Temperament, Plasticity, and Emotions in Defensive Behaviour of Paca (Mammalia, Hystricognatha)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
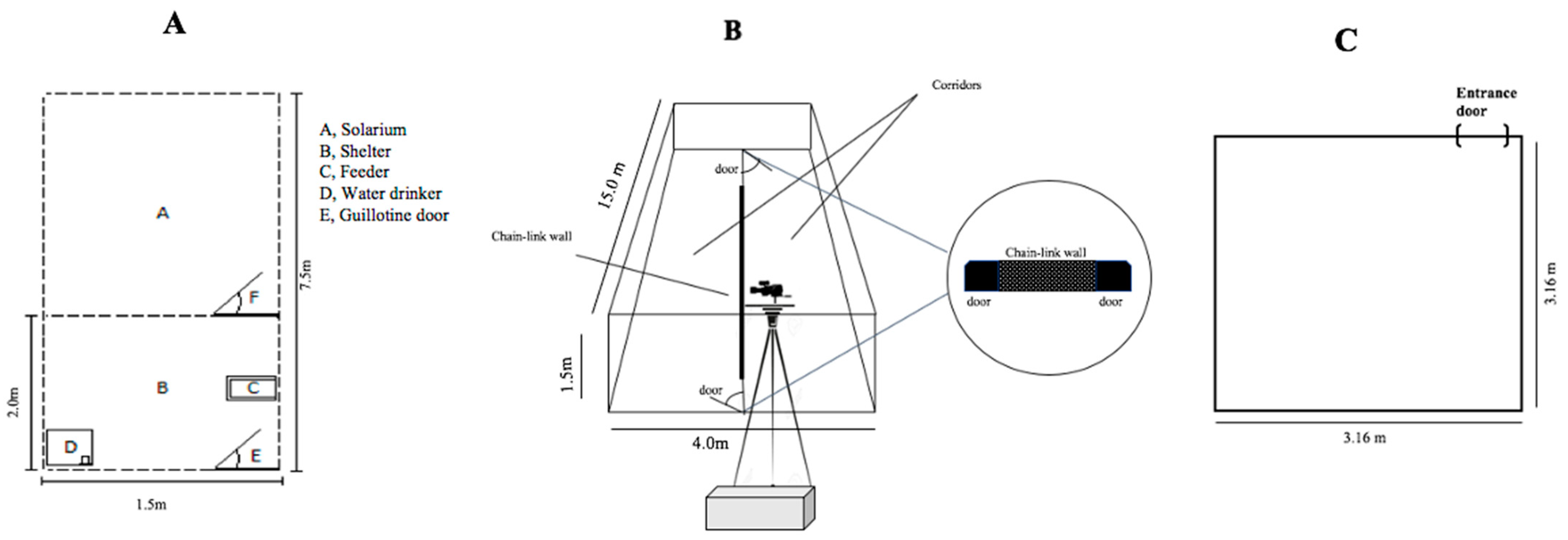
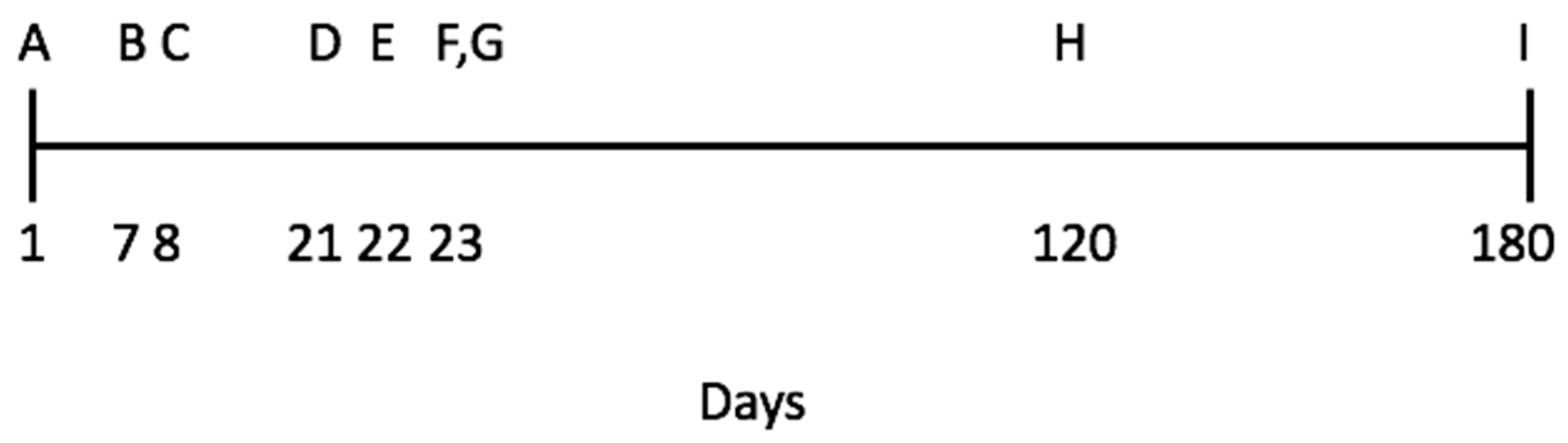
2.2. Study Site, Animals, and Sequence of Experimental Procedures
2.3. Temperament Assessment
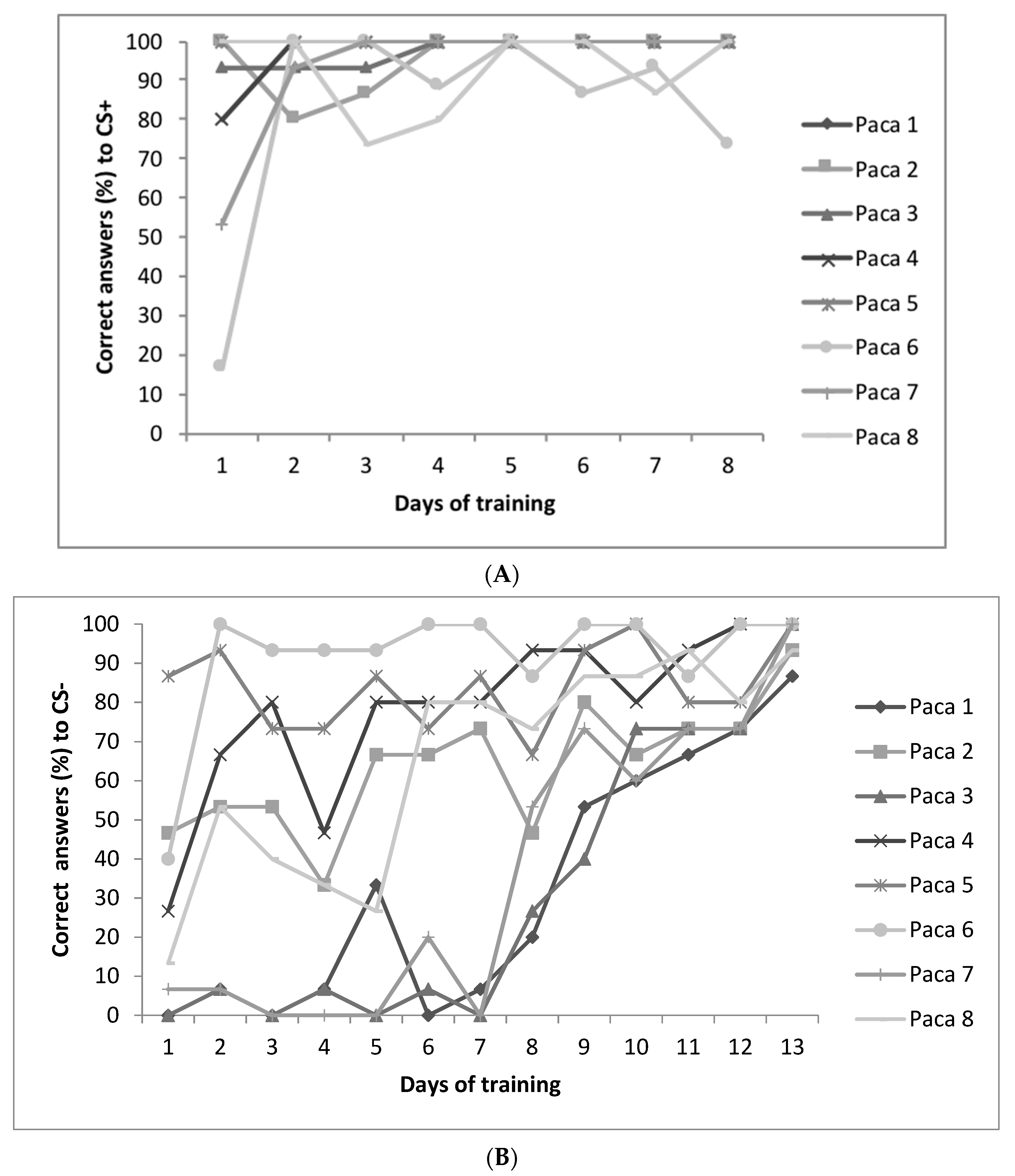
2.4. Training Sessions for the Judgment Bias Test (JBT)
2.5. Judgment Bias Test (JBT)
2.6. Modified Defence Test Battery—MDTB
2.7. Endocrine Stress Responses: Faecal Glucocorticoid Metabolites
2.8. Data Analyses and Statistics
3. Results
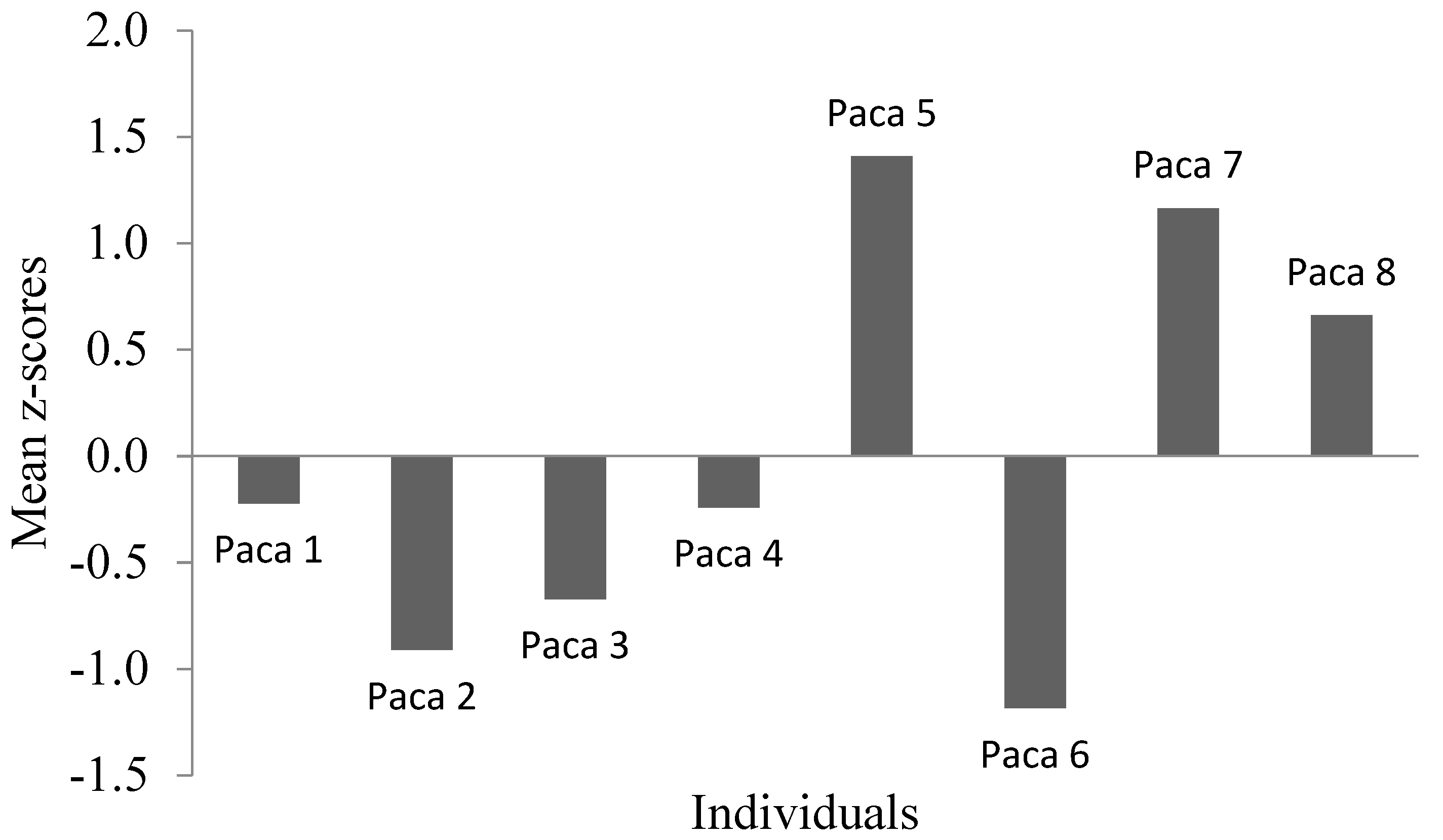
3.1. Temperament Tests
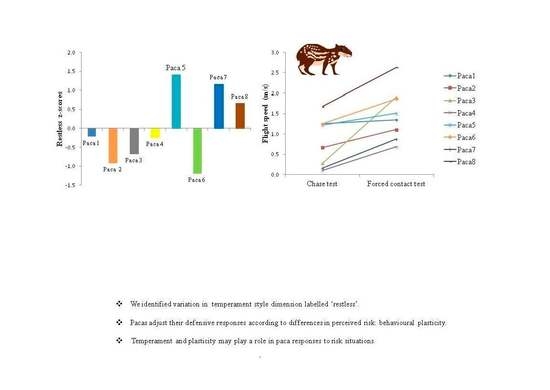
3.2. Modified Defence Test Battery (MDTB) Behaviour and Its Relationship to Temperament
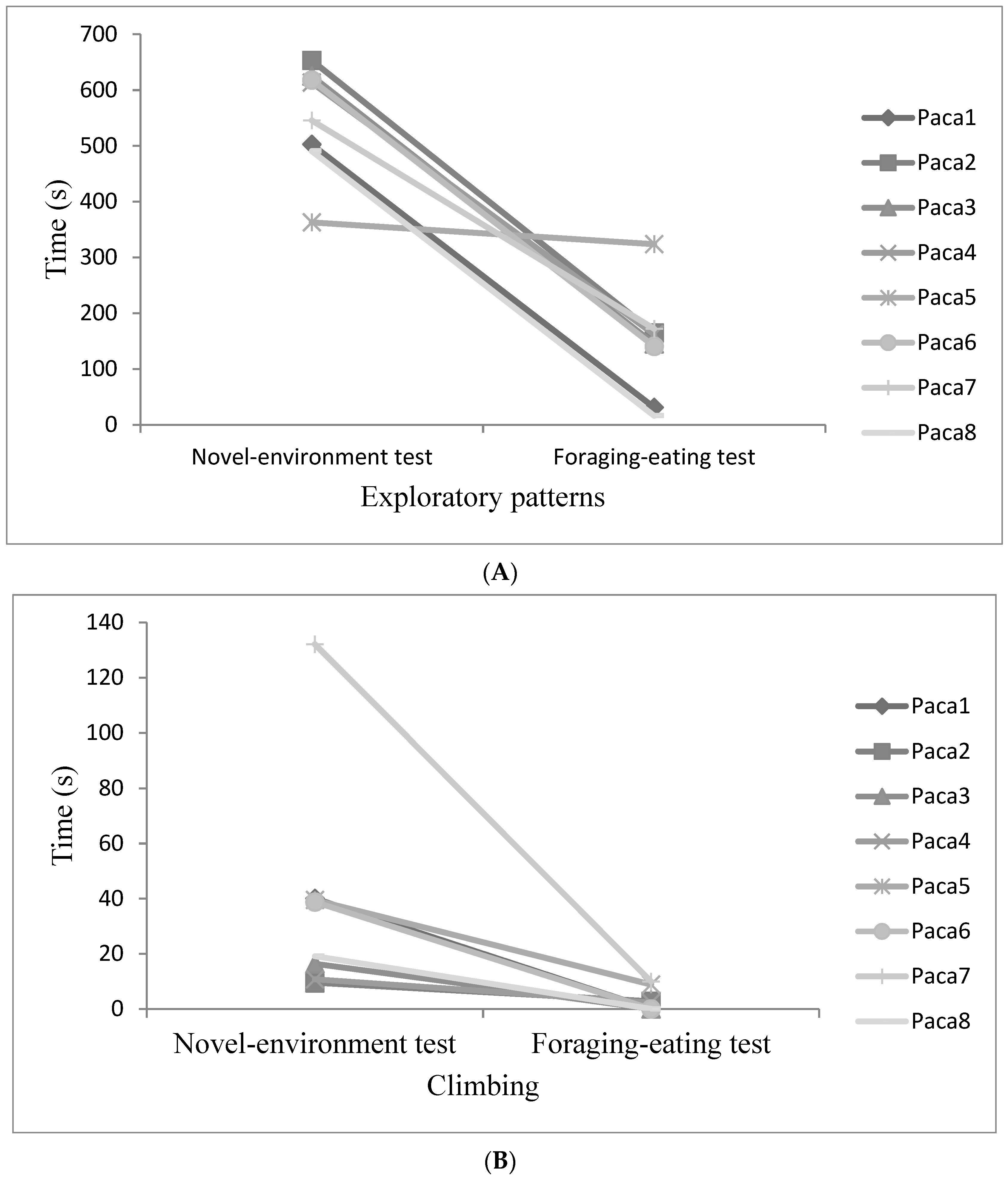
