Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. jubatus) and Giraffe (G. c. reticulata and G. c. tippelskirchii)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Focal Animals
2.2.1. Cheetah (A. jubatus)
2.2.2. Giraffe (G.c. reticulata and G. tippelskirchi)
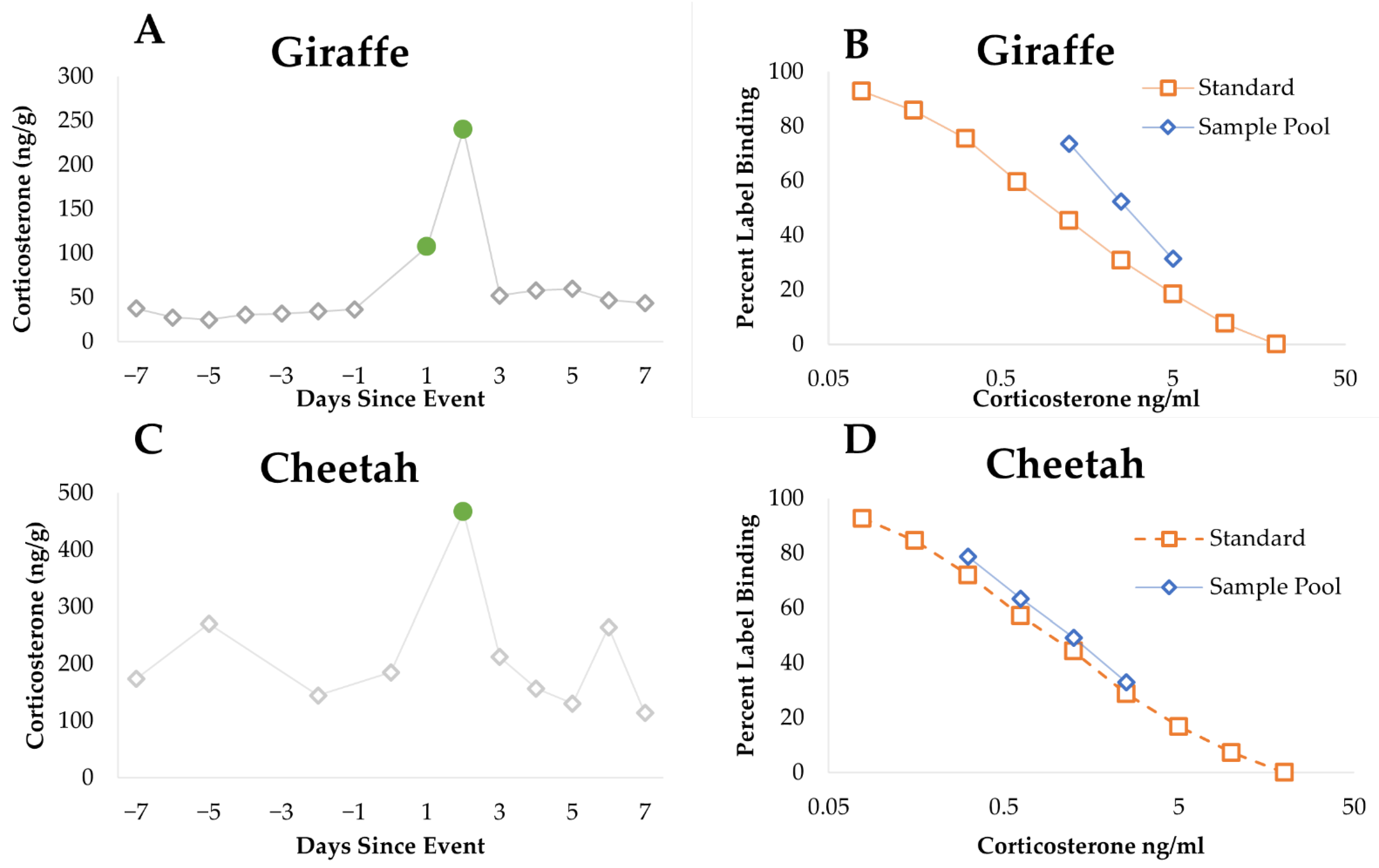
2.3. Endocrinology
2.3.1. Sample Collection
2.3.2. Steroid Extraction
2.3.3. Enzyme Immunoassay (EIA) Analysis
2.3.4. Data Analysis
2.4. Behavior
Data Analysis
3. Results
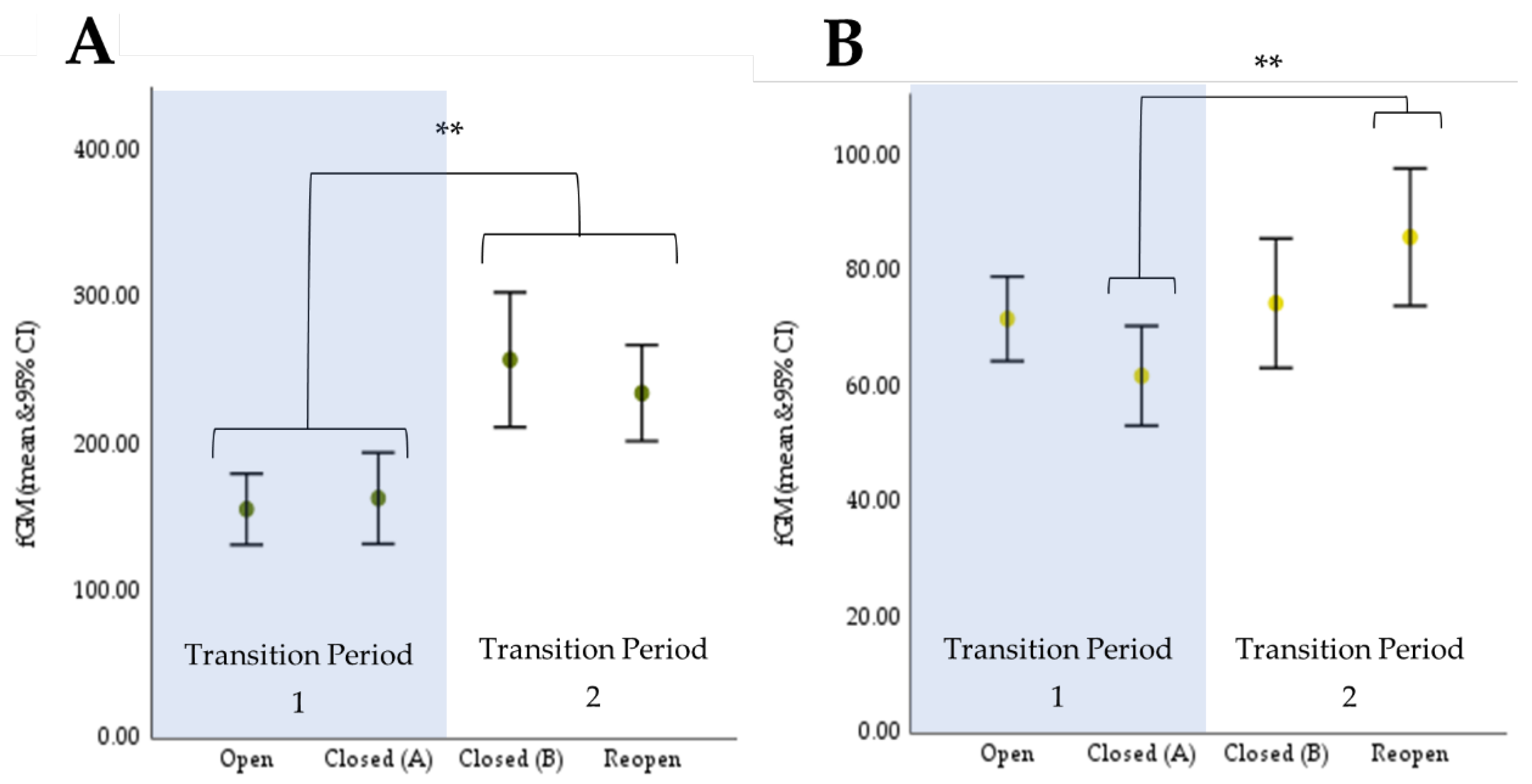
3.1. Endocrinology
3.1.1. Cheetah
3.1.2. Giraffe
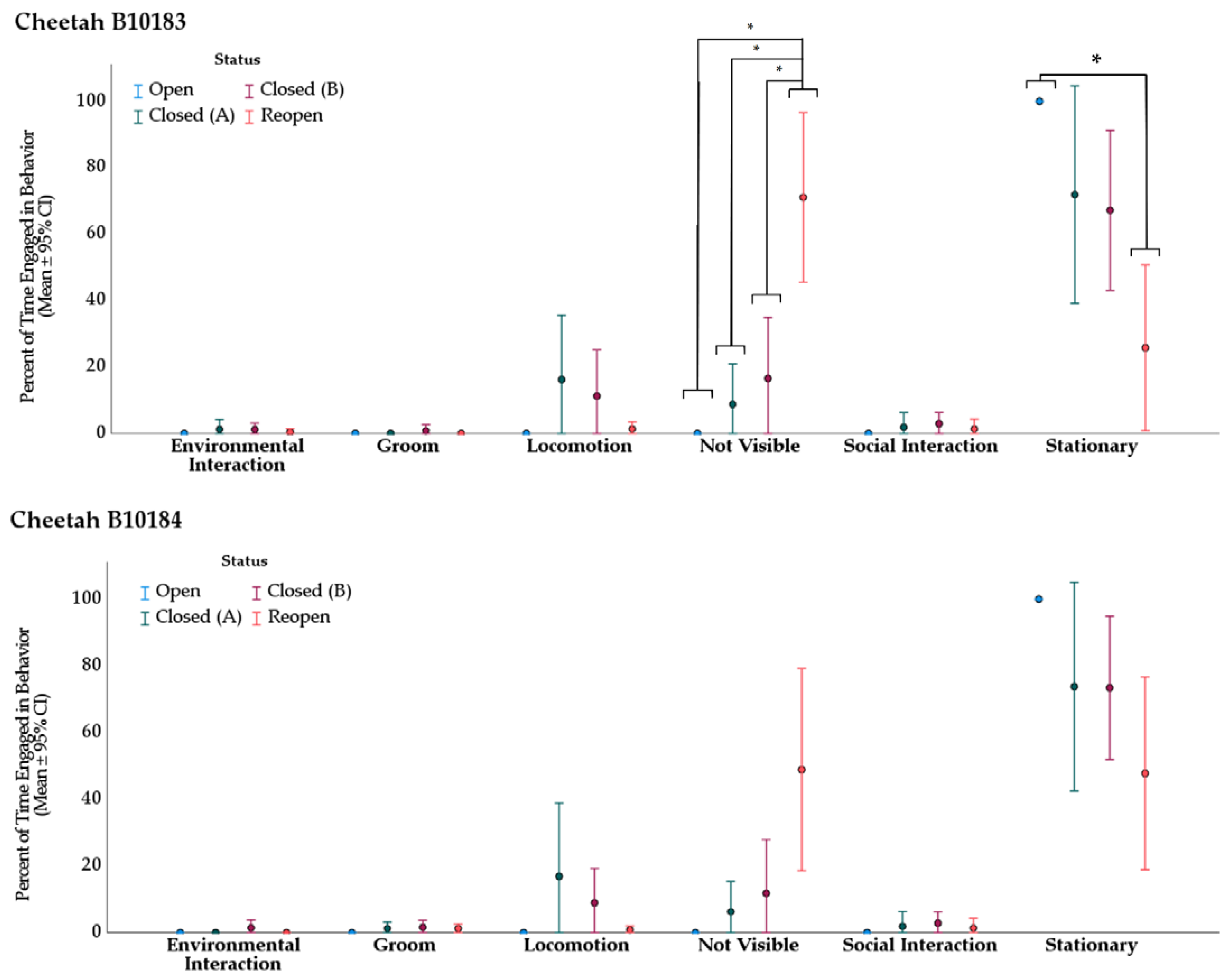
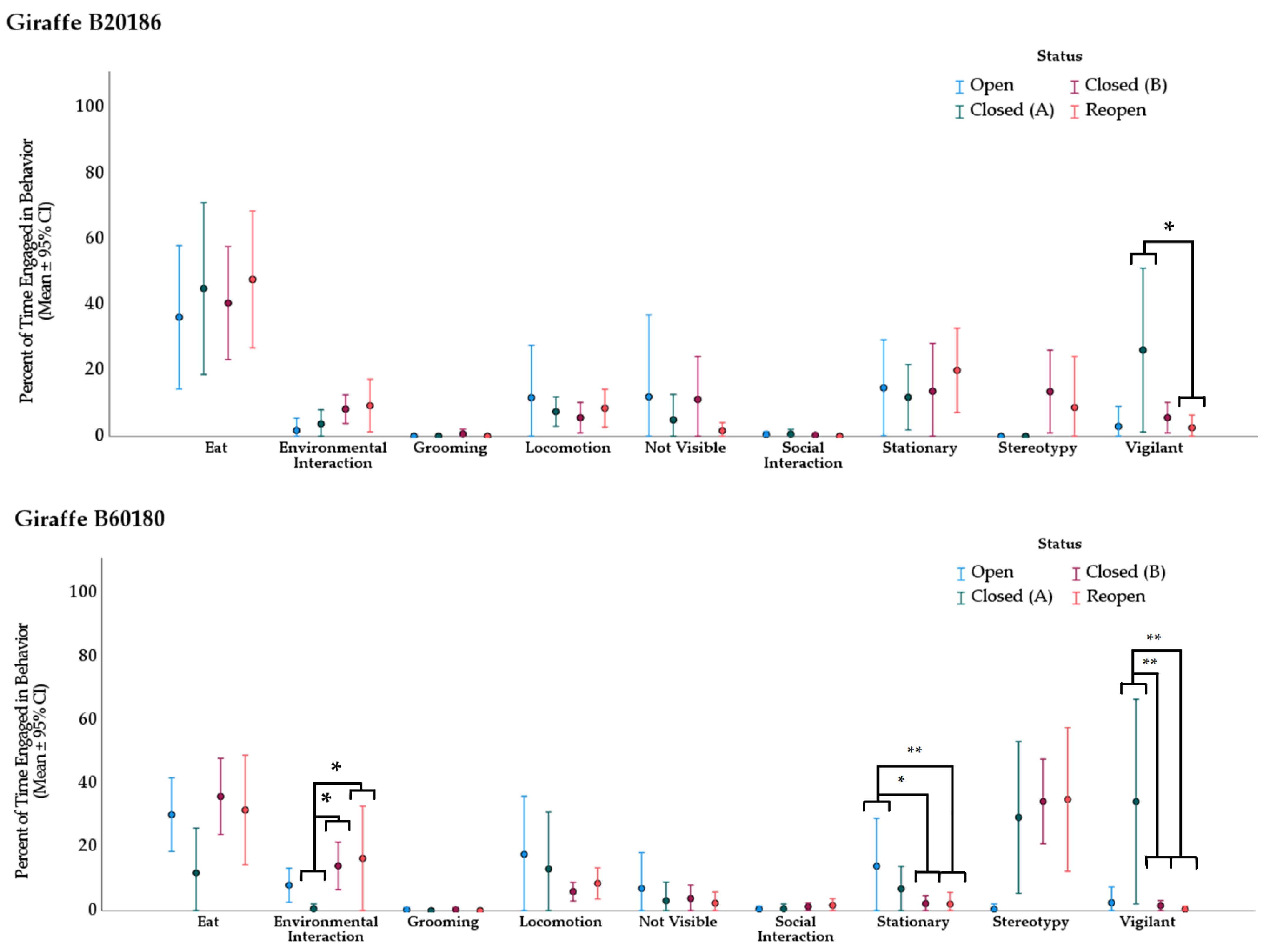
3.2. Behavior
3.2.1. Cheetah
3.2.2. Giraffe
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosey, G.R. Zoo Animals and Their Human Audiences: What Is the Visitor Effect? Anim. Welf. 2000, 9, 343–357. [Google Scholar]
- Choo, Y.; Todd, P.A.; Li, D. Visitor effects on zoo orangutans in two novel, naturalistic enclosures. Appl. Anim. Behav. Sci. 2011, 133, 78–86. [Google Scholar] [CrossRef]
- Larsen, M.J.; Sherwen, S.L.; Rault, J.L. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl. Anim. Behav. Sci. 2014, 154, 76–82. [Google Scholar] [CrossRef]
