Simple Summary
For sustainable animal agriculture, we need to find ways to increase growth efficiency without using feed antibiotics. Bacteriophages, which are only harmful to specific bacterial strains, have been suggested as a feed additive replacing antibiotics. β-mannanase, which degrades mannan, is known to promote nutrient digestibility, animal growth, or both, thus improving feed efficiency. The objective of this study was to evaluate the effects of dietary supplementation with bacteriophage and β-mannanase on health and growth performance in calves. We assigned 36 pre-weaning male Holstein calves to one of four treatments with a 2 × 2 factorial arrangement: no supplementation, 0.1% β-mannanase, 0.1% bacteriophage, and both 0.1% bacteriophage and 0.1% β-mannanase supplementation in a starter on a dry matter basis. Compared to unsupplemented, the bacteriophage supplemented group showed a tendency to improve the survival rate without growth promotion. Supplementation of β-mannanase, on the other hand, increased the starter intake and the weekly body weight (BW) gain and tended to increase the final BW. Our study indicated that bacteriophage supplementation has a positive effect on survival rate, while β-mannanase supplementation improves growth performance in calves.
Abstract
The objective of this study was to evaluate the effects of dietary supplementation with bacteriophage and β-mannanase on health and growth performance in calves. Thirty-six pre-weaning male Holstein calves were randomly allocated to one of four dietary treatments with a 2 × 2 factorial arrangement: no supplementation, 0.1% β-mannanase, 0.1% bacteriophage, and both 0.1% bacteriophage and 0.1% β-mannanase supplementation in a starter on a dry matter basis. The experiment lasted from 2 weeks before weaning to 8 weeks after weaning. Twenty-two calves survived to the end of the experiment. No interaction was observed between the two different feed additives. The bacteriophage supplementation tended to increase the odds ratio of survival (p = 0.09). The number of Escherichia coli in feces significantly decreased by bacteriophage supplementation one week after weaning. β-mannanase supplementation increased the concentrate intake (p < 0.01) and tended to increase the final BW (p = 0.08). Analysis of repeated measures indicated β-mannanase supplementation increased weekly body weight gain (p = 0.018). We conclude that bacteriophage supplementation may have a positive effect on calf survival rate, while β-mannanase supplementation may increase the growth rate and starter intake by calves just before and after weaning.
1. Introduction
Diarrhea and respiratory disease are the main causes of death in calves [1] and are caused by infection with viral, protozoal, and bacterial pathogens [2,3]. Calf mortality rates are higher in winter than in summer [4], mainly due to cold temperatures and poor ventilation [5]. Supplementation with antibiotics helps to prevent disease and promote growth in livestock [6]. However, several countries have restricted the use of antibiotics in the diets of livestock [7], and some others are in the process of banning antibiotics due to concern for antimicrobial resistance.
Several studies have been conducted to develop feed additives to replace the use of antibiotics as feed additives. For example, bacteriophages can repress the growth of specific or narrow groups of bacteria [8]. Bacteriophages multiply in bacteria by exploiting the biosynthetic machinery of the host [8]. Bacteriophages have shown the potential for various applications for human and animal health [8,9]. The bacteriophage supplementation in the diets of monogastric animals has been reported to reduce pathogenic bacteria in the intestine [10,11]. However, few studies have investigated the use of bacteriophages as a feed additive for ruminants. Prebiotics, such as mannan oligosaccharides (MOS), can also be added to the diet to enhance the health-improving activities of gut microbes [12,13]. The MOS can be produced by hydrolysis of mannan in feed by supplementation of β-mannanase, which can indirectly provide MOS to animals. Supplementation of β-mannanase in a diet improved feed utilization and animal performance in chicken [14,15], pigs [16,17], and ruminants [18,19,20]. Both supplementations are expected to improve gut health but via different modes of action.
Thus, the objective of the present study was to investigate the effects of bacteriophage and β-mannanase supplementation and their synergies, if any, on health, growth performance, intestinal pathogenic bacteria, and survival rate of calves reared in winter.
2. Materials and Methods
This study was conducted at the Center for Animal Science Research, Chungnam National University, Korea. Animal use and experimental protocols were reviewed and pre-approved by the Chungnam National University Animal Research Ethics Committee (approval number CNU-00188).
2.1. Animals and Housing
A total of 36 Holstein male calves (45.1 ± 7.30 kg of body weight (BW), averaged 3 to 4 weeks old) were purchased from several commercial dairy farms and used in this study. The calves were housed in individual wood pens (1.2 m width × 1.4 m length × 1.2 m height) at the Center for Animal Science Research, Chungnam National University. Pens were designed to prevent physical contact between calves but allow visual and auditory contact. The front of each pen had two openings for access to pails (diameter = 34 cm, height = 29 cm) mounted on the outside. The first seven days were used to adapt the calves to the diet by providing them free access to feed and water in individual indoor pens bedded with straw. During the experimental period, the pens were bedded with copra meal pellets. The bedding was replaced every 2 weeks throughout the experimental period.
