Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Collection of Specimens
2.2. DNA Extraction and PCR Amplification
2.3. Sequencing and Molecular Analysis
2.4. Phylogenetic Analysis
2.5. Nucleotide Sequence Accession Numbers
3. Results
3.1. Occurrence of E. bieneusi in Stray Dogs
3.2. Genetic Characterizations of E. bieneusi in Stray Dogs
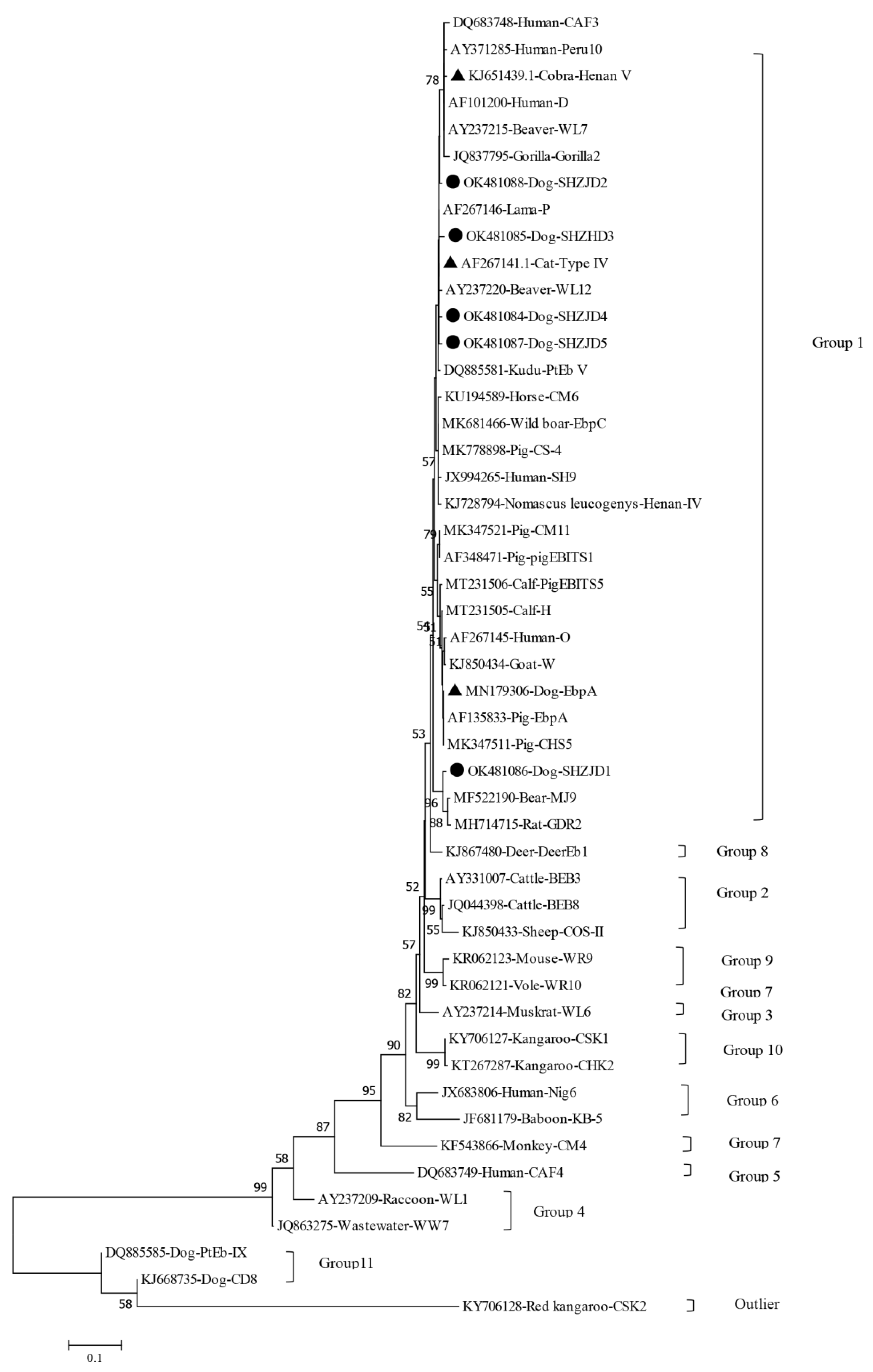
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amer, S.; Kim, S.; Han, J.I.; Na, K.J. Prevalence and genotypes of Enterocytozoon bieneusi in wildlife in Korea: A public health concern. Parasites Vectors 2019, 12, 160. [Google Scholar] [CrossRef]
- Deng, L.; Yue, C.J.; Chai, Y.J.; Wang, W.Y.; Su, X.Y.; Zhou, Z.Y.; Wang, L.Q.; Li, L.Y.; Liu, H.F.; Zhong, Z.J.; et al. New genotypes and molecular characterization of Enterocytozoon bieneusi in pet birds in Southwestern China. Int. J. Parasitol. Parasites Wildl. 2019, 10, 164–169. [Google Scholar] [CrossRef]
- Karim, M.R.; Dong, H.; Yu, F.; Jian, F.; Zhang, L.; Wang, R.; Zhang, S.; Rume, F.I.; Ning, C.; Xiao, L. Genetic diversity in Enterocytozoon bieneusi isolates from dogs and cats in China: Host specificity and public health implications. J. Clin. Microbiol. 2014, 52, 3297–3302. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Shen, Y.; Yin, J.; Yuan, Z.; Jiang, Y.; Xu, Y.; Pan, W.; Hu, Y.; Cao, J. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infect. Dis. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Jiang, Z.; Yuan, Z.; Yin, J.; Wang, Z.; Yu, B.; Zhou, D.; Shen, Y.; Cao, J. Infection by and genotype characteristics of Enterocytozoon bieneusi in HIV/AIDS patients from Guangxi Zhuang autonomous region, China. BMC Infect. Dis. 2017, 17, 684. [Google Scholar] [CrossRef]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, O.; Lobo, M.L.; Xiao, L. Epidemiology of Enterocytozoon bieneusi Infection in Humans. J. Parasitol. Res. 2012, 2012, 981424. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, J.; Song, Q.; Meng, F.; Ba, X.; Liu, W. A survey of the current status of pet market in Wuhan. China. Anim. Husb. Vet. Med. 2007, 11, 145–147. [Google Scholar]
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.D.; Traub, R.J.; Lappin, M.; Baneth, G. Zoonotic Parasites of Sheltered and Stray Dogs in the Era of the Global Economic and Political Crisis. Trends Parasitol. Trends Parasitol. 2017, 33, 813–825. