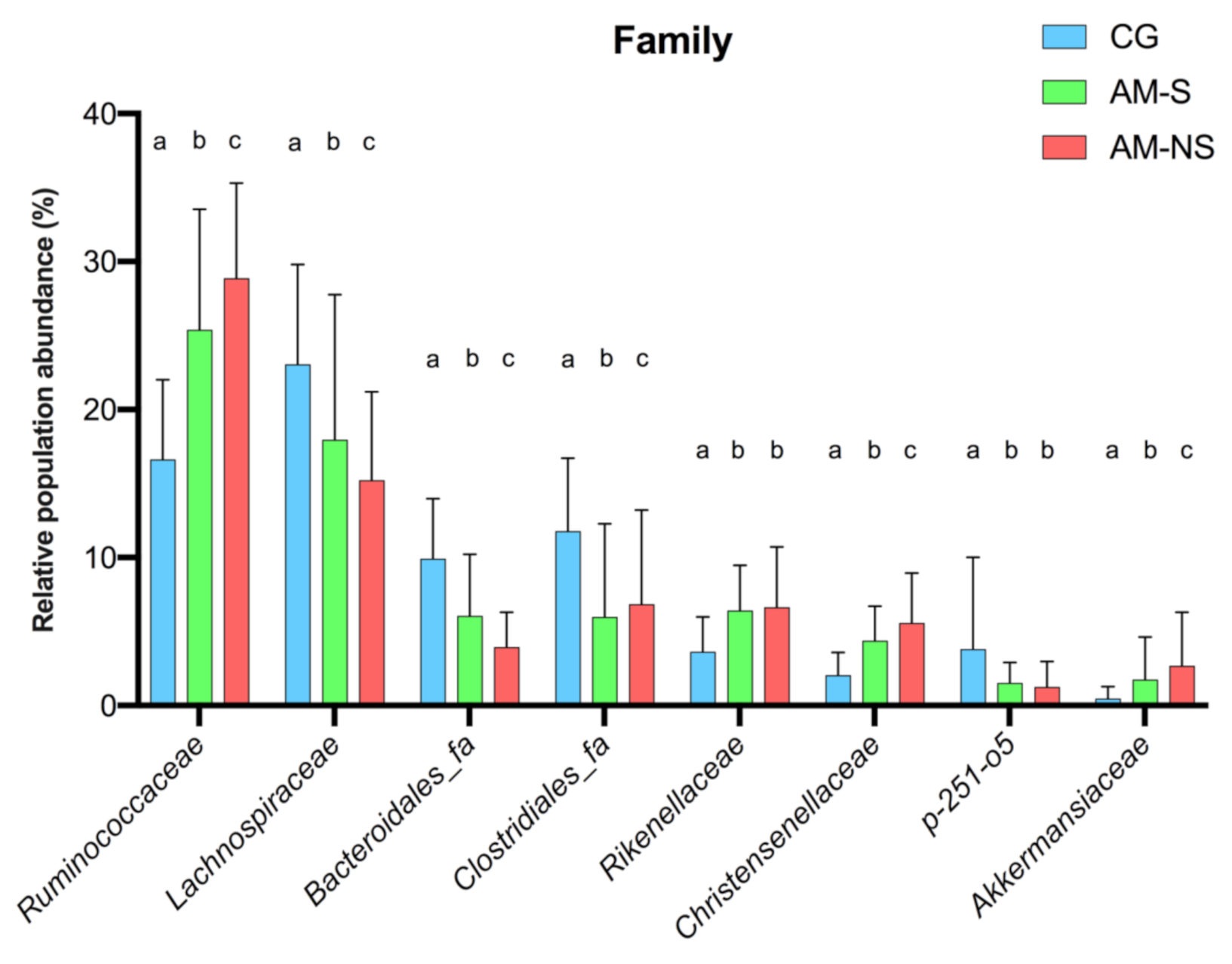
| Family | Global p-Values (Corrected) | CG vs. AM-S | CG vs. AM-NS | AM-S vs. AM-NS |
|---|---|---|---|---|
| Ruminococcaceae | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Lachnospiraceae | 0.0053 | <0.0001 | <0.0001 | <0.0001 |
| Bacteroidales_fa | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Clostridiales_fa | 0.0402 | <0.0001 | <0.0001 | 0.0331 |
| Rikenellaceae | 0.0062 | <0.0001 | <0.0001 | 0.2263 |
| Christensenellaceae | <0.0001 | <0.0001 | <0.0001 | 0.0176 |
| p-251-o5 | 0.0203 | <0.0001 | <0.0001 | 0.2147 |
| Akkermansiaceae | 0.0016 | 0.0067 | <0.0001 | 0.0249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimmer-Scherr, C.; Taminiau, B.; Renaud, B.; van Loon, G.; Palmers, K.; Votion, D.; Amory, H.; Daube, G.; Cesarini, C. Comparison of Fecal Microbiota of Horses Suffering from Atypical Myopathy and Healthy Co-Grazers. Animals 2021, 11, 506. https://doi.org/10.3390/ani11020506
Wimmer-Scherr C, Taminiau B, Renaud B, van Loon G, Palmers K, Votion D, Amory H, Daube G, Cesarini C. Comparison of Fecal Microbiota of Horses Suffering from Atypical Myopathy and Healthy Co-Grazers. Animals. 2021; 11(2):506. https://doi.org/10.3390/ani11020506
Chicago/Turabian StyleWimmer-Scherr, Christina, Bernard Taminiau, Benoît Renaud, Gunther van Loon, Katrien Palmers, Dominique Votion, Hélène Amory, Georges Daube, and Carla Cesarini. 2021. "Comparison of Fecal Microbiota of Horses Suffering from Atypical Myopathy and Healthy Co-Grazers" Animals 11, no. 2: 506. https://doi.org/10.3390/ani11020506
APA StyleWimmer-Scherr, C., Taminiau, B., Renaud, B., van Loon, G., Palmers, K., Votion, D., Amory, H., Daube, G., & Cesarini, C. (2021). Comparison of Fecal Microbiota of Horses Suffering from Atypical Myopathy and Healthy Co-Grazers. Animals, 11(2), 506. https://doi.org/10.3390/ani11020506