2.2. Experimental Design and Diets
The experiment was carried out with 1 week adaptation and 10 week experimental periods. The experimental periods included 2 weeks prior to weaning and 8 weeks after weaning. Calves were divided into four groups and used in a balanced and completely randomized design with a 2 × 2 factorial arrangement. The dietary treatments were as follows: (1) control diet with neither supplementation (CON), (2) no bacteriophage but 0.1% β-mannanase (EZ), (3) 0.1% bacteriophage but no β-mannanase (BP), and (4) both 0.1% bacteriophage and 0.1% β-mannanase supplementation (BP_EZ) in a calf starter on a dry matter basis. The bacteriophage and β-mannanase used in this experiment were commercial feed additive BacterPhage C (CTCBIO, Inc., Seoul, Korea) and CTCZYME (CTCBIO, Inc., Seoul, Korea), respectively. BacterPhage C was composed of various bacteriophages targeting Salmonella typhimurium, Salmonella enteritidis, Salmonella dublin, Salmonella derby, Staphylococcus aureus, Escherichia coli k99, and f41, and Clostridium perfringens type A and C. CTCZYME contains Endo 1-4 β-mannanase (800,000 U/kg), which is produced using a patented strain of Bacillus subtills WL-7 (patent 100477456; CTCBIO, Inc., Seoul, Korea).
During the adaptation period, the calves were fed only a commercial milk replacer (Telilac; LNB International Feed B. V., Nisterlrode, The Netherlands; 21% crude protein (CP), 16% crude fat (CF)). The milk replacer was prepared with 125 g/L as the manufacturer’s recommendation and given to the calves at 10% BW. After a 1 week of adaptation, calf starter and timothy hay (Table 1) were offered for ad libitum intake before weaning, and thus, the feeding of the experimental diets started 2 weeks before weaning. The amount of milk replacer was progressively reduced by 20% daily from five days before weaning. From 1 week after weaning, the amount of starter was restricted to 1.5% body weight (BW) to prevent digestive disorders and enhance forage intake. The calves were fed twice daily at 0800 h and 1800 h with equal amounts of each meal throughout the experimental period. Drinking water was freely accessible to calves throughout the experimental period. Individual daily dry matter intake (DMI) was recorded by measuring the feed offered, and the feed refused. Body weight was measured before morning feeding at the start of the adaptation period, 2 weeks before weaning, at weaning, and weekly thereafter.

Table 1.
Chemical composition of the experimental diets (g/kg DM or as stated).
2.3. Enumeration of Intestinal Pathogenic Bacteria
Approximately 100 g of fecal samples were collected directly from the rectum following BW measurements 2 weeks before weaning, at weaning, and weekly thereafter. Collected fecal samples were placed on ice, transferred to the laboratory, and stored at −80 °C until analysis. One-gram of feces was diluted in 9 mL of phosphate-buffered saline (PBS) and homogenized by vortexing. Serial dilutions in PBS were plated in triplicate on the following selective media: eosin methylene blue agar (EMB agar, Oxoid Ltd., Basingstoke, UK), Salmonella Shigella agar (SS agar, Difco Laboratories, Detroit, MI, USA), and Perfringens agar (OPSP agar, Oxoid Ltd., Basingstoke, UK), which were used to isolate E. coli, Salmonella spp., and C. perfringens, respectively. All plates were incubated in an anaerobic chamber (Coy Laboratory Products Inc., Ann Arbor, MI, USA). E. coli and Salmonella spp. were incubated for 24 h at 37 °C, and C. perfringens was incubated for 18–24 h at 35 °C. On the EMB agar, colonies with a metallic green sheen were suspected to be E. coli and were counted. Colonies of Salmonella spp. on the SS agar had black centers without color and were counted. Clostridium perfringens produces black colonies on Perfringens agar.
2.4. Hematological Parameters
Before the beginning of the experiment, blood samples were taken from the jugular veins of all calves into a Vacutainer tube containing EDTA (Becton Dickinson Vacutainer Systems, Plymouth, UK). Approximately 2 mL blood samples were analyzed for initial immunoglobulin G (IgG) using Bovine IgG ELISA Core Kit (Koma Biotech, Seoul, Korea). After that, blood samples were taken from the jugular veins of 16 calves, which included four calves randomly selected from each treatment group. A total of six samples were taken from each calf throughout the experiment: 2 weeks before weaning, at weaning, and 1, 2, 5, and 8 weeks after weaning. Approximately 10 mL blood samples were taken from the jugular vein and collected into a Vacutainer tube containing EDTA (Becton Dickinson Vacutainer Systems, Plymouth, UK), as well as a serum tube containing clot activator (Becton Dickinson Vacutainer Systems, Plymouth, UK) before morning feeding. The EDTA tubes were placed on ice and then immediately transferred to the analytical laboratory of the Animal Hospital at Chungnam National University for complete blood count (CBC) analysis, which included white blood cells (WBC), lymphocytes, monocytes, neutrophils, eosinophils, basophils, the neutrophil to lymphocyte ratio (N:L), red blood cell (RBC), mean cell volume (MCV), hematocrit (HCT), mean cell hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), hemoglobin (Hgb), red blood cell distribution width (RDW), platelets (PLT), and mean platelet volume (MPV). Serum was obtained by centrifugation at 1300× g for 15 min at 4 °C and frozen at −80 °C until later analysis. The serum was analyzed for total protein, blood urea nitrogen (BUN), and glucose using kits purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and a clinical auto analyzer (Toshiba Acute Biochemical Analyzer-TBA-40FR, Toshiba Medical instruments, Tokyo, Japan) following the procedures described by Wang et al. [21].