- Suárez, P.; Recuerda, P.; Arias-De-Reyna, L. Behaviour and welfare: The visitor effect in captive felids. Anim. Welf. 2017, 26, 25–34. [Google Scholar] [CrossRef]
- Davey, G. Visitors’ Effects on the Welfare of Animals in the Zoo: A Review. J. Appl. Anim. Welf. Sci. 2007, 10, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Polgár, Z.; Wood, L.; Haskell, M.J. Individual differences in zoo-housed squirrel monkeys’ (Saimiri sciureus) reactions to visitors, research participation, and personality ratings. Am. J. Primatol. 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Wolf, T.E.; Bennett, N.C.; Burroughs, R.; Ganswindt, A. The impact of age-class and social context on fecal glucocorticoid metabolite levels in free-ranging male giraffes. Gen. Comp. Endocrinol. 2018, 255, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Razal, C.; Bryant, J.; Miller, L. Monitoring the behavioral and adrenal activity of giraffe (Giraffa camelopardalis) to assess welfare during seasonal housing changes. Anim. Behav. Cogn. 2017, 4, 154–164. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Young, R.J. Housing. In Environmental Enrichment for Captive Animals; Blackwell Science Ltd.: Oxford, UK, 2003; pp. 122–141. ISBN 0-632-06407-2. [Google Scholar]