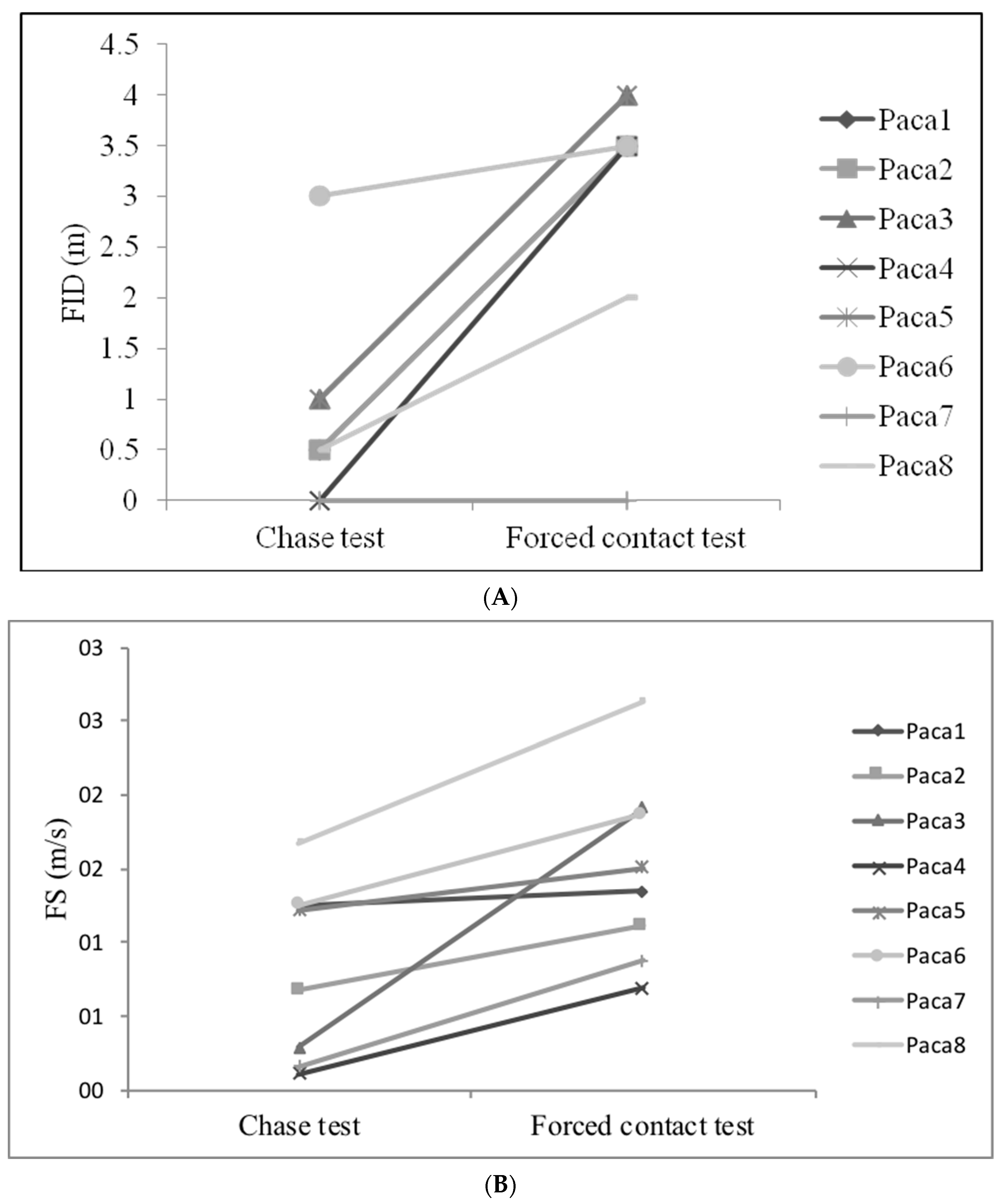
3.3. Evaluation of Behavioural Plasticity
3.4. Judgment Bias Test (JBT) and Temperament
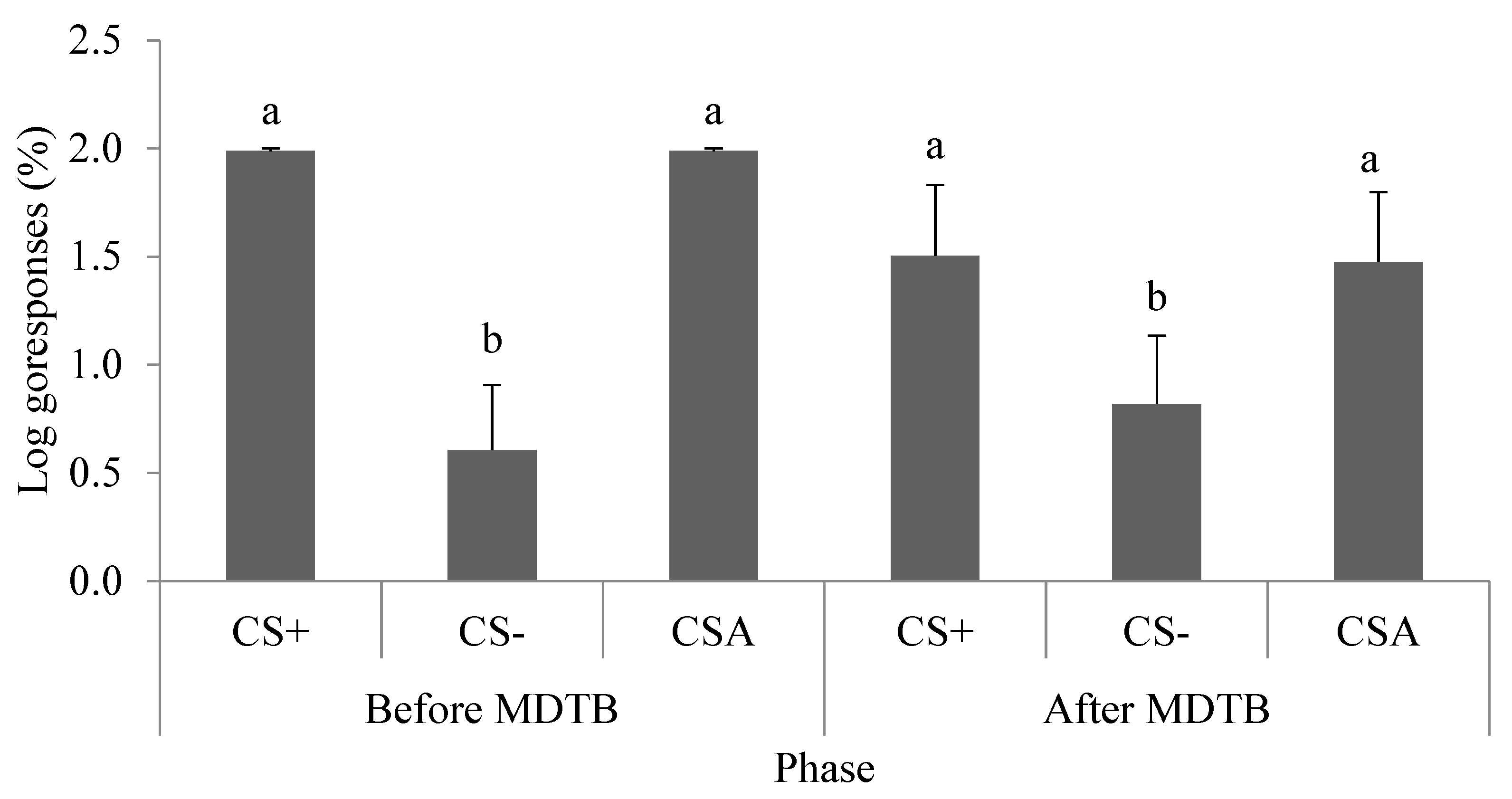
3.5. Concentration of Faecal Glucocorticoid Metabolites and Subjective Dimensions of Temperament
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, L.; Martin, J. The personality of escape. In Escaping from Predators: An Integrative View of Escape Decisions; Cooper, W.E., Blumstein, D.T., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 385–404. [Google Scholar]
- Réale, D.; Reader, S.; Sol, D.; Mcdougall, P.; Dingemanse, N. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, W.; Quinn, J.L.; Whittingham, M.J.; Butler, S. Good foragers can also be good at detecting predators. Proc. R. Soc. B Biol. Sci. 2003, 270, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; de