Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Tissue Collection
- Group 1 (control)—Intraperitoneal injection (i.p.) of 2 mL of saline solution (0.9% NaCl);
- Group 3 (Glu)—Injection (i.p.) of Glu (G1626, Sigma-Aldrich, St. Louis, USA), dose 5.07 mg/kg b.w. (30 μM), i.p. in a volume of 2 mL of 0.9% NaCl;
- Group 4 (Glu + stress)—Injection (i.p.) of Glu (as described in Group 3) and a single stressor factor (as described in Group 2);
- Group 5 (Glu antagonist)—Injection (i.p.) of glutamate receptor antagonist (LY-341495, Sigma-Aldrich), dose 7.36 mg/kg b.w. (30 μM), i.p. in a volume of 2 mL of 0.9% NaCl;
- Group 6 (Glu antagonist + stress)—Injection (i.p.) of glutamate receptor antagonist (as described in Group 5) and a single stressor factor (as described in Group 2).
2.2. Statistical Treatment of Results
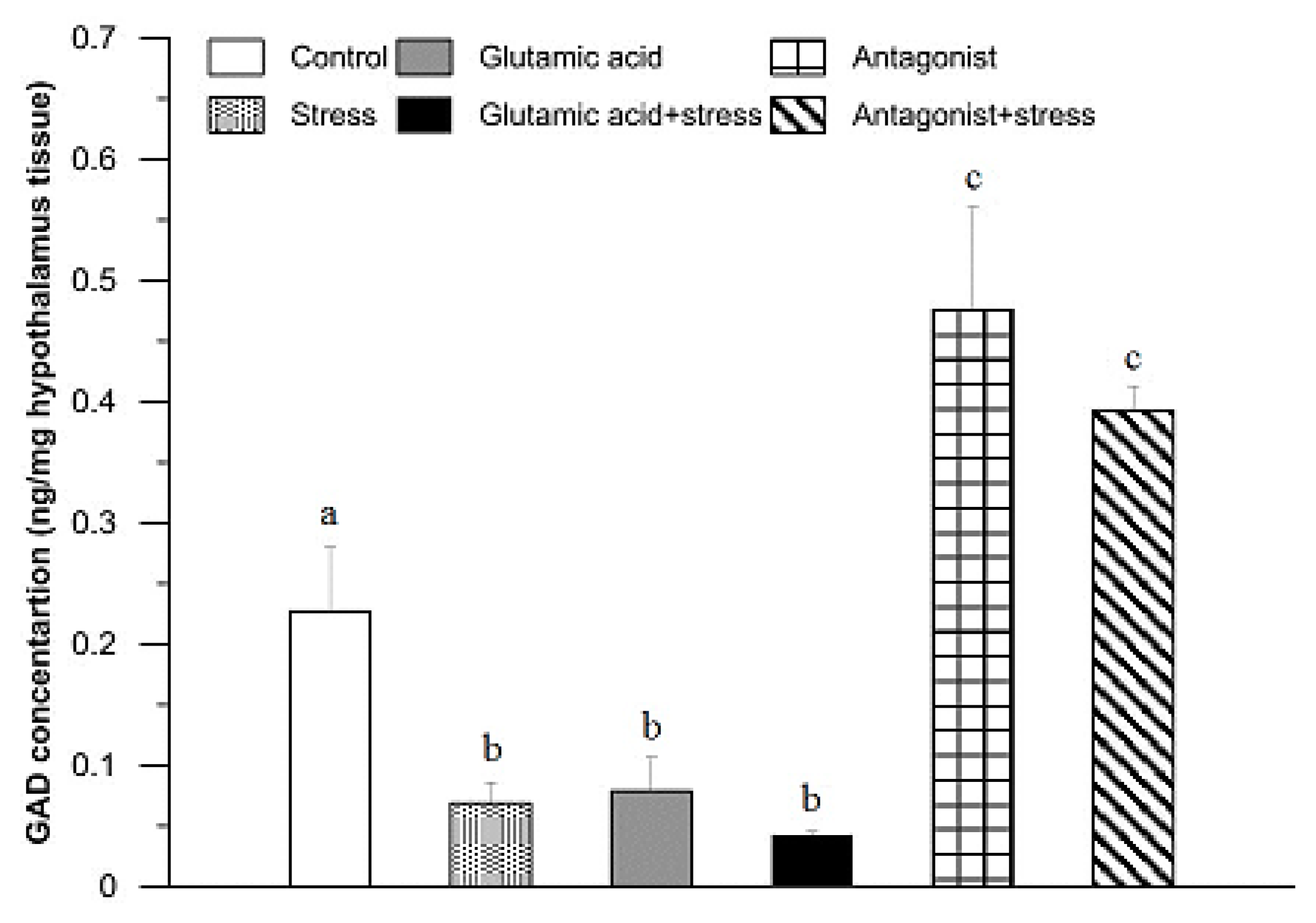
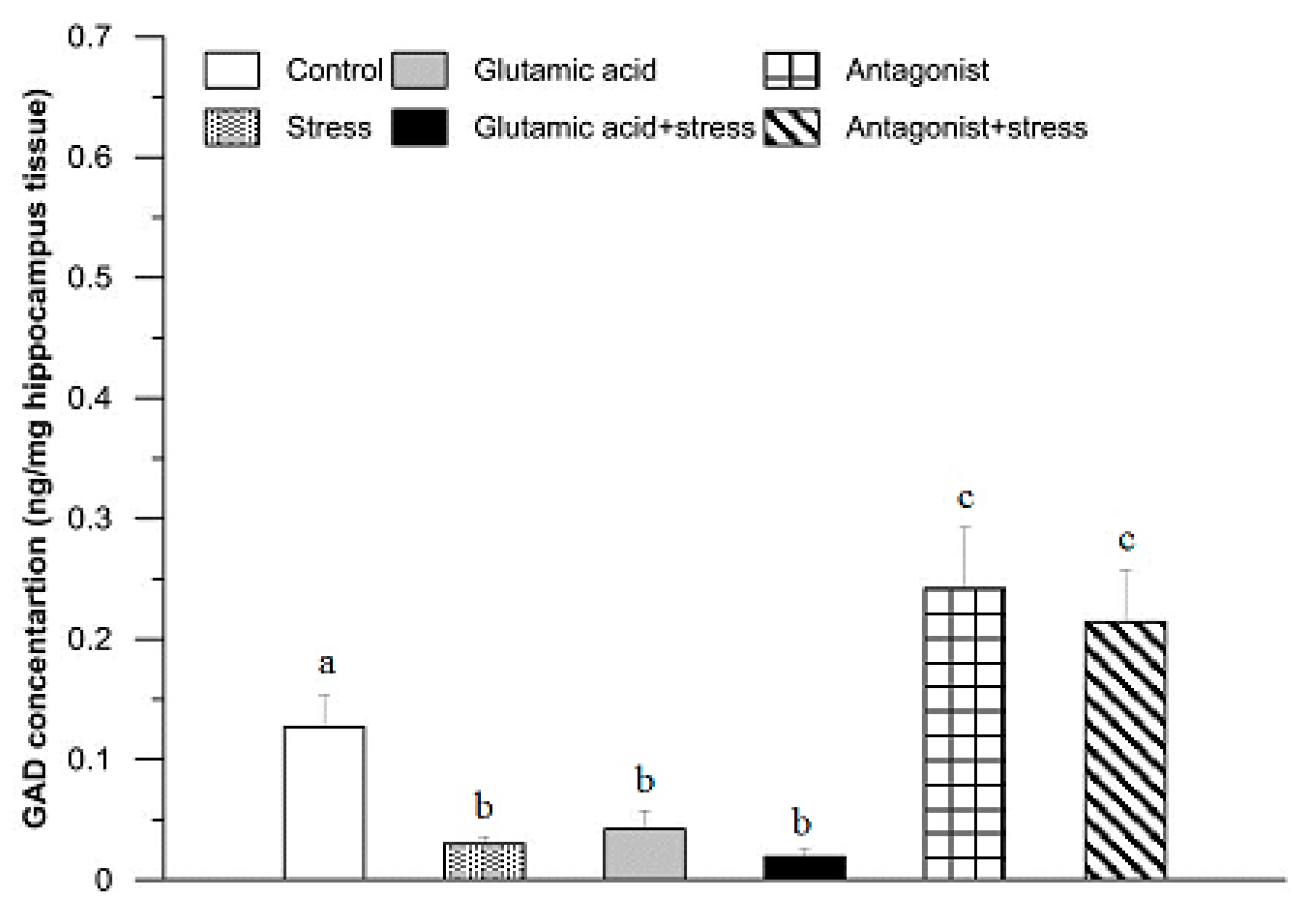
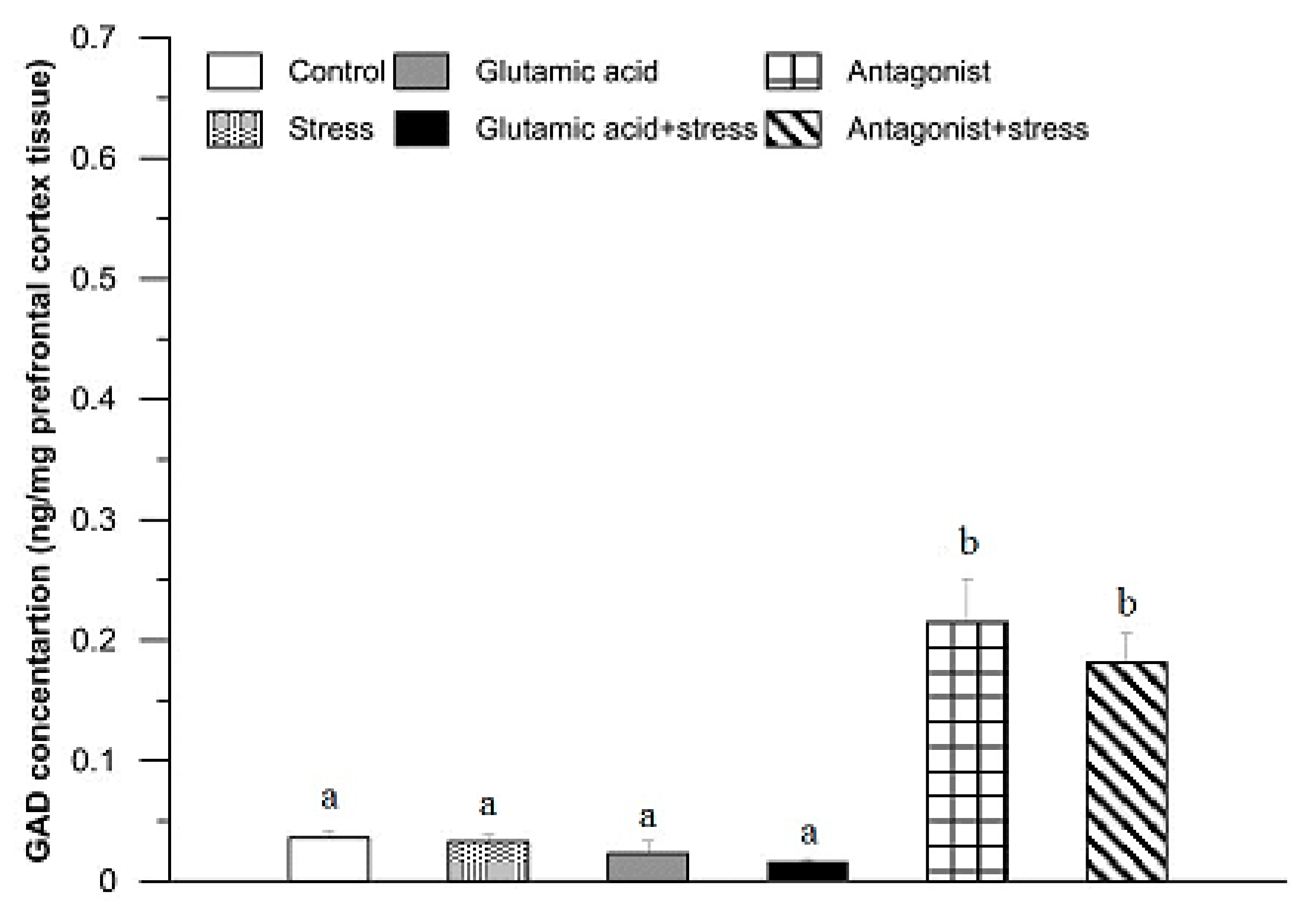
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lüthi, A.; Lüscher, C. Pathological circuit function underlying addiction and anxiety disorders. Nat. Neurosci. 2014, 17, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.J.; Hutchison, K.E. Alcohol, aging and the stress response. Alcohol Res. Health 1999, 23, 272–283. [Google Scholar] [PubMed]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.T.; Olive, M.F. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 2008, 75, 218–265. [Google Scholar] [CrossRef]
- Lujan, R.; Shigemoto, R.; Lopez-Bendito, G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience 2005, 86, 125–137. [Google Scholar] [CrossRef]
- Cunningham, M.D.; Ferlrany, J.W.; Enna, S.J. Excitatory amino acid receptors: A gallery of new targets for pharmacological intervention. Life Sci. 1993, 54, 135–145. [Google Scholar] [CrossRef]