- Farrand, A. The Effect of Zoo Visitors on the Behaviour and Welfare of Zoo Mammals; University of Stirling: Stirling, UK, 2007. [Google Scholar]
- Robbins, L.; Margulis, S.W. The effects of auditory enrichment on gorillas. Zoo Biol. 2014, 33, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Hardy, A.R.; Barber, J.R.; Fristrup, K.M.; Crooks, K.R.; Angeloni, L.M. The effect of human activities and their associated noise on ungulate behavior. PLoS ONE 2012, 7, e40505. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.; Passos, L.; Vecci, M.A.M.; Young, R.J. Zoo visitor effect on mammal behaviour: Does noise matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Mallapur, A.; Sinha, A.; Waran, N. Influence of visitor presence on the behaviour of captive lion-tailed macaques (Macaca silenus) housed in Indian zoos. Appl. Anim. Behav. Sci. 2005, 94, 341–352. [Google Scholar] [CrossRef]
- Chiew, S.J.; Butler, K.L.; Fanson, K.V.; Eyre, S.; Coleman, G.J.; Sherwen, S.L.; Melfi, V.; Hemsworth, P.H. Effects of the presence of zoo visitors on zoo-housed little penguins (Eudyptula minor). N. Z. J. Zool. 2021, 1–22. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Halloran, D.J.; Shanahan, F. The stress response and the hypothalamic-pituitary-adrenal axis: From molecule to melancholia. QJM-Mon. J. Assoc. Physicians 2000, 93, 323–333. [Google Scholar] [CrossRef]