Boer, S.F.; Bohus, B. Motivational systems or motivational states: Behavioural and physiological evidence. Appl. Anim. Behav. Sci. 1997, 53, 131–143. [Google Scholar] [CrossRef]
- Quinn, J.; Cresswell, W. Escape response delays in wintering redshank, Tringa totanus, flocks: Perceptual limits and economic decisions. Anim. Behav. 2005, 69, 1285–1292. [Google Scholar] [CrossRef]
- Sih, A.; Del Giudice, M. Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philos. Trans. R. Soc. B 2012, 367, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Price, T.D.; Qvarnström, A.; Irwin, D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. Roy. Soc. B Biol. Sci. 2003, 270, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.A. Hystricognath rodents. In Orders and Families of Recent Mammals of the World; Anderson, J.K., Jones, S., Jr., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1984; pp. 389–445. [Google Scholar]
- Gallina, S.; Pérez-torres, J.; Guzmán-Aguirre, C.C. Use of the paca, Cuniculus paca (Rodentia: Agoutidae) in the Sierra de Tabasco State Park, Mexico. Rev. Biol. Trop. 2012, 60, 1345–1355. [Google Scholar] [CrossRef]
- Leuchtenberger, C.; Tirelli, F.; Mazim, F.; Peters, F.; de Oliveira, E.; Cariolatto, L.; Queirolo, D. New records of Cuniculus paca (Rodentia: Cuniculidae) in a temperate grassland dominated landscape of the Pampa region of Brazil and Uruguay. Mammalia 2016, 81, 425–428. [Google Scholar] [CrossRef]
- Queirolo, D.; Emmons, V.; Emmons, L.; Samudio, R. Cuniculus paca. In 2008 IUCN Red List of Threatened Species. Version 2016-2. Available online: www.iucnredlist.org (accessed on 28 August 2019).