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Nourrisson, C.; Moniot, M.; De Kyvon, M.A.; Bonnin, V.; De La Bretonniere, M.E.; Morange, V.; Bailly, E.; Lemaignen, A.; Morio, F.; et al. Genotyping Approach for Potential Common Source of Enterocytozoon bieneusi Infection in Hematology Unit. Emerg. Infect. Dis. 2019, 25, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Santin, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Fayer, R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: A consensus. J. Eukaryot. Microbiol. 2009, 56, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, L. Ecological and public health significance of Enterocytozoon bieneusi. One Health 2021, 12, 100209. [Google Scholar] [CrossRef]
- Del, A.C.; Izquierdo, F.; Navajas, R.; Pieniazek, N.J.; Miro, G.; Alonso, A.I.; Da, S.A.; Fenoy, S. Enterocytozoon bieneusi in animals: Rabbits and dogs as new hosts. J. Eukaryot. Microbiol. 1999, 46, 8S–9S. [Google Scholar]
- Mathis, A.; Breitenmoser, A.C.; Deplazes, P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite 1999, 6, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delrobaei, M.; Jamshidi, S.; Shayan, P.; Ebrahimzade, E.; Ashrafi, T.I.; Rezaeian, M.; Rezaeian, M.; Mirjalali, H. Molecular Detection and Genotyping of Intestinal Microsporidia from Stray Dogs in Iran. Iran J. Parasitol. 2019, 14, 159–166. [Google Scholar]
- Santin, M.; Cortes, V.J.; Fayer, R. Enterocytozoon bieneusi genotypes in dogs in Bogota, Colombia. Am. J. Trop. Med. Hyg. 2008, 79, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, N.; Kimata, I.; Iseki, M. Molecular evidence of Enterocytozoon bieneusi in Japan. J. Vet. Med. Sci. 2009, 71, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Y.; Song, M.; Lu, Y.; Yang, J.; Tao, W.; Jiang, Y.; Wan, Q.; Zhang, S.; Xiao, L. Prevalence and genetic characteristics of Cryptosporidium, Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang province, China. Vet. Parasitol. 2015, 208, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, W.; Yang, Z.; Liu, A.; Zhang, L.; Yang, F.; Wang, R.; Ling, H. Genotyping of Enterocytozoon bieneusi in Farmed Blue Foxes (Alopex lagopus) and Raccoon Dogs (Nyctereutes procyonoides) in China. PLoS ONE. 2015, 10, e0142611. [Google Scholar]
- Yang, Y.; Lin, Y.; Li, Q.; Zhang, S.; Tao, W.; Wan, Q.; Jiang, Y.; Li, W. Widespread presence of human-pathogenic Enterocytozoon bieneusi genotype D in farmed foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in China: First identification and zoonotic concern. Parasitol. Res. 2015, 114, 4341–4348. [Google Scholar] [CrossRef]
- Xu, C.; Ma, X.; Zhang, H.; Zhang, X.X.; Zhao, J.P.; Ba, H.X.; Du, R.; Xing, X.M.; Wang, Q.K.; Zhao, Q. Prevalence, risk factors and molecular characterization of Enterocytozoon bieneusi in raccoon dogs (Nyctereutes procyonoides) in five provinces of Northern China. Acta Trop. 2016, 161, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Qin, J.; Wang, K.; Gu, Y.F. Genotypes of Enterocytozoon bieneusi in Dogs and Cats in Eastern China. Iran J. Parasitol. 2018, 13, 457–465. [Google Scholar] [PubMed]