- Kania, B.F.; Wrońska, D. Glutamate and metabotropic glutamate receptors: Physiology, function, and roles in neurological disorders. In Metabotropic Glutamate Receptors: Classification, Structure and Roles in Disease; O’Keeffe, J., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 1–78. [Google Scholar]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Conn, P.J. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann. N. Y. Acad. Sci. 2003, 1003, 12–21. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Julio-Pieper, M.; Flor, P.J.; Dinan, T.G.; Cryan, J.F. Exciting times beyond the brain: Metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol. Rev. 2011, 63, 35–58. [Google Scholar] [CrossRef]
- Brandon, C.; Criswell, M.H. Displaced starburst amacrine cells of the rabbit retina contain the 67-kDa isoform, but not the 65-kDa isoform, of glutamate decarboxylase. Vis. Neurosci. 1995, 12, 1053–1061. [Google Scholar] [CrossRef]
- Johnson, M.A.; Vardi, N. Regional differences in GABA and GAD immunoreactivity in rabbit horizontal cells. Vis. Neurosci. 1998, 15, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; de Graaf, R.A.; Mason, G.F.; Rothman, D.L.; Shulman, R.G.; Behar, K.L. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.; Tillakaratne, N.J.; Kaufman, D.L.; Tobin, A.J.; Houser, C.R. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J. Neurosci. 1994, 14, 1834–1855. [Google Scholar] [CrossRef] [PubMed]
- Louzoun-Kaplan, V.; Zuckerman, M.; Perez-Polo, J.R.; Golan, H.M. Prenatal hypoxia down regulates the GABA pathway in newborn mice cerebral cortex; partial protection by MgSO4. Int. J. Dev. Neurosci. 2008, 26, 77–85. [Google Scholar] [CrossRef]
- Kaazi, A.I.; Oomen, A. Chronic noise stress-induced alterations of glutamate and gamma-aminobutyric acid and their metabolism in the rat brain. Noise Health 2014, 16, 343–349. [Google Scholar] [CrossRef]
- El-Tarabany, M.S.; Ahmed-Farid, O.A.; El-Tarabany, A.A. Impact of space allowance on performance traits, brain neurotransmitters and blood antioxidant activity of New Zealand White Rabbits. Prev. Vet. Med. 2018, 163, 44–50. [Google Scholar] [CrossRef]