- Romero, L.M.; Reed, J.M. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol. Mol. Integr. Physiol. 2005, 140, 73–79. [Google Scholar] [CrossRef]
- Stead, S.K.; Meltzer, D.G.A.; Palme, R. The measurement of glucocorticoid concentrations in the serum and faeces of captive African elephants (Loxodonta africana) after ACTH stimulation: Research communication. J. S. Afr. Vet. Assoc. 2012, 71, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, M.J.; Krebs, C.J.; Boonstra, R. Assessing stress in animal populations: Do fecal and plasma glucocorticoids tell the same story? Gen. Comp. Endocrinol. 2010, 166, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring Fecal Glucocorticoid Metabolites in Mammals and Birds: The Importance of Validation. Ann. N. Y. Acad. Sci 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wielebnowski, N.; Watters, J. Applying Fecal Endocrine Monitoring to Conservation and Behavior Studies of Wild Mammals: Important Considerations and Preliminary Tests. Isr. J. Ecol. Evol. 2007, 53, 439–460. [Google Scholar] [CrossRef]
- Wielebnowski, N. Stress and distress: Evaluating their impact for the well-being of zoo animals. J. Am. Vet. Med. Assoc. 2003, 223, 973–977. [Google Scholar] [CrossRef]
- The Oregon Zoo. Oregon Zoo Foundation 2010–2011 Gratitude Report. Available online: https://www.oregonzoo.org/about/about-oregon-zoo (accessed on 1 January 2021).
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Understanding impacts of zoo visitors: Quantifying behavioural changes of two popular zoo species during COVID-19 closures. Appl. Anim. Behav. Sci. 2021, 236, 1–8. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Impacts of COVID-19 on Animals in Zoos: A Longitudinal Multi-Species Analysis. J. Zool. Bot. Gard. 2021, 2, 130–145. [Google Scholar] [CrossRef]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.C.; Soltis, J.; Leeds, A. Evaluating the Effect of Visitor Presence on Nile Crocodile (Crocodylus niloticus) Behavior. J. Zool. Bot. Gard. 2021, 2, 115–129. [Google Scholar] [CrossRef]
- Wright, R. Some Zoos, and Some of Their Animals, May Not Survive the Pandemic. Available online: https://www.newyorker.com/news/our-columnists/some-zoos-and-some-of-their-animals-may-not-survive-the-pandemic (accessed on 1 January 2021).