- Ma, Y.Y.; Ma, Y.T.; Nie, L.B.; Li, T.S.; Peng, J.J.; Cong, W.; Zou, Y.; Zhu, X.Q. Prevalence and genotype distribution of Enterocytozoon bieneusi in farmed raccoon dogs (Nyctereutes procyonoides) in Shandong Province, eastern China. Parasitol. Res. 2020, 119, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Su, Y.; Liang, X.; Sun, X.; Peng, S.; Lu, H.; Jiang, N.; Yin, J.; Xiang, M.; et al. Identification and genotyping of Enterocytozoon bieneusi in China. J. Clin. Microbiol. 2011, 49, 2006–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Jin, Y.; Wu, W.; Li, P.; Wang, L.; Li, N.; Feng, Y.; Xiao, L. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasites Vectors 2016, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Tong, Q.; Zhao, C.; Maimaiti, A.; Chuai, L.; Wang, J.; Ma, D.; Qi, M. Molecular detection and genotyping of Enterocytozoon bieneusi in pet dogs in Xinjiang, Northwestern China. Parasite 2021, 28, 57. [Google Scholar] [CrossRef]
- Lores, B.; Del, A.C.; Arias, C. Enterocytozoon bieneusi (microsporidia) in faecal samples from domestic animals from Galicia, Spain. Mem. Inst. Oswaldo Cruz. 2002, 97, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Galvan-Diaz, A.L.; Magnet, A.; Fenoy, S.; Henriques-Gil, N.; Haro, M.; Gordo, F.P.; Millan, J.; Miro, G.; Del, A.C.; Izquierdo, F. Microsporidia detection and genotyping study of human pathogenic E. bieneusi in animals from Spain. PLoS ONE 2014, 9, e92289. [Google Scholar] [CrossRef] [Green Version]
- Al-Herrawy, A.Z.; Gad, M.A. Microsporidial Spores in Fecal Samples of Some Domesticated Animals Living in Giza, Egypt. Iran J. Parasitol. 2016, 11, 195–203. [Google Scholar]
- Piekarska, J.; Kicia, M.; Wesolowska, M.; Kopacz, Z.; Gorczykowski, M.; Szczepankiewicz, B.; Kvac, M.; Sak, B. Zoonotic microsporidia in dogs and cats in Poland. Vet. Parasitol. 2017, 246, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Santin, M.; Cano, L.; de Lucio, A.; Bailo, B.; de Mingo, M.H.; Koster, P.C.; Fernandez-Basterra, J.A.; Aramburu-Aguirre, J.; Lopez-Molina, N.; et al. Occurrence and genetic diversity of Enterocytozoon bieneusi (Microsporidia) in owned and sheltered dogs and cats in Northern Spain. Parasitol. Res. 2019, 118, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Koehler, A.V.; Wang, T.; Cunliffe, D.; Gasser, R.B. Enterocytozoon bieneusi genotypes in cats and dogs in Victoria, Australia. BMC Microbiol. 2019, 19, 183. [Google Scholar] [CrossRef] [Green Version]