- Liste, G.; Villarroel, M.; Chacón, G.; Sañudo, C.; Olleta, J.L.; García-Belenguer, S.; Alierta, S.; María, G.A. Effect of lairage duration on rabbit welfare and meat quality. Meat Sci. 2009, 82, 71–76. [Google Scholar] [CrossRef]
- Graf, S.; Biglera, L.; Failing, K.; Würbelc, H.; Buchwaldera, T. Regrouping rabbit does in a familiar or novel pen: Effects on agonistic behaviour, injuries and core body temperature. Appl. Anim. Behav. Sci. 2011, 135, 121–127. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Expression of GABA in the fetal, postnatal, and adult human retinas: An immunohistochemical study. Vis. Neurosci. 1997, 14, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H.; Fredette, B.J.; Mugnaini, E. Parasolitary nucleus: A source of GABAergic vestibular information to the inferior olive of rat and rabbit. J. Comp. Neurol. 1998, 392, 352–372. [Google Scholar] [CrossRef]
- Chellappan, D.; Joseph, J.; Shabi, M.M.; Krishnamoorthy, G.; Ravindhran, D.; Uthrapathy, S.; Rajamanickam, G.V.; Dubey, P.G. Psycho-emotional stress–A cause of coronary artery disease. Acta Sci. Vet. 2008, 36, 133–139. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 2014, 11, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Sujatha, R.; Paul, V.; Asokan, C.; Govindasamy, S.; Jayakumar, R. Role of nitric oxide on GABA, glutamic acid, activities of GABA-T and GAD in rat brain cerebral cortex. Brain Res. 1999, 837, 229–235. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Martin, L.J.; Kuncl, R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992, 326, 1464–1468. [Google Scholar] [CrossRef]
- Eid, T.; Thomas, M.J.; Spencer, D.D.; Rundén-Pran, E.; Lai, J.C.; Malthankar, G.V.; Kim, J.H.; Danbolt, N.C.; Ottersen, O.P.; de Lanerolle, N.C. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Wallace, M.; Buren, C.; Martyniuk, K.; Andreyev, A.Y.; Li, E.; Fields, J.A.; Cordes, T.; Reynolds, I.J.; Bloodgood, B.L.; et al. Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J. Cell Biol. 2017, 216, 1091–1105. [Google Scholar] [CrossRef]