- Frank, B.J. Phoenix Zoo Working to Combat Loneliness Felt by Animals. Available online: https://www.azcentral.com/story/news/local/phoenix/2020/04/04/phoenix-zoo-working-combat-loneliness-felt-animals-during-coronavirus/2948477001/ (accessed on 19 February 2021).
- Normando, S.; Pollastri, I.; Florio, D.; Ferrante, L.; Macchi, E.; Isaja, V.; de Mori, B. Assessing animal welfare in animal-visitor interactions in zoos and other facilities. A pilot study involving giraffes. Animals 2018, 8, 153. [Google Scholar] [CrossRef]
- Donovan, D.O.; Hindle, J.E.; Mckeown, S.; Donovan, O. Effect of visitors on the behaviour of female Cheetahs and cubs. Int. Zoo Yearb. 1993, 32, 238–244. [Google Scholar] [CrossRef]
- Orban, D.A.; Siegford, J.M.; Snider, R.J. Effects of guest feeding programs on captive giraffe behavior. Zoo Biol. 2016, 35, 157–166. [Google Scholar] [CrossRef]
- Quirke, T.; O’riordan, R.M.; Zuur, A. Factors influencing the prevalence of stereotypical behaviour in captive cheetahs (Acinonyx jubatus). Appl. Anim. Behav. Sci. 2012, 142, 189–197. [Google Scholar] [CrossRef]
- Fanson, K.V.; Lynch, M.; Vogelnest, L.; Miller, G.; Keeley, T. Response to long-distance relocation in Asian elephants (Elephas maximus): Monitoring adrenocortical activity via serum, urine, and feces. Eur. J. Wildl. Res. 2013, 59, 655–664. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of Stress during Handling and Transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Volfová, M.; Machovcová, Z.; Schwarzenberger, F.; Voslářová, E.; Bedáňová, I.; Večerek, V. The effects of transport stress on the behaviour and adrenocortical activity of the black-and-white ruffed lemur (Varecia variegata). Acta Vet. Brno 2019, 88, 85–92. [Google Scholar] [CrossRef]
- Fazio, J.M.; Freeman, E.W.; Bauer, E.; Rockwood, L.; Brown, J.L.; Hope, K.; Siegal-Willott, J.; Parsons, E.C.M. Longitudinal fecal hormone monitoring of adrenocortical function in zoo housed fishing cats (Prionailurus viverrinus) during institutional transfers and breeding introductions. PLoS ONE 2020, 15, e0230239. [Google Scholar] [CrossRef] [PubMed]
- Loeding, E.; Thomas, J.; Bernier, D.; Santymire, R. Using Fecal Hormonal and Behavioral Analyses to Evaluate the Introduction of Two Sable Antelope at Lincoln Park Zoo. J. Appl. Anim. Welf. Sci. 2011, 14, 220–246. [Google Scholar] [CrossRef]
- Rothschild, D.M.; Serfass, T.L.; Seddon, W.L.; Hegde, L.; Fritz, R.S. Using Fecal Glucocorticoids to Assess Stress Levels in Captive River Otters. J. Wildl. Manag. 2008, 72, 138–142. [Google Scholar] [CrossRef]
- Bryant, J.; Wielebnowski, N.C. Environmental impact on activity level and fecal glucocorticoid metabolite concentration of African elephants and black rhinoceros at brookfield zoo. Int. Int. J. Avian Wildl. Biol. 2018, 3, 101–107. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Sicks, F.; Palme, R.; Schwarzenberger, F.; Tordiffe, A.S.W.; Ganswindt, A. Non-invasive assessment of adrenocortical activity as a measure of stress in giraffe (Giraffa camelopardalis). BMC Vet. Res. 2016, 12, 235. [Google Scholar] [CrossRef]