- Valsecchi, J.; El Bizri, H.R.; Figueira, J.E.C. Subsistence hunting of Cuniculus paca in the middle of the Solimões River, Amazonas, Brazil. Braz. J. Biol. 2014, 74, 560–568. [Google Scholar] [CrossRef]
- Mayor, P.; El Bizri, H.; Bodmer, R.E.; Bowler, M. Assessment of mammal reproduction for hunting sustainability through community-based sampling of species in the wild. Conserv. Biol. 2017, 31, 912–923. [Google Scholar] [CrossRef]
- El Bizri, H.R.; Monteiro, F.O.B.; Andrade, R.D.S.; Valsecchi, J.; Araújo Guimarães, D.A.; Mayor, P. Embryonic and fetal morphology in the lowland paca (Cuniculus paca): A precocial hystricomorph rodent. Theriogenology 2017, 104, 7–17. [Google Scholar] [CrossRef] [PubMed]
- El Bizri, H.R.; Fa, J.E.; Bowler, M.; Valsecchi, J.; Bodmer, R.; Mayor, P. Breeding seasonality in the lowland paca (Cuniculus paca) in Amazonia: Interactions with rainfall, fruiting, and sustainable hunting. J. Mammal. 2018, 99, 1101–1111. [Google Scholar] [CrossRef]
- El Bizri, H.R.; Fa, J.E.; Valsecchi, J.; Bodmer, R.; Mayor, P. Age at sexual maturity, first parturition and reproductive senescence in wild lowland pacas (Cuniculus paca): Implications for harvest sustainability. Anim. Reprod. Sci. 2019, 205, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Emmons, L. Cuniculus paca. The IUCN Red List of Threatened Species 2016: E.T699A22197347. 2016. Available online: https://doi.org/10.2305/IUCN (accessed on 28 August 2019).
- van Vliet, N.; Nasi, R. What do we know about the life-history traits of widely hunted tropical mammals? Oryx 2018, 53, 670–676. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Redford, K.H. Mammals of The Neotropics. Volume 3: The Central Neotropics: Ecuador, Peru, Bolivia, Brazil; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Patton, J.L.; Pardiñas, U.F.; D’Elía, G. Mammals of South. America, Volume 2: Rodents; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Gómez, H.; Wallace, R.B.; Ayala, G.; Tejada, R. Dry season activity periods of some Amazonian mammals. Stud. Neotrop. Fauna Environ. 2005, 40, 91–95. [Google Scholar] [CrossRef]
- Michalski, F.; Norris, D. Activity pattern of Cuniculus paca (Rodentia: Cuniculidae) in relation to lunar illumination and other abiotic variables in the southern Brazilian Amazon. Zoologia 2011, 28, 701–708. [Google Scholar] [CrossRef]
- Smythe, N.; Brown de Guanti, O. La Domesticación y Cría de la Paca (Agouti paca)—Domestication and Captive Reeding of the Paca (Agouti paca); FAO: Rome, Italy, 1995; pp. 23–38. [Google Scholar]
- Emmons, L.; Feer, F. Neotropical Rainforest Mammals: A Field Guide, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Nogueira-Filho, S.L.G.; Nogueira, S.S.C. Criação de Pacas (Agouti Paca)—Captive Breeding of Pacas (Agouti Paca), 1st ed.; Fundação de Estudos Agrários-FEALQ: Piracicaba, São Paulo, Brazil, 1999; pp. 23–30. [Google Scholar]
- Lima, S.G.C.; Sousa-Lima, R.S.; Tokumaru, R.S.; Nogueira-Filho, S.L.G.; Nogueira, S.S.C. Vocal complexity and sociality in spotted paca (Cuniculus paca). PLoS ONE 2018, 13, e0190961. [Google Scholar] [CrossRef]
- Barros, K.; Tokumaru, R.; Pedroza, J.; Nogueira, S.S.C. Vocal repertoire of captive capybara (Hydrochoerus hydrochaeris): Structure, context and function. Ethology 2011, 117, 83–94. [Google Scholar] [CrossRef]
- Ebensperger, L.; Cofré, H. On the evolution of group-living in the New World cursorial hystricognath rodents. Behav. Ecol. 2001, 12, 227–236. [Google Scholar] [CrossRef]
- Ebensperger, L.; Blumstein, D. Sociality in New World hystricognath rodents is linked to predators and burrow digging. Behav. Ecol. 2006, 17, 410–418. [Google Scholar] [CrossRef]
- Feist, G.J. Creativity and the Big Two model of personality: Plasticity and stability. Curr. Opin. Behav. Sci. 2019, 27, 31–35. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Griebel, G.; Blanchard, R.J. The Mouse Defense Test Battery: Pharmacological and behavioral assays for anxiety and panic. Eur. J. Pharm. 2003, 463, 97–116. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Drent, P.J.; Van Oers, K.; Van Noordwijk, A.J. Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. B Biol. Sci. 2003, 270, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, M.; Quevedo, K. The Neurobiology of Stress and Development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef]
- Harding, E.J.; Paul, E.S.; Mendl, M. Cognitive bias and affective state. Nature 2004, 427, 312. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- MacLeod, C.; Rutherford, E.; Campbell, L.; Ebsworthy, G.; Holker, L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J. Abnorm. Psychol. 2002, 111, 107–123. [Google Scholar] [CrossRef]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef]
- Nogueira, S.S.C.; Macedo, J.; Sant’Anna, A.; Nogueira-Filho, S.L.G.; da Costa, M.J.R.P. Assessment of temperament traits of white-lipped (Tayassu pecari) and collared peccaries (Pecari tajacu) during handling in a farmed environment. Anim. Welf. 2015, 24, 291–298. [Google Scholar] [CrossRef]
- Matzel, L.D.; Sauce, B. Individual differences: Case studies of rodent and primate intelligence. J. Exp. Psychol. Animal Learn. Cogn. 2017, 43, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A. Behavioral inhibition in rodents: A model to study causes and health consequences of temperament. In Behavioral Inhibition; Perez-Edgar, K., Fox, N.A., Eds.; Springer: Cham, Switzerland, 2018; pp. 35–58. [Google Scholar]
- Lima, C.C.; Silva, F.E.; Chaves Filho, A.M.; Queiroz, A.I.D.G.; Okamura, A.; Fries, G.R.; Quevedo, J.; Sousa, F.; Vasconcelos, S.; Lucena, D.; et al. Influence of temperament and exposure of periadolescent rats to paradoxical sleep deprivation combined with unpredictable stress to memory and hippocampal immune-inflammatory/oxidative changes in adult age. Front. Psychiatry 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-de León, A.; Naranjo, E.J.; Perales, H.; Santos-Moreno, A.; Lorenzo, C. Availability and characterization of cavities used by pacas (Cuniculus paca) in the Lacandon Rainforest, Chiapas, Mexico. Rev. Mex. Biodivers. 2016, 87, 1062–1068. [Google Scholar] [CrossRef]