2.5. Chemical Analysis
Feed samples were sampled one before the beginning of the experiments and were ground through a cyclone mill (Foss, Hillerød, Denmark), fitted with a 1 mm screen prior to chemical analysis. Contents of dry matter (DM #934.01), crude protein (CP; #976.05), ether extract (EE; #920.39), acid detergent fiber (ADF; #973.18), and ash (#942.05) were determined as previously described AOAC (Association of Official Analytical Chemists) [22]. CP was calculated as 6.25 times the nitrogen content, and total nitrogen was measured by the Kjeldahl method using a DK 20 Heating Digester and Semi-Automatic Distillation Unit Model UDK 139 (VELP Scientifica, Usmate, Italy). Neutral detergent fiber (NDF) was analyzed using a heat stable amylase and expressed inclusive of residual ash (aNDF) as described by Van Soest et al. [23].
2.6. Statistical Analysis
Data were analyzed using the MIXED procedure in SAS (SAS Institute Inc., Cary, NC, USA) for comparison of means. The linear model was as follows
where Yij is jth observation (j = 1–12) in ith treatment (i = 1–4), μ is the overall mean, τi is the fixed effect of the ith treatment, and eij is the unexplained random effect on the jth observation in the ith treatment.
Yij = μ + τi + eij
Three orthogonal contrasts were tested: the difference with or without bacteriophage supplementation (i.e., CON and EZ versus BP and BP_EZ), the difference with or without β-mannanase supplementation (i.e., CON and BP versus EZ and BP_EZ), and the interaction between bacteriophage and β-mannanase supplementation (i.e., CON and BP_EZ versus EZ and BP). When no interaction was observed, we further tested the main effect of the two supplementations orthogonally without considering the interaction between them [24].
The effects of treatments on BW and hematological parameters were analyzed using the MIXED procedure with repeated measures. Initial values were included in the model as covariates in each analysis. The best variance-covariance structures for each analysis were unstructured, UN, and auto-regression, AR(1), for BW and hematological parameters, respectively, as assessed by the lowest Akaike information criterion (AIC) and Bayesian information criterion (BIC). In the BW analysis, the treatment × week interaction, body weight gain (BWG, kg/week), was of particular interest. To estimate the effects of bacteriophage and β-mannanase supplementation on survival rate, we used the logistic procedure in SAS to estimate the strength of the association between each variable and death based on the odds. Pair-wise comparisons of the least square means were conducted using the PDIFF option with Tukey–Kramer adjustment when there was a significant overall treatment effect. Significance was declared at p < 0.05, and a trend was discussed at 0.05 ≤ p < 0.10.
3. Results
3.1. Effects of Supplementation of Bacteriophage
Twenty-two calves survived to the end of the experiment. Among the treatments, five, four, seven, and six calves survived in the CON, EZ, BP, and BP_EZ groups, respectively. No calves died later than 2 weeks post-weaning. Although not statistically significant (p = 0.21), the bacteriophage supplemented group (BP and BP_EZ) showed a numerical increase in calf survival rate, which was defined as the number of surviving calves as a percentage of the total number of calves at the beginning of the experiment, compared to the bacteriophage unsupplemented group (CON and EZ). After weaning, the survival rate rapidly declined in the bacteriophage unsupplemented group, compared to that in the bacteriophage supplemented group (Figure 1). Up to 2 weeks after weaning, 50% of the calves in the bacteriophage unsupplemented group survived, while 70% of the calves in the bacteriophage supplemented group survived.
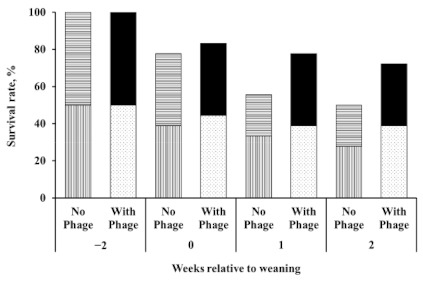
Figure 1.
Calf survival rate. Striped bars and dotted or solid bars represent the survival rate in the bacteriophage unsupplemented group (i.e., control and β-mannanase) and the bacteriophage supplemented group (i.e., bacteriophage and bacteriophage plus β-mannanase supplementation), respectively. No further calf deaths occurred from two weeks after weaning.