- Uetake, K.; Une, Y.; Ito, S.; Yamabe, M.; Toyoda, H.; Tanaka, T. Relationship of climatic conditions to fecal corticosterone levels of captive cheetahs reared in Japan. Anim. Sci. J. 2014, 85, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Wielebnowski, N. (Oregon Zoo, OR, USA) Oregon Zoo General Monitoring Ethogram—Cheetah. Unpublished work. 2020. [Google Scholar]
- Lewis, K.; Wielebnowski, N. (Oregon Zoo, OR, USA) Oregon Zoo General Monitoring Ethogram—Giraffe. Unpublished work. 2020. [Google Scholar]
- Wark, J.D.; Wierzal, N.K.; Cronin, K.A. Gaps in Live Inter-Observer Reliability Testing of Animal Behavior: A Retrospective Analysis and Path Forward. J. Zool. Bot. Gard. 2021, 2, 207–221. [Google Scholar] [CrossRef]
- Cameron, E.Z.; du Toit, J.T. Social influences on vigilance behaviour in giraffes, Giraffa camelopardalis. Anim. Behav. 2005, 69, 1337–1344. [Google Scholar] [CrossRef]
- Vonderen, I.K.; Kooistra, H.S.; Rijnberk, A. Influence of Veterinary Care on the Urinary Corticoid: Creatinine Ratio in Dogs. J. Vet. Intern. Med. 1998, 12, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Baird, B.A. Ambassador Animal Welfare: Using Behavioral and Physiological Indicators to Assess the Well-Being of Animals Used for Education Programs in Zoos; Case Western Reserve University: Cleveland, HI, USA, 2018. [Google Scholar]
- Rajagopal, T.; Archunan, G.; Sekar, M. Impact of Zoo Visitors on the Fecal Cortisol Levels and Behavior of an Endangered Species: Indian Blackbuck (Antelope cervicapra L.). J. Appl. Anim. Welf. Sci. 2011, 14, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Zwijacz-Kozica, T.; Selva, N.; Barja, I.; Silván, G.; Martínez-Fernández, L.; Illera, J.C.; Jodłowski, M. Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol. (Warsz.) 2012, 58, 215–222. [Google Scholar] [CrossRef]
- Cizauskas, C.A.; Turner, W.C.; Pitts, N.; Getz, W.M. Seasonal Patterns of Hormones, Macroparasites, and Microparasites in Wild African Ungulates: The Interplay among Stress, Reproduction, and Disease. PLoS ONE 2015, 10, e0120800. [Google Scholar] [CrossRef]
- Average Weather in Portland, Oregon, United States, Year Round-Weather Spark. Available online: https://weatherspark.com/y/757/Average-Weather-in-Portland-Oregon-United-States-Year-Round (accessed on 6 July 2021).
- Conte, C. Do Visitors Affect Zebra Behavior in Zoos? Biol. Sci. 2014, 3, 1–30. [Google Scholar]
- Scheijen, C.P.J.; van der Merwe, S.; Ganswindt, A.; Deacon, F. Anthropogenic influences on distance traveled and vigilance behavior and stress-related endocrine correlates in free-roaming giraffes. Animals 2021, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B.L. Zoo Giraffe Welfare: A Literature Review and the Behavioral Effects of Guest Feeding Programs; University of California Davis: Davis, CA, USA, 2018. [Google Scholar]
- USA National Phenology Network Historical Annual Spring Indices (2016-Previous Year), First Leaf-Spring Index. 2020. Region 49.9375, -66.4791667, 24.0625, -125.0208333 2017. Available online: https://data.usanpn.org/geoserver-request-builder?service=wms&layer=si-x:average_leaf_ncep_historic&year=2020&format=image/jpeg&projection=4269&width=1700&height=800&state_border=1 (accessed on 31 October 2021).