- Smythe, N. The paca (Cuniculus paca) as a domestic source of protein for the neotropical, humid lowlands. Appl. Anim. Behav. Sci. 1987, 17, 155–170. [Google Scholar] [CrossRef]
- Aldrigui, L.; Nogueira-Filho, S.L.G.; Altino, V.S.; Mendes, A.; Clauss, M.; Nogueira, S.S.C. Direct and indirect caecotrophy behaviour in paca (Cuniculus paca). J. Anim. Physiol. Anim. Nutr. 2018, 102, 1774–1782. [Google Scholar] [CrossRef]
- Aldrigui, L.; Nogueira-Filho, S.L.G.; Mendes, A.; Altino, V.S.; Ortmann, S.; Nogueira, S.S.C.; Clauss, M. Effect of different feeding regimes on cecotrophy behavior and retention of solute and particle markers in the digestive tract of paca (Cuniculus paca). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 226, 57–65. [Google Scholar] [CrossRef]
- Nogueira, S.S.C.; Reis, A.M.; Marsaro, S.G.; Duarte, J.M.; Moreto, V.; Lima, S.C.G.; Costa, T.S.O.; Nogueira-Filho, S.L.G. The defensive behavioral patterns of captive white-lipped and collared peccary (Mammalia, Tayassuidae): An approach for conservation of the species. Acta Ethol. 2017, 20, 127–136. [Google Scholar] [CrossRef]
- Turner, S.; Navajas, E.; Hyslop, J.; Ross, D.; Richardson, R.; Prieto, N.; Roehe, R. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Mogil, J.S. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 2014, 11, 629–632. [Google Scholar] [CrossRef]
- Davis, H.; Taylor, A.A.; Norris, C. Preference for familiar humans by rats. Psychon. Bull. Rev. 1997, 4, 118–120. [Google Scholar] [CrossRef]
- Young, R.J. Environmental Enrichment for Captive Animals, 1st ed.; Blackwell Science: Oxford, UK, 2003; pp. 185–218. [Google Scholar]
- McGuire, J.L.; Coyner, J.L.; Johnson, L.R. Rodent models of conditioned fear: Behavioral measures of fear and memory. In TRP Channels in Drug Discovery; Szallasi, A., Bíró, T., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume II, pp. 187–202. [Google Scholar]
- Mason, G.; Burn, C.C.; Dallaire, J.A.; Kroshko, J.; Kinkaid, H.M.; Jeschke, J.M. Plastic animals in cages: Behavioural flexibility and responses to captivity. Anim. Behav. 2013, 85, 1113–1126. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Hunter, T.E.; Mendl, M.T.; Lawrence, A.B. Assessing the ‘whole animal’: A free choice profiling approach. Anim. Behav. 2001, 62, 209–220. [Google Scholar] [CrossRef]
- Feaver, J.; Mendl, M.; Bateson, P. A method for rating the individual distinctiveness of domestic cats. Anim. Behav. 1986, 101, 1016–1025. [Google Scholar] [CrossRef]
- Webster, M.W. Merriam-Webster Dictionary. Available online: https://www.merriam-webster.com/dictionary (accessed on 4 April 2019).
- Oliveira, F.; Nogueira-Filho, S.L.G.; Sousa, M.; Dias, C.T.S.; Mendl, M.T.; Nogueira, S.S.S.C. Measurement of cognitive bias and cortisol levels to evaluate the effects of space restriction on captive collared peccary (Mammalia, Tayassuidae). Appl. Anim. Behav. Sci. 2016, 181, 76–82. [Google Scholar] [CrossRef]
- Ribeiro-Barbosa, E.R.; Canteras, N.S.; Cezário, A.F.; Blanchard, R.J.; Blanchard, D.C. An alternative experimental procedure for studying predator-related defensive responses. Neurosci. Biobehav. Rev. 2005, 29, 1255–1263. [Google Scholar] [CrossRef]
- Altino, V.S. Validação de Métodos não Invasivos para Monitorar o Estresse em Pacas (Cuniculus paca) Criadas em Cativeiro—Validation of Non-Invasive Methods to Monitor Stress in Captive Pacas (Cuniculus paca). Ph.D. Thesis, Universidade Estadual de Santa Cruz, Ilhéus, Bahia, Brazil, 2018. [Google Scholar]
- Coradello, M.A.; Morais, R.N.; Roper, J.; Spercoski, K.M.; Massuda, T.; Nogueira, S.S.C.; Nogueira-Filho, S.L.G. Validation of a fecal glucocorticoid metabolite assay for collared peccaries (Pecari tajacu). J. Zoo Wildl. Med. 2012, 43, 275–282. [Google Scholar] [CrossRef]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Monfort, S.L. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine, coffee and ephedrine: Impact on exercise performance and metabolism. Can. J. Appl. Physiol. 2001, 26, S186–S191. [Google Scholar] [CrossRef]
- Young, E.A.; Tolman, R.; Witkowski, K.; Kaplan, G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol. Psychiatry 2004, 55, 621–626. [Google Scholar] [CrossRef]
- Brown, J.; Walke, S.; Steinman, K. Endocrine manual for the reproductive assessment of domestic and non-domestic species. In Endocrine Research Laboratory, 1st ed.; Department of Reproductive Sciences, Conservation and Research Center, National Zoological Park, Smithsonian Institution: Washington, DC, USA, 2004; pp. 1–93. [Google Scholar]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. Ann. N. Y. Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef]
- Hänninen, L.; Pastell, M. CowLog; open-source software for coding behaviors from digital video. Behav. Res. Methods 2009, 41, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.S.; Faucitano, L.; Macitelli, F.; Sant’Anna, A.C.; Méthot, S.; da Costa, M.J.R.P. Temperament effects on performance and adaptability of Nellore young bulls to the feedlot environment. Livest. Sci. 2018, 216, 88–93. [Google Scholar] [CrossRef]
- Flörcke, C.; Engle, T.E.; Grandin, T.; Deesing, M.J. Individual differences in calf defence patterns in Red Angus beef cows. Appl. Anim. Behav. Sci. 2012, 139, 203–208. [Google Scholar] [CrossRef]
- Dielenberg, R.A.; McGregor, I.S. Defensive behavior in rats towards predatory odors: A review. Neurosci. Biobehav. Rev. 2001, 25, 597–609. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Individual consistency in flight initiation distances in burrowing owls: A new hypothesis on disturbance-induced habitat selection. Biol. Lett. 2009, 6, 167–170. [Google Scholar] [CrossRef]