Differences in the odds ratio of survival between the bacteriophage unsupplemented and supplemented groups were also compared using the logistic procedure with initial BW as a covariate. Initial BW significantly affected the odds ratio of survival. The estimated effect of initial BW on the odds ratio of survival was 1.41 (p < 0.01). Therefore, a 1-kg increase in BW 2 weeks before weaning resulted in a 1.41-fold increase in survival. Accounting for differences in the initial BW of the calves, the odds ratio of survival tended to increase approximately 6.15-fold in the bacteriophage supplemented group (p = 0.09).
The supplemented bacteriophage did not show a difference in growth performance except for the tendency to decrease the forage DMI (p = 0.07) (Table 2).

Table 2.
The effects of bacteriophage and β-mannanase supplementation on feed intake and growth performance in male Holstein calves.
3.2. Effects of Supplementation of β-Mannanase
No significant differences were observed among treatments for the initial BW, BW at weaning, and final BW of calves (Table 2). In contrast, comparisons showed that the calves supplemented with β-mannanase tended to have a heavier final BW (p = 0.08) than those unsupplemented with β-mannanase. Total DMI did not differ among the treatment groups (p > 0.10); however, the DMI of concentrate was significantly increased by β-mannanase supplementation (p < 0.01). No significant differences were observed among treatments for feed efficiency or the average daily gain (g) to DMI (g) ratio (p > 0.05).
The effects of dietary treatments on changes in BW were analyzed using the 22 surviving calves. In contrast to the health of the calves, no trend was observed for the effect of bacteriophage supplementation on growth by orthogonal contrasts analysis; however, BWG was significantly greater in the β-mannanase supplemented group (p = 0.018). The estimated BWG was 3.05 and 4.09 kg/week, equivalent to 436 and 584 g/d, in the β-mannanase unsupplemented and supplemented groups, respectively (Figure 2).
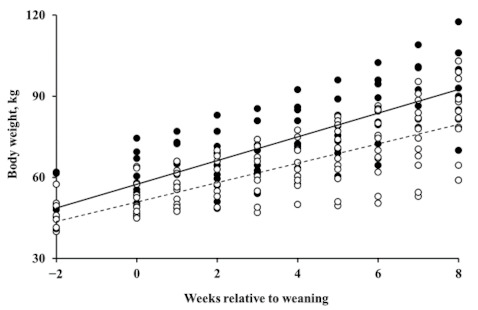
Figure 2.
Body weight (BW) of calves at weeks relative to weaning. The open circle (○) and dotted line represent BW of the β-mannanase unsupplemented group (i.e., control and bacteriophage supplementation) and the closed circle (●) and solid line represent BW of the β-mannanase supplemented group (i.e., β-mannanase and bacteriophage plus β-mannanase supplementation).
3.3. Intestinal Pathogenic Bacteria and Hematological Parameters
The total number of fecal E. coli decreased linearly throughout the experimental period (p < 0.01, Figure 3). Among treatments, there was a significant difference in the number of E. coli 1 week after weaning (p = 0.01). After 1 week weaning, the bacteriophage supplemented group had a significantly reduced population of E. coli in feces compared with the bacteriophage unsupplemented group (3.5 versus 4.7 log colony forming unit [CFU]/g, p < 0.01). Thereafter, no difference between groups was found. Salmonella spp. and C. perfringens were barely detectable in fecal samples from calves throughout the experimental period (data not shown).
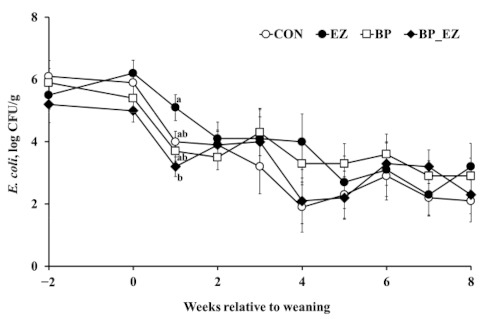
Figure 3.
Changes in the number (log CFU/g) of Escherichia coli in calf feces. The open-circle (○), closed-circle (●), open-square (□), and closed-diamond (♦) represent least square means of the control, β-mannanase supplementation, bacteriophage supplementation, and bacteriophage plus β-mannanase supplementation groups, respectively. Error bars represent standard errors of the means at each week. a,b Means that do not have common superscripts differ (p < 0.01); CFU: colony forming unit.
Most of the tested hematological parameters were not significantly different among the treatment groups; however, the lymphocyte concentration and the neutrophil to lymphocyte (N:L) ratio tended to differ by treatment (p = 0.07 and 0.08, respectively; Table 3). This was due to the interaction between β-mannanase and bacteriophage supplementation. Unlike supplementation of β-mannanase alone to control (CON vs. EZ), supplementation of β-mannanase in addition to bacteriophage (BP vs. BP_EZ) decreased the concentration of lymphocytes in blood (p < 0.01). There was a significant interaction between β-mannanase and bacteriophage supplementations (p = 0.02). When only β-mannanase was supplemented to control (CON vs. EZ), the N:L ratio substantially increased. However, this increase was lowered when β-mannanase was co-supplemented with bacteriophage (BP vs. BP_EZ).