- Fendt, S.M.; Verstreken, P. Neurons eat glutamate to stay alive. J. Cell Biol. 2017, 216, 863–865. [Google Scholar] [CrossRef]
- Chen, H.S.V.; Lipton, S. The chemical biology of clinically tolerated NMDA ceceptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef]
- Soghomonian, J.J.; Martin, D.L. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol. Sci. 1998, 19, 500–505. [Google Scholar] [CrossRef]
- Battaglioli, G.; Liu, H.; Martin, D.L. Kinetic differences between the isoforms of glutamate decarboxylase: Implications for the regulation of GABA synthesis. J. Neurochem. 2003, 86, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Yu, S.; Wong, W.; McGeer, E.G.; McGeer, P.L. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 1073–1088. [Google Scholar] [CrossRef]
- Langendorf, C.G.; Tuck, K.L.; Key, T.L.; Fenalti, G.; Pike, R.N.; Rosado, C.J.; Wong, A.S.M.; Buckle, A.M.; Law, R.H.P.; Whisstock, J.C. Structural characterization of the mechanism through which human glutamic acid decarboxylase auto-activates. Biosci. Rep. 2013, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.L.; Houser, C.R.; Tobin, A.J. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 1991, 56, 720–723. [Google Scholar] [CrossRef]
- Pinal, C.S.; Tobin, A.J. Uniqueness and redundancy in GABA production. Perspect. Dev. Neurobiol. 1998, 5, 109–118. [Google Scholar] [PubMed]
- Bowers, G.; Cullinan, W.E.; Herman, J.P. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J. Neurosci. 1998, 18, 5938–5947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kass, I.; Hoke, D.E.; Costa, M.G.S.; Reboul, C.F.; Benjamin, T.; Porebski, B.T.; Nathan, P.; Cowieson, N.P.; Leh, H.; Pennacchietti, E.; et al. Cofactor-dependent conformational heterogeneity of GAD65 and its role in autoimmunity and neurotransmitter homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, E2524–E2529. [Google Scholar] [CrossRef]
- Krystal, J.H.; Sanacora, G.; Duman, R.S. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry 2013, 73, 1133–1141. [Google Scholar] [CrossRef]