- Myers, R.E.; Hyman, J. Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J. Ethol. 2016, 34, 197–206. [Google Scholar] [CrossRef]
- Papciak, J.; Popik, P.; Fuchs, E.; Rygula, R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous cue interpretation paradigm. Behav. Brain Res. 2013, 256, 305–310. [Google Scholar] [CrossRef]
- Oosthuizen, M.K.; Scheibler, A.G.; Bennett, N.C.; Amrein, I. Effects of laboratory housing on exploratory behaviour, novelty discrimination and spatial reference memory in a subterranean, solitary rodent, the Cape mole-rat (Georychus capensis). PLoS ONE 2013, 8, e75863. [Google Scholar] [CrossRef]
- Beck-King, H.; von Helversen, O.; Beck-King, R. Home Range, population density, and food resources of Agouti paca (Rodentia: Agoutidae) in Costa Rica: A study using alternative methods. Biotropica 1999, 31, 675–685. [Google Scholar] [CrossRef]
- Blanchard, D.C. Translating dynamic defense patterns from rodents to people. Neurosci. Biobehav. Rev. 2017, 76, 22–28. [Google Scholar] [CrossRef]
- Mcnaughton, N. Fear, anxiety and their disorders: Past, present and future neural theories. Psychol. Neurosci. 2011, 4, 173–181. [Google Scholar] [CrossRef]
- Steimer, T. The biology of fear-and anxiety-related behaviors. Dialogues Clin. Neurosci. 2002, 1878, 231–249. [Google Scholar]
- Nogueira, S.S.C.; Fernandes, I.K.; Costa, T.S.O.; Nogueira-Filho, S.L.G.; Mendl, M. Does trapping influence decision-making under ambiguity in white-lipped peccary (Tayassu pecari)? PLoS ONE 2015, 10, e0127868. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.; Bateson, M.; Walsh, C.; Bédué, A.; Edwards, S.A. Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 2012, 139, 65–73. [Google Scholar] [CrossRef]
- Trimmer, P.C.; Ehlman, S.M.; Sih, A. Predicting behavioural responses to novel organisms: State-dependent detection theory. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162108. [Google Scholar] [CrossRef]
- Trimmer, P.C.; Ehlman, S.M.; Mcnamara, J.M.; Sih, A. The erroneous signals of detection theory. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171852. [Google Scholar] [CrossRef]
- Stankowich, T.; Coss, R.G. Effects of predator behavior and proximity on risk assessment by Columbian black-tailed deer. Behav. Ecol. 2005, 17, 246–254. [Google Scholar] [CrossRef]
- Doyle, R.E. Judgement Bias: A Cognitive Measure of Affective State in Sheep. Ph.D. Thesis, University of New England, Biddeford, ME, USA, 2010. [Google Scholar]
- Burman, O.; McGowan, R.; Mendl, M.; Norling, Y.; Paul, E.; Rehn, T.; Keeling, L. Using judgement bias to measure positive affective state in dogs. Appl. Anim. Behav. Sci. 2011, 132, 160–168. [Google Scholar] [CrossRef]
- Iigaya, K.; Jolivald, A.; Jitkrittum, W.; Gilchrist, I.D.; Dayan, P.; Paul, E.; Mendl, M. Cognitive bias in ambiguity judgements: Using computational models to dissect the effects of mild mood manipulation in humans. PLoS ONE 2016, 11, e0165840. [Google Scholar] [CrossRef]
- Novaro, A.J.; Redford, K.H.; Bodmer, R.E. Effect of hunting in source-sink systems in the neotropics. Conserv. Biol. 2000, 14, 713–721. [Google Scholar] [CrossRef]
- Brown, G.; Rive, A.; Ferrari, M.; Chivers, D. The dynamic nature of antipredator behavior: Prey fish integrate threat-sensitive antipredator responses within background levels of predation risk. Behav. Ecol. Sociobiol. 2006, 61, 9–16. [Google Scholar] [CrossRef]
- Cooper, W.E.; Blumstein, D.T. Escaping from Predators: An Integrative View of Escape Decisions; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Vincenz, D.; Kerstin, W.E.; Fendt, M.; Goldschmidta, J. Habenula and interpeduncular nucleus differentially modulate predator odor-induced innate fear behaviour in rats. Behav. Brain Res. 2017, 332, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Schubach, K.M.; Marques, R.S.; Peres, R.F.G.; Silva, L.G.T. Effects of temperament on physiological, productive, and reproductive responses in Bos indicus beef cows. J. Anim. Sci. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niermann, H.C.M.; Figner, B.; Tyborowska, A.; Cillessen, A.H.N.; Roelofs, K.; Sullivan, R.M. Investigation of the Stability of Human Freezing-Like Responses to Social Threat From Mid to Late Adolescence. Front. Behav. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carreras, R.; Arroyo, L.; Mainau, E.; Valent, D.; Bassols, A.; Dalmau, A.; Faucitano, L.; Manteca, X.; Velarde, A. Can the way pigs are handled alter behavioural and physiological measures of affective state? Behav. Process. 2017, 142, 91–98. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; Galvão, A.C.M.; Silva, F.S.; Sousa, M.B.C. Common marmosets: A potential translational animal model of juvenile depression. Front. Psychol. 2017, 8, 175. [Google Scholar] [CrossRef]
- Frank, E.; Salchner, P.; Aldag, J.M.; Salomé, N.; Singewald, N.; Landgraf, R.; Wigger, A. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav. Neurosci. 2006, 120, 60. [Google Scholar] [CrossRef]
- Herborn, K.A.; Coffey, J.; Larcombe, S.D.; Alexander, L.; Arnold, K.E. Oxidative profile varies with personality in European greenfinches. J. Exp. Biol. 2011, 214, 1732–1739. [Google Scholar] [CrossRef][Green Version]
- Ferrari, C.; Pasquaretta, C.; Carere, C.; Cavallone, E.; von Hardenberg, A.; Réale, D. Testing for the presence of coping styles in a wild mammal. Anim. Behav. 2013, 85, 1385–1396. [Google Scholar] [CrossRef]
- Patronek, G.J.; Bradley, J. No better than flipping a coin: Reconsidering canine behavior evaluations in animal shelters. J. Vet. Behav. 2016, 15, 66–77. [Google Scholar] [CrossRef]
- Patronek, G.J.; Bradley, J.; Arps, E. What is the evidence for reliability and validity of behavior evaluations for shelter dogs? A prequel to “No better than flipping a coin”. J. Vet. Behav. 2019, 31, 43–58. [Google Scholar] [CrossRef]