Table 3.
The effects of bacteriophage and β-mannanase supplementation on hematological responses in Holstein calves (n = 4 per treatment). Means of five samples are presented (at weaning and at 1, 2, 5, and 8 weeks after weaning).
4. Discussion
The most important causes of death in the early life of cattle are diarrhea and respiratory disease, resulting in significant economic losses to the cattle industry [1]. Many substances (e.g., antibiotics, probiotics, and prebiotics) have been proposed to reduce the calf mortality rate through direct antimicrobial effects or through improved animal health. In line with these efforts, this study investigated the effects of dietary supplementation with a commercial bacteriophage product, a commercial β-mannanase, and their associative effects on health and growth performance in male Holstein calves. This is the first study to evaluate the effect of bacteriophage supplementation and its interaction with β-mannanase supplementation in ruminants.
By the end of the experiment, 14 out of 36 calves had died up to 2 weeks after weaning, and the overall calf mortality rate was 39%. The major cause of death was infection by coronavirus and coccidiosis, as diagnosed by the Animal Disease Diagnostic Division of the Ministry for Food, Agriculture, Forestry and Fisheries (Anyang, Gyeonggi, Korea). Although no national statistics on calf mortality are available in Korea, this value was higher than expected. Mortality rates in the early life of cattle can vary widely by season, region, and country [1]. Reported values vary from 1.5% [25] to 25% [26]. Even in developed countries, the mortality rate could be up to 31% [27].
One possible reason for the high calf mortality rate is the inadequate passive transfer of immunoglobulins as a result of the insufficient consumption of colostrum. Calf immunity depends on the passive transfer of colostrum immunoglobulins from dams. However, failure of the passive transfer of immunoglobulins was reported in a considerable proportion of dairy calves [28], which occurred even when the calves remained with their dam after birth [29]. Insufficient passive transfer of immunoglobulins is associated with increases in morbidity and mortality before and after weaning [28]. At the time when this trial was conducted, the price of male Holstein calves was so low that many dairy farmers paid little attention to the supply of adequate amounts of colostrum to male calves. The mean (± standard deviation (SD)) serum IgG concentration of the calves, measured by ELISA on the day the experiment, was initiated was 10.8 (±3.34 g/L). This implied that the passive transfer of IgG was inadequate (<16 g/L; [30]), probably due to insufficient consumption of colostrum after birth.
The cold weather may be another reason for the high calf mortality rate. The experiment was conducted between January and March in unusually cold winter. The weekly mean average (minimum) daily temperature during the 2 weeks before weaning, 1 week before weaning, 1 week after weaning, and 2 weeks after weaning were −2.2 °C (−5.8), 1.9 °C (−2.7), −4.1 °C (−8.5), and −0.1 °C (−4.8), respectively. To maintain the indoor temperature, ventilation was relatively poor. Calf mortality rate is higher in winter than in summer [1,4], and poor ventilation is known to increase the calf mortality rate during cold winters [5]. Therefore, we cannot exclude the possibility that the positive effects of the supplementations observed in this study can be limited to harsh conditions (e.g., failure of passive immunity transfer and inadequate environmental conditions).
Although statistical significance was not attained on survival rate among the treatments, bacteriophage supplementation tended to increase the odds ratio of survival of calves when the effect of initial BW was considered as a covariate. Supplementation of diets with bacteriophage also suppressed the population of E. coli (3.5 versus 4.7 log CFU/g) at 1 week after weaning, when four calves died in the bacteriophage unsupplemented group, and one calf died in the bacteriophage supplemented group. Studies have shown that the population of E. coli, Salmonella spp., and C. perfringens in feces is associated with the survival rate of young ruminants [31,32].
The use of bacteriophages as bacteria-control agents has been proposed in several studies [33,34]. Inoculation of phage in sheep decreased populations of E. coli O157:H7 in the rumen, cecum, and rectum [35]. When challenged with pathogens, the application of pathogen-specific bacteriophages decreased the number of intestinal pathogens and reduced mortality in chickens [36]. Supplementation of commercial feed additive with bacteriophages has reduced fecal E. coli counts in laying hens [37], broilers [38], and pigs [39]. However, increased E. coli counts following supplementation have also been reported in laying hens [10].
Although supplementation with bacteriophages may improve the health status of calves, it may not increase their growth performance. In the present study, the growth of calves was not affected by bacteriophage supplementation. Inconsistent effects of bacteriophage supplementation have been reported on the growth of farm animals. In pigs, DMI, average daily gain (ADG), and feed efficiency were not improved by bacteriophage supplementation at 0.5 g/kg although apparent total tract digestibility (ATTD) of DM and nitrogen were increased [39]. On the other hand, Kim et al. [11] and Hosseindoust et al. [40] reported that 1.0 and 1.5 g/kg bacteriophage had significantly improved ADG and the fecal score of pigs. Bacteriophage supplementation did not improve the performance of chickens. No significant differences in growth and ATTD of nutrients were observed in broilers [38]. Little improvement in laying performance and egg quality was observed following supplementation in laying hens [10].