- Paul, I.A.; Skolnick, P. Glutamate and depression: Clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003, 1003, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A.; Niciu, M.J. Ketamine for depression: Evidence, challenges and promise. World Psychiatry 2015, 14, 348–350. [Google Scholar] [CrossRef]
- Highland, J.N.; Zanos, P.; Georgiou, P.; Gould, T.D. Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Mango, D.; Caruso, A.; Saidi, A.; Nisticò, R.; Scaccianoce, S. The positive allosteric modulator at mGlu2 receptors, LY487379, reverses the effects of chronic stress-induced behavioral maladaptation and synaptic dysfunction in the adulthood. Synapse 2019, 73, e22101. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Podkowa, K.; Rafało-Ulińska, A. The group II mGlu receptor antagonist LY341495 induces a rapid antidepressant-like effect and enhances the effect of ketamine in the chronic unpredictable mild stress model of depression in C57BL/6J mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110239. [Google Scholar] [CrossRef]
- Olive, M.F. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr. Drug Abus. Rev. 2009, 2, 83–98. [Google Scholar] [CrossRef]
- El-Faramawy, Y.A.; El-banouby, M.H.; Sergeev, P.; Mortagy, A.K.; Amer, M.S.; Abdel-tawab, A.M. Changes in glutamate decarboxylase enzyme activity and tau-protein phosphorylation in the hippocampus of old rats exposed to chronic mild stress: Reversal with the neuronal nitric oxide synthase inhibitor 7-nitroindazole. Pharmacol. Biochem. Behav. 2009, 91, 339–344. [Google Scholar] [CrossRef]
- Pochwat, B.; Nowak, G.; Szewczyk, B. Brain glutamic acid decarboxylase-67 kDa alterations induced by magnesium treatment in olfactory bulbectomy and chronicmild stress models in rats. Pharmacol. Rep. 2016, 68, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Larson, B.R. Differential regulation of forebrain glutamic acid decarboxylase mRNA expression by aging and stress. Brain Res. 2001, 912, 60–66. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Liu, H.T.; Hollmann, M.W.; Liu, W.H.; Hoenemann, C.W.; Durieux, M.E. Modulation of NMDA receptor function by ketamine and magnesium: Part I. Anesth. Analg. 2001, 92, 1173–1181. [Google Scholar] [CrossRef]