- Lobo, M.L.; Xiao, L.; Cama, V.; Stevens, T.; Antunes, F.; Matos, O. Genotypes of Enterocytozoon bieneusi in mammals in Portugal. J. Eukaryot. Microbiol. 2006, 53 (Suppl. S1), S61–S64. [Google Scholar] [CrossRef] [PubMed]
- Askari, Z.; Mirjalali, H.; Mohebali, M.; Zarei, Z.; Shojaei, S.; Rezaeian, T.; Rezaeian, M. Molecular Detection and Identification of Zoonotic Microsporidia Spore in Fecal Samples of Some Animals with Close-Contact to Human. Iran J. Parasitol. 2015, 10, 381–388. [Google Scholar] [PubMed]
- Phrompraphai, T.; Itoh, N.; Iijima, Y.; Ito, Y.; Kimura, Y. Molecular detection and genotyping of Enterocytozoon bieneusi in family pet dogs obtained from different routes in Japan. Parasitol. Int. 2019, 70, 86–88. [Google Scholar] [CrossRef]
- Liu, H.; Xu, N.; Yin, J.; Yuan, Z.; Shen, Y.; Cao, J. Prevalence and multilocus genotyping of potentially zoonotic Giardia duodenalis in pigs in Shanghai, China. Parasitology 2019, 146, 1199–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ni, H.; Xu, J.; Wang, R.; Li, Y.; Shen, Y.; Cao, J.; Yin, J. Genotyping and zoonotic potential of Cryptosporidium and Enterocytozoon bieneusi in pigs transported across regions in China. Microb. Pathog. 2021, 154, 104823. [Google Scholar] [CrossRef]
- Jamshidi, S.; Tabrizi, A.S.; Bahrami, M.; Momtaz, H. Microsporidia in household dogs and cats in Iran; a zoonotic concern. Vet. Parasitol. 2012, 185, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Zhao, X.; Zhang, L.; Zhang, G.; Guo, M.; Liu, L.; Feng, Y.; Xiao, L. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 2013, 51, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.R.; Wang, R.; He, X.; Zhang, L.; Li, J.; Rume, F.I.; Dong, H.; Qi, M.; Jian, F.; Zhang, S.; et al. Multilocus sequence typing of Enterocytozoon bieneusi in nonhuman primates in China. Vet. Parasitol. 2014, 200, 13–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, L.; Zhao, A.; Xu, C.; Wang, T.; Jing, B.; Qi, M. Molecular detection and genotypes of Enterocytozoon bieneusi in farmed mink (Neovison vison), blue foxes (Alopex lagopus), and raccoon dogs (Nyctereutes procyonoides) in Xinjiang, China. Int. J. Parasitol. Parasites Wildl. 2021, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yin, H.; Zhang, Y.; Li, Z.; Chi, X.; Wang, S. Research progress on co-infection of H9N2 subtype Avian Influenza Virus with Other Pathogens. China Anim. Husb. Vet. Med. 2021, 48, 3447–3455. [Google Scholar]
- Macpherson, C.N. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005, 35, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Szwabe, K.; Blaszkowska, J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann. Agric Environ. Med. 2017, 24, 39–43. [Google Scholar] [CrossRef]