| Adjective | Definition * |
|---|---|
| Active | The active individual moves around a lot or engages in a range of different activities, such as walking and foraging |
| Curious | The individual appears to be investigating its surroundings, smelling the soil or the air |
| Apathetic | The individual appears to show a lack of interest in or concern about the environment |
| Fearful | The individual appears to be alert and apprehensive when facing challenging situations, walking slowly, but stopping from time to time |
| Agitated | The individual appears to be extremely disturbed or excited, moving around rapidly, stopping and walking suddenly |
| Calm | The individual appears to be peaceful and tranquil, showing relaxed jaw muscles and walking at a regular speed |
| Tense | The individual shows rigid jaw muscles and bristly hair, as if about to react strongly |
| Docile | The individual shows cooperative and submissive behavior. The animal shows no bristly hair, no aggression, and relaxed jaw muscles |
| Anxious | The individual appears to be vigilant and is highly responsive to changes in the environment. It may run or try to hide close to a wall, and move back and forth quickly |
| Relaxed | The individual appears to be at ease, with relaxed muscles and no bristly hair |
| Shy | The individual is cautious and tends to hold back in challenging situations |
| Bold | The individual readily explores its surroundings, even when facing dangerous and challenging situations |
| Satisfied | The individual appears to be relaxed and calm following an event. It shows relaxed muscles, no bristly hair, and moves at a regular speed |
| Stressed | The individual is restless, vigilant and shows panting, bristly hair and movement of jaw muscles. |
| Behaviour | Description |
|---|---|
| Exploratory patterns | |
| Roaming | The animals move about or travel aimlessly or unsystematically, especially over the arena |
| Raising forelegs | The paca lifts both forepaws, sniffing the arena walls. The animal seems relaxed, without fur bristled |
| Trying to escape patterns | |
| Running | The animal makes fast movement to escape |
| Climbing | The animal climbs the test arena walls with forepaws, while the fur is bristled |
| Freezing | Stationary state lasting more than 3 s regardless of posture |
| Eating patterns | |
| Eating banana | The paca takes pieces of banana with its mouth, chews, and swallows it |
| Abnormal behaviour | The paca eats inappropriate food (grass) found on the dirt floor of the arena |
| Inter-Observer | Inter-Challenge Tests | |||
|---|---|---|---|---|
| Adjective * | W | Significance (p) | W | Significance (p) |
| Active | 0.70 | p< 0.05 | 0.86 | p< 0.05 |
| Relaxed | 0.52 | n.s. | - | - |
| Agitated | 0.95 | p< 0.05 | 0.93 | p< 0.05 |
| Fearful | 0.70 | p< 0.05 | 0.80 | p< 0.05 |
| Calm | 0.70 | p< 0.05 | 0.57 | p < 0.05 |
| Tense | 0.72 | p< 0.05 | 0.98 | p< 0.05 |
| Anxious | 0.70 | p< 0.05 | 0.64 | p < 0.05 |
| Satisfied | 0.03 | n.s. | - | - |
| Stressed | 0.73 | p< 0.05 | 0.14 | n.s. |
| Nervous | 0.60 | n.s. | - | - |
| Apathetic | 0.98 | p< 0.05 | 0.33 | n.s. |
| Curious | 0.86 | p< 0.05 | 0.98 | p< 0.05 |
| Shy | 0.90 | p< 0.05 | 0.09 | n.s. |
| Bold | 0.99 | p< 0.05 | 0.99 | p< 0.05 |
| Active | Fearful | Agitated | Tense | Bold | Curious | |
|---|---|---|---|---|---|---|
| Active | - | −0.33 (p > 0.05) | −0.24 (p > 0.05) | −0.19 (p > 0.05) | −0.24 (p > 0.05) | −0.29 (p > 0.05) |
| Fearful | - | 0.95 (p < 0.05) | 0.88 (p < 0.05) | −0.10 (p > 0.05) | 0.24 (p > 0.05) | |
| Agitated | - | 0.98 (p < 0.05) | 0.02 (p > 0.05) | 0.24 (p > 0.05) | ||
| Tense | - | 0.07 (p > 0.05) | 0.17 (p > 0.05) | |||
| Bold | - | 0.60 (p < 0.05) | ||||
| Curious | - |
| Animal | New-Environment Test | Foraging-Eating Test | Chase Test | Forced Contact Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| z-Score 1 | FGC 2a | FGC 2b | Exp 3 | Esc 4 | Ab. Behav. 5 | Exp 3 | Esc 4 | Ab. Behav. 5 | Eating Banana | FID 6 | FS 7 | Freez. 8 | FID 6 | FS 7 | Freez. 8 | |
| Paca 1 | −0.22 | 86.0 | 240.4 | 502 | 41 | 29 | 31 | 0 | 0 | 639 | 0.5 | 1.3 | 7.6 | 3.5 | 1.3 | 12.0 |
| Paca 2 | −0.91 | 22.6 | 116.6 | 659 | 37 | 18 | 165 | 3 | 0 | 498 | 0.5 | 0.7 | 1.2 | 3.5 | 1.1 | 3.6 |
| Paca 3 | −0.67 | 13.7 | 32.1 | 632 | 101 | 58 | 145 | 0 | 0 | 362 | 1.0 | 0.3 | 0.2 | 4.0 | 1.9 | 4.4 |
| Paca 4 | −0.24 | 54.6 | 161.2 | 616 | 18 | 14 | 162 | 2 | 0 | 324 | 0.0 | 0.1 | 6.1 | 3.5 | 0.7 | 6.0 |
| Paca 5 | 1.41 | 34.7 | 102.9 | 365 | 88 | 102 | 324 | 9 | 0 | 489 | 1.0 | 1.5 | 29.7 | 4 | 1.2 | 6.6 |
| Paca 6 | −1.19 | 30.4 | 49.2 | 632 | 44 | 6 | 141 | 0 | 0 | 365 | 3.0 | 1.3 | 6.9 | 3.5 | 1.9 | 4.4 |
| Paca 7 | 1.16 | 44.7 | 152.7 | 546 | 132 | 56 | 172 | 10 | 0 | 484 | 0.0 | 0.2 | 31.2 | 0.0 | 0.9 | 19.0 |
| Paca 8 | 0.66 | 227.4 | 1812.8 | 490 | 35 | 52 | 16 | 0 | 0 | 426 | 0.5 | 1.7 | 4.7 | 2.0 | 2.6 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, S.S.C.; Nogueira-Filho, S.L.G.; Duarte, J.M.B.; Mendl, M. Temperament, Plasticity, and Emotions in Defensive Behaviour of Paca (Mammalia, Hystricognatha). Animals 2021, 11, 293. https://doi.org/10.3390/ani11020293
Nogueira SSC, Nogueira-Filho SLG, Duarte JMB, Mendl M. Temperament, Plasticity, and Emotions in Defensive Behaviour of Paca (Mammalia, Hystricognatha). Animals. 2021; 11(2):293. https://doi.org/10.3390/ani11020293
Chicago/Turabian StyleNogueira, Selene S. C., Sérgio L. G. Nogueira-Filho, José M. B. Duarte, and Michael Mendl. 2021. "Temperament, Plasticity, and Emotions in Defensive Behaviour of Paca (Mammalia, Hystricognatha)" Animals 11, no. 2: 293. https://doi.org/10.3390/ani11020293
APA StyleNogueira, S. S. C., Nogueira-Filho, S. L. G., Duarte, J. M. B., & Mendl, M. (2021). Temperament, Plasticity, and Emotions in Defensive Behaviour of Paca (Mammalia, Hystricognatha). Animals, 11(2), 293. https://doi.org/10.3390/ani11020293