- Vargas-Caballero, M.; Robinson, H.P.C. Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: The asymmetric trapping block model. J. Neurosci. 2004, 24, 6171–6180. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.; Mazure, C.M.; Sinha, R. Neural circuits responsible for conscious self-control are highly vulnerable to even mild stress. When they shut down, primal impulses go unchecked and mental paralysis sets in. Sci. Am. 2012, 306, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Balesar, R.; Lu, J.; Farajnia, S.; Zhu, Q.; Huang, M.; Bao, A.M.; Swaab, D.F. Increased glutamic acid decarboxylase expression in the hypothalamic suprachiasmatic nucleus in depression. Brain Struct. Funct. 2017, 222, 4079–4088. [Google Scholar] [CrossRef] [PubMed]
- Linden, A.M.; Johnson, B.G.; Trokovic, N.; Korpi, E.R.; Schoepp, D.D. Use of MGLUR2 and MGLUR3 knockout mice to explore in vivo receptor specificity of the MGLUR2/3 selective antagonist LY341495. Neuropharmacology 2009, 57, 172–182. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpręgiel, I.; Wrońska, D.; Kmiecik, M.; Pałka, S.; Kania, B.F. Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures. Animals 2021, 11, 455. https://doi.org/10.3390/ani11020455
Szpręgiel I, Wrońska D, Kmiecik M, Pałka S, Kania BF. Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures. Animals. 2021; 11(2):455. https://doi.org/10.3390/ani11020455
Chicago/Turabian StyleSzpręgiel, Izabela, Danuta Wrońska, Michał Kmiecik, Sylwia Pałka, and Bogdan F. Kania. 2021. "Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures" Animals 11, no. 2: 455. https://doi.org/10.3390/ani11020455
APA StyleSzpręgiel, I., Wrońska, D., Kmiecik, M., Pałka, S., & Kania, B. F. (2021). Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures. Animals, 11(2), 455. https://doi.org/10.3390/ani11020455