- Arechiga, C.N.; Karunaratna, D.; Aguilar, S.A. Control of canine rabies in developing countries: Key features and animal welfare implications. Rev. Sci. Tech. 2014, 33, 311–321. [Google Scholar] [CrossRef]
- Gong, Q.L.; Ge, G.Y.; Wang, Q.; Tian, T.; Liu, F.; Diao, N.C.; Nie, L.B.; Zong, Y.; Li, J.M.; Shi, K.; et al. Meta-analysis of the prevalence of Echinococcus in dogs in China from 2010 to 2019. PLoS Negl. Trop. Dis. 2021, 15, e0009268. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.; Gibson, A.D.; Shervell, K.; Lohr, F.; Otter, I.; Mellanby, R.J. The problem of stray dogs. Rev. Sci. Tech. 2018, 37, 543–550. [Google Scholar] [CrossRef] [PubMed]
| Infection | Genotypes | No. of Positive |
|---|---|---|
| Known | EbpA | 3 |
| Type IV | 10 | |
| Novel | SHZJD1 | 6 |
| SHZJD2 | 1 | |
| SHZJD3 | 1 | |
| SHZJD4 | 1 | |
| Mixed | HenanV + SHZJD4 | 1 |
| HenanV + Type IV | 1 |
| Genotype | Nucleotide at Position (ITS) | Genbank No. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 137 | 187 | 193 | 199 | 201 | 207 | 219 | 223 | 237 | 239 | 241 | 244 | 245 | 250 | 264 | 268 | 271 | 282 | ||
| Novel | |||||||||||||||||||
| SHZJD1 | A | T | T | T | G | A | T | T | A | G | G | A | A | A | T | A | T | G | OK481086 |
| SHZJD2 | G | C | T | T | G | A | C | G | G | G | G | G | G | A | T | A | G | A | OK481088 |
| SHZJD3 | G | C | T | T | G | A | C | G | G | G | G | G | A | A | C | G | G | A | OK481085 |
| SHZJD4 | G | C | T | T | G | G | C | G | G | G | G | G | A | A | T | A | G | A | OK481084 |
| SHZJD5 | G | C | C | T | G | A | C | G | G | G | G | G | A | A | T | A | G | A | OK481087 |
| Known | |||||||||||||||||||
| EbpA | A | T | T | T | T | A | T | T | G | G | G | G | A | G | T | A | G | A | MN179306 |
| Type IV | G | C | T | T | G | A | C | G | G | G | G | G | A | A | T | A | G | A | AF267141 |
| HenanV | G | C | T | C | G | A | C | G | G | A | A | G | A | A | T | A | G | A | KJ651439 |
| County/Province | Source; Infection Rate | Genotype (No.) | Reference |
|---|---|---|---|
| Colombia | S, 15.0 (18/120) | 1: Type IV (1), WL11 (1); 11: PtEb IX (16) | [17] |
| Poland | H, 4.9 (4/82) | 1: D (2); 11: PtEb IX (2) | [31] |
| Spain | H, 19.2 (14/73) | 1: A (7) | [29] |
| Spain | H, 0.8 (2/237) | 2: BEB6 (1); 11: PtEb IX (1) | [32] |
| Spain | H, 11.8 (2/17) | N/A | [28] |
| Spain | H, 8.7 (4/46) | N/A | [14] |
| Switzerland | F, 8.3 (3/36) | 11: PtEb IX (3) | [15] |
| Australia | H, 4.4 (15/342) | 1: D (5); 3: VIC_dog1 (1); 11: PtEb IX (9) | [33] |