Co-supplementation of β-mannanase and bacteriophage seemed to alter the inflammatory response of calves, although changes in hematological parameters are hard to interpret due to large variations among individual animals. When both bacteriophage and β-mannanase supplementation were provided, a reduction in the lymphocyte count without an increase in the neutrophil count was observed. This may imply that chronic inflammation was reduced, although the mode of action is not clear. Chronic viral infections, inflammation, and autoimmune diseases increase the numbers of both neutrophils and lymphocytes [41]. Both neutrophil and lymphocyte counts were also increased in vaccinated animals [42]. Although temporary decreases in the number of lymphocytes can occur [43], this may not be the case in the present study since the lymphocyte counts were measured six times throughout the 10 week experimental period, and a concurrent decrease in the number of neutrophils was not observed. Stress may also cause lymphocyte counts to decrease; however, under stressed situations, the number of neutrophils is generally increased [44]. Other studies reported no differences in hematological parameters following bacterial supplementation in pigs [39] and broilers [38].
Conversely, β-mannanase supplementation increased BWG (p = 0.018). Numerous studies in monogastric animals have reported that β-mannanase supplementation improved feed utilization and growth performance in broilers [14,45] and pigs [16,46]. Positive effects of β-mannanase supplementation on growth performance have also been reported in ruminants. The inclusion of β-mannanase in the starter feed tended to improve the feed efficiency of calves [20]. In addition, β-mannanase supplementation improved ADG, feed efficiency, and nitrogen retention in growing goats [18]. In dairy cows, the supplementation of β-mannanase has increased the feed efficiency by lowering the somatic cell count [19].
The mechanism of growth improvement in response to β-mannanase supplementation in ruminants remains to be determined. In monogastric animals, β-mannanase supplementation has been proposed to improve growth performance by improving nutrient digestibility and utilization in broilers and in pigs [16,47]. Another proposed mechanism of action of β-mannanase supplementation is associated with mannan oligosaccharides, which are released following the breakdown of β-mannan in the diet and may stimulate the innate immune system of animals [14]. However, in ruminants, Lee et al. [18] observed a decrease in nutrients digestibility and proposed that increased growth following β-mannanase supplementation might be associated with improved nitrogen metabolism in goats. Furthermore, β-mannanase supplementation alone did not alter the innate immune system in the present study. Further studies are needed to obtain a better understanding of the mechanism of action of β-mannanase supplementation in ruminants.
5. Conclusions
Supplementation of feed with bacteriophage may have a positive effect on calf survival rate but not on growth performance. The levels of some pathogenic bacteria (e.g., E. coli) may be reduced following bacteriophage supplementation. β-mannanase supplementation, on the other hand, may increase the growth rate and intake of a starter feed in calves. No synergistic effects were observed between the two dietary supplements on intake, growth performance, and mortality of calves, although a reduction in the lymphocyte count without an increase in the neutrophil count was observed when the diet was supplemented with both bacteriophage and β-mannanase.
Author Contributions
Conceptualization: J.-J.L., J.-H.L., and S.S.; Methodology: S.S.; Validation: S.J.; Formal Analysis: S.J. and S.S.; Investigation: S.J. and N.J.; Resources: J.-J.L., J.-H.L., and D.-K.K.; Data Curation: S.J. and S.S.; Writing—Original Draft preparation: S.J.; Writing—Review and Editing: N.J., D.-K.K., J.S., E.K., and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bioindustry Technology Development Program (Project No. 312030-04-3-HD060), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Institutional Review Board Statement
The animal use and experimental protocols were approved by the Committees of Animal Research Ethics of Chungnam National University, Daejeon, Republic of Korea (approval number CNU-00188).
Acknowledgments
The authors would like to express sincere appreciation to the late Woonsu Kim for his scientific assistance and friendship during the preparation of the manuscript.
Conflicts of Interest
Author J.-J.L., J.-H.L., and E.K. are listed as inventors on patent applications filed by CTCBIO Inc. All other authors declare no conflict of interest.
References
- Svensson, C.; Linder, A.; Olsson, S.-O. Mortality in Swedish dairy calves and replacement heifers. J. Dairy Sci. 2006, 89, 4769–4777. [Google Scholar] [CrossRef]
- Taylor, M. Protozoal disease in cattle and sheep. In Pract. 2000, 22, 604–617. [Google Scholar] [CrossRef]
- Cho, Y.-I.; Yoon, K.-J. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Uetake, K. Newborn calf welfare: A review focusing on mortality rates. Anim. Sci. J. 2013, 84, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.; McGuirk, S.M.; Bennett, T.B.; Cook, N.B.; Nordlund, K.V. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. J. Dairy Sci. 2006, 89, 4014–4025. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334–336. [Google Scholar] [CrossRef]
- Gebru, E.; Lee, J.S.; Son, J.C.; Yang, S.Y.; Shin, S.A.; Kim, B.; Kim, M.K.; Park, S.C. Effect of probiotic-, bacteriophage-, or organic acid-supplemented feeds or fermented soybean meal on the growth performance, acute-phase response, and bacterial shedding of grower pigs challenged with serotype Typhimurium. J. Anim. Sci. 2010, 88, 3880–3886. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Brüssow, H. Phage therapy: The Escherichia coli experience. Microbiology 2005, 151, 2133–2140. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Shin, H.S.; Kim, M.C.; Lee, J.H.; Kim, G.B.; Kil, D.Y. Effect of dietary supplementation of bacteriophage on performance, egg quality and caecal bacterial populations in laying hens. Br. Poult. Sci. 2015, 56, 132–136. [Google Scholar] [CrossRef]
- Kim, J.S.; Hosseindoust, A.; Lee, S.H.; Choi, Y.H.; Kim, M.J.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage cocktail and multi-strain probiotics in the feed for weanling pigs: Effects on intestine morphology and targeted intestinal coliforms and Clostridium. Animal 2017, 11, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Martin-Orue, S.; Taylor-Pickard, J.; Perez, J.; Gasa, J. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J. Anim. Sci. 2008, 86, 94–101. [Google Scholar] [CrossRef]
- Hume, M.E. Food safety symposium: Potential impact of reduced antibiotic use and the roles of prebiotics, probiotics, and other alternatives in antibiotic-free broiler production. Poult. Sci. 2011, 90, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.E.; Geronian, K.; Knox, A.; McNab, J.; McCartney, E. A dose-response study with the feed enzyme beta-mannanase in broilers provided with corn-soybean meal based diets in the absence of antibiotic growth promoters. Poult. Sci. 2004, 83, 1992–1996. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Effects of beta-mannanase supplementation in combination with low and high energy dense diets for growing and finishing broilers. Livest. Sci. 2013, 154, 137–143. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Yang, Y.X.; Shinde, P.L.; Choi, J.Y.; Kim, J.S.; Kim, Y.W.; Yun, K.; Jo, J.K.; Lee, J.H.; Ohh, S.J.; et al. Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs. J. Anim. Sci. 2010, 88, 181–191. [Google Scholar] [CrossRef]
- Kim, J.; Ingale, S.; Lee, S.; Kim, K.; Lee, J.; Chae, B. Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Anim. Feed Sci. Technol. 2013, 186, 64–70. [Google Scholar] [CrossRef]
- Lee, J.-J.; Seo, J.; Jung, J.K.; Lee, J.; Lee, J.-H.; Seo, S. Effects of β-mannanase supplementation on growth performance, nutrient digestibility, and nitrogen utilization of Korean native goat (Capra hircus coreanae). Livest. Sci. 2014, 169, 83–87. [Google Scholar] [CrossRef]
- Tewoldebrhan, T.; Appuhamy, J.; Lee, J.-J.; Niu, M.; Seo, S.; Jeong, S.; Kebreab, E. Exogenous β-mannanase improves feed conversion efficiency and reduces somatic cell count in dairy cattle. J. Dairy Sci. 2017, 100, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lee, S.-M.; Kim, J.-H.; Ki, K.-S.; Kim, H.-S.; Kam, D.-K.; Lee, J.-H.; Lee, J.-J.; Bae, G.-S.; Seo, S.-W. Effect of β-mannanase (CTCZYME®) on the growth of young calf. Korean J. Agric. Sci. 2010, 37, 239–243. [Google Scholar] [CrossRef]
- Wang, T.; Lee, K.-H.; Jung, U.-S.; Jin, Y.-C.; Lee, S.-B.; Lee, J.-S.; Hwang, J.-H.; Lim, J.-N.; Kim, M.-J.; Choi, S.-H. Responses of blood metabolites and proteins to different vitamin A levels in Korean native steers. Pak. Vet. J. 2014, 34, 527–531. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Jeon, S.; Ha, J.K. Guidelines for experimental design and statistical analyses in animal studies submitted for publication in the Asian-Australasian Journal of Animal Sciences. Asian-Australas. J. Anim. Sci. 2018, 31, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Torsein, M.; Lindberg, A.; Sandgren, C.H.; Waller, K.P.; Törnquist, M.; Svensson, C. Risk factors for calf mortality in large Swedish dairy herds. Prev. Vet. Med. 2011, 99, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.N.; Bonfoh, B.; Schelling, E.; Bengaly, S.; Tembely, S.; Tanner, M.; Zinsstag, J. Calf mortality rate and causes of death under different herd management systems in peri-urban Bamako, Mali. Livest. Sci. 2006, 100, 169–178. [Google Scholar] [CrossRef]