| Iran | S, 5.3 (4/75) | 1: D (4) | [16] |
| Iran | P, 11.8 (2/17) | N/A | [35] |
| Iran | C, 25.8 (8/100) | N/A | [40] |
| Egypt | H, 13 (14/108) | N/A | [30] |
| Japan | P, 5.0 (1/20); S, 1.7 (1/59) | 11: PtEb IX (2) | [18] |
| Japan | P, 4.4 (26/597) | 11: PtEb IX(26) | [36] |
| Korea | U, 43.8 (21/48) | 1: D(8); Korea-WL1(8); Korea-WL1(6); Korea-WL1(1) | [1] |
| China | - | - | - |
| Jilin | P, 7.7 (2/26) | 2: CHN5 (1), CHN6 (1) | [44] |
| Henan | P, 11.7 (23/197); S, 20.5 (31/151) | 1: O (4), D (3), EbpA (2), macaque3 (2), CD1-CD4, EbpC, Peru8, PigEBITS5 and Type IV (1 each); 2: CD6 (1); 7: CD5 (1); 11: PtEb IX (26), CD8 (4), CD7 (2), WW8 (1) | [39] |
| Heilongjiang | P, 7.2 (18/249); S, 0.0 (0/18) | 1: D (1), EbpC (2), NED1 (1), NED2 (1); 11: PtEb IX (14), NED3 (1), NED4 (1) | [19] |
| Shanghai | H, 7.8 (8/102); P, 5.5 (21/383) | 1: D (1); 11: PtEb IX (28) | [22] |
| Anhui | C, 9.3(20/215) | 1: PtEb IX(12),EbpC(4),CHD1(2),CHD2 (2) | [23] |
| Zhejiang | C, 7(7/100) | 1: PtEb IX(4), CHD 3(3) | [19] |
| Shandong | F, 6.5 (23/356) | 1: D(8), Peru8(3), Type IV(11); 2:CHG1(1) | [24] |
| Heilongjiang | F, 4.1 (2/49) | 1: D (1), CHN-R1 (1) | [20] |
| Heilongjiang | F, 10.5 (17/162) | 1: D (14), CHN-DC1 (1), and CHN-DC1/WildBoar3 (1) | [21] |
| Heilongjiang | F, 2.5 (1/40) | CHN-DC1 (1) | [22] |
| Hebei | F, 40.74(22/54) | 1: CHN-DC1 (1), CHN–F1 (3), NCF2 (15), NCR1 (2) | [20] |
| Jilin | F, 30.91 (34/110) | 1: CHN-DC1 (7), CHN–F1 (6), D (5), NCF2 (11), NCR2 (5) | [20] |
| Liaoning | F, 15.28 (11/72) | 1: CHN–F1 (1), D (4), NCF2 (6) | [20] |
| Xinjiang | H, 2.6(1/39) | 1: EbpA(1) | [43] |
| Xinjiang | P, 6.3(38/604) | 11: PtEb IX (19); 1: EbpC (12), D (2), CD9 (1), Type IV (1), CD11 (1), CD12 (1), CD13 (1) | [27] |
| Shanghai | S, 8.8(24/272) | 1: TypeIV(10); EbpA(3); SHZJD1(6); SHZJD2(1); SHZJD3(1); SHZJD5(1); SHZJD4 + HenanV (1); HenanV + Type IV (1) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xu, J.; Shen, Y.; Cao, J.; Yin, J. Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China. Animals 2021, 11, 3571. https://doi.org/10.3390/ani11123571
Liu H, Xu J, Shen Y, Cao J, Yin J. Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China. Animals. 2021; 11(12):3571. https://doi.org/10.3390/ani11123571
Chicago/Turabian StyleLiu, Hua, Jie Xu, Yujuan Shen, Jianping Cao, and Jianhai Yin. 2021. "Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China" Animals 11, no. 12: 3571. https://doi.org/10.3390/ani11123571
APA StyleLiu, H., Xu, J., Shen, Y., Cao, J., & Yin, J. (2021). Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China. Animals, 11(12), 3571. https://doi.org/10.3390/ani11123571