- Wells, S.J.; Dargatz, D.A.; Ott, S.L. Factors associated with mortality to 21 days of life in dairy heifers in the United States. Prev. Vet. Med. 1996, 29, 9–19. [Google Scholar] [CrossRef]
- Godden, S. Colostrum management for dairy calves. Vet. Clin. N. Am. Food Anim. 2008, 24, 19–39. [Google Scholar] [CrossRef]
- Beam, A.; Lombard, J.; Kopral, C.; Garber, L.; Winter, A.; Hicks, J.; Schlater, J. Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations. J. Dairy Sci. 2009, 92, 3973–3980. [Google Scholar] [CrossRef]
- Waldner, C.L.; Rosengren, L.B. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can. Vet. J. 2009, 50, 275–281. [Google Scholar] [CrossRef][Green Version]
- Dean-Nystrom, E.A.; Bosworth, B.T.; Cray, W.; Moon, H.W. Pathogenicity of Escherichia coli O157: H7 in the intestines of neonatal calves. Infect. Immun. 1997, 65, 1842–1848. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.D.; Keen, J.E.; Anderson, R.C.; Rossman, M.L.; Engler, M.J.; Genovese, K.J.; Gwartney, B.L.; Reagan, J.O.; et al. Fecal prevalence of Escherichia coli O157, Salmonella, Listeria, and Bacteriophage Infecting E. coli O157:H7 in feedlot cattle in the Southern Plains region of the United States. Foodborne Pathog. Dis. 2006, 3, 234–244. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–819. [Google Scholar] [CrossRef]
- Lim, T.-H.; Kim, M.-S.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Youn, H.-N.; Lee, H.-J.; Yang, S.-Y.; Cho, Y.-W.; Lee, J.-B.; et al. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012, 93, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.D.; Anderson, R.C.; Rossman, M.L.; Engler, M.J.; Carr, M.A.; Genovese, K.J.; Keen, J.E.; Looper, M.L.; et al. Bacteriophage isolated from feedlot cattle can reduce Escherichia coli O157:H7 populations in ruminant gastrointestinal tracts. Foodborne Pathog. Dis. 2008, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.; Sieo, C.; Tan, W.; Hair-Bejo, M.; Jalila, A.; Ho, Y. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poult. Sci. 2010, 89, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.Y.; Baek, H.Y.; Kim, I.H. Effects of bacteriophage supplementation on egg performance, egg quality, excreta microflora, and moisture content in laying hens. Asian-Australas. J. Anim. Sci. 2012, 25, 1015–1020. [Google Scholar] [CrossRef]
- Wang, J.P.; Yan, L.; Lee, J.H.; Kim, I.H. Evaluation of bacteriophage supplementation on growth performance, blood characteristics, relative organ weight, breast muscle characteristics and excreta microbial shedding in broilers. Asian-Australas. J. Anim. Sci. 2013, 26, 573–578. [Google Scholar] [CrossRef]
- Kim, K.H.; Ingale, S.L.; Kim, J.S.; Lee, S.H.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage and probiotics both enhance the performance of growing pigs but bacteriophage are more effective. Anim. Feed Sci. Technol. 2014, 196, 88–95. [Google Scholar] [CrossRef]
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Noh, H.S.; Lee, J.H.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Dietary bacteriophages as an alternative for zinc oxide or organic acids to control diarrhoea and improve the performance of weanling piglets. Vet. Med. 2017, 62, 53–61. [Google Scholar] [CrossRef]
- Jones, M.L.; Allison, R.W. Evaluation of the ruminant complete blood cell count. Vet. Clin. N. Am. Food Anim. 2007, 23, 377–402. [Google Scholar] [CrossRef]
- Jo, N.C.; Jung, J.; Kim, J.N.; Lee, J.; Jeong, S.Y.; Kim, W.; Sung, H.G.; Seo, S. Effect of vaccination against foot-and-mouth disease on growth performance of Korean native goat (Capra hircus coreanae). J. Anim. Sci. 2014, 92, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, B.; Azkur, A.K.; Gazyagci, S. Inactivated bovine viral diarrhea virus vaccine trigger leucopenia and lymphopenia on calves. Acta Sci. Vet. 2011, 39, 994–999. [Google Scholar]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Chen, Y.; Li, Z.; Cao, Y. Effects of β-mannanase expressed by Pichia pastoris in corn–soybean meal diets on broiler performance, nutrient digestibility, energy utilization and immunoglobulin levels. Anim. Feed Sci. Technol. 2010, 159, 59–67. [Google Scholar] [CrossRef]
- Lv, J.; Chen, Y.; Guo, X.; Piao, X.; Cao, Y.; Dong, B. Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 579. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, J.W.; Kim, J.U.; Kim, I.H. Effects of dietary supplementation of bacteriophage on growth performance, nutrient digestibility, blood profiles, carcass characteristics and fecal microflora in broilers. Korean J. Poult. Sci. 2013, 40, 75–81. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).