- Martin, P.; Bateson, P. Measuring Behavior: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-53563-2. [Google Scholar]
- Ross, S.R.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Appl. Anim. Behav. Sci. 2009, 121, 42–50. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Harvey, T.J.; Magrath, M.J.L.; Butler, K.L.; Fanson, K.V.; Hemsworth, P.H. Effects of visual contact with zoo visitors on black-capped capuchin welfare. Appl. Anim. Behav. Sci. 2015, 167, 65–73. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Magrath, M.J.L.; Butler, K.L.; Hemsworth, P.H. Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors. Appl. Anim. Behav. Sci. 2015, 168, 71–76. [Google Scholar] [CrossRef]
- Queiroz, M.B.; Young, R.J. The different physical and behavioural characteristics of zoo mammals that influence their response to visitors. Animals 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, B.; Broekhuis, F. Living on the edge: Multiscale habitat selection by cheetahs in a human-wildlife landscape. Ecol. Evol. 2018, 8, 7611–7623. [Google Scholar] [CrossRef] [PubMed]
- Terio, K.A.; Marker, L.; Munson, L. Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J. Wildl. Dis. 2004, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
| Cheetah | ||||
|---|---|---|---|---|
| Category | Sample Dates | Sample Count (B10183) | Sample Count (B10184) | |
| Transition Period 1 | Open | 2/20/2020–3/15/2020 | 15 | 15 |
| Closed (A) | 3/16/2020–4/4/2020 | 15 | 15 | |
| Transition Period 2 | Closed (B) | 6/16/2020–7/12/2020 | 15 | 15 |
| Reopen | 7/13/2020–8/7/2020 | 15 | 15 | |
| Giraffe | ||||
| Category | Sample Dates | Sample Count (B20186) | Sample Count (B60180) | |
| Transition Period 1 | Open | 2/13/2020–3/15/2020 | 15 | 15 |
| Closed (A) | 3/16/2020–4/12/2020 | 15 | 15 | |
| Transition Period 2 | Closed (B) | 6/9/2020–7/12/2020 | 15 | 15 |
| Reopen | 7/13/2020–8/8/2020 | 15 | 15 | |
| All-Occurrence Behavior | Description |
|---|---|
| charge glass | Charge towards glass ending within one body length of the glass; may or may not include a strike or hiss |
| glass strike | Forceful paw contact with glass |
| hiss | Lips pulled back to bare teeth and emit sound |
| Interval Behaviors | Description |
| not visible | Individual out of sight or unable to determine behavior at interval |
| keeper visible | Keeper is present—can be actively interacting with focal animal or just walking past |
| environmental interaction | Individual is actively engaged with an element of its environment; does not include interaction with zoo visitors or inactive contact with environment (e.g., laying down on rocks) or incidental contact with exhibit furniture |
| stereotypy | Locomotor stereotypy: walking from one point to another, turning and walking back to the starting point, or walking in a loop/to-and-fro, for more than three repetitions without interruption. |
| social interaction | Any active social interaction with another cheetah, regardless of who instigated it |
| locomotion | Any movement that transports the animal more than one body length forward, backward, or sideways at any speed; includes walk, trot, run or jump |
| groom | Focal animal is engaged in self-grooming, licking, chewing, scratching (self) |
| stationary | Not deliberately exhibiting locomotion behaviors for at least three seconds; can be alert (head up, eyes open) and resting (head down or head up with closed eyes) |
| All-Occurrence Behavior | Description |
|---|---|
| urine testing | Using flehmen reaction specifically at urine |
| flehmen | Upper lip curled back and inhalation |
| interaction with Speke’s gazelle | Any interaction between giraffes and resident Speke’s gazelle |
| interaction with hornbills | Any interactions between giraffes and resident hornbills |
| run | Cantering or sprinting |
| lay down | Any instance when the giraffe has its stomach on the ground |
| Interval Behaviors | Description |
| not visible | Individual out of sight or unable to determine behavior at interval |
| keeper visible | Keeper is present—can be actively interacting with focal animal or just walking past |
| eat | Individual is actively eating from designated food stations or keeper-provided browse elements |
| environmental interaction | Individual is actively engaged with an element of its environment; does not include interaction with zoo visitors or inactive contact with the environment (e.g., laying down on rocks) or incidental contact with exhibit furniture. |
| stereotypy | Locomotor stereotypy: walking from one point to another, turning and walking back to the starting point, or walking in a loop/to-and-fro, for more than three repetitions without interruption; non-locomotor stereotypy: repetitive licking/tongue flagging |
| social interaction | Any active social interaction with another giraffe, regardless of who instigated it |
| locomotion | Any movement that transports the animal more than one body length forward, backward, or sideways at any speed; includes walk, trot, run or jump |
| groom | Focal animal is engaged in self-grooming, licking, chewing, scratching (self) |
| vigilant | Standing still with an erect neck and actively observing (rather than scanning) the environment (similar to that defined by Cameron and du Toit [49]) |
| stationary | Not deliberately exhibiting locomotion behaviors for at least three seconds; can be alert (head up, eyes open) and resting (head down or head up with closed eyes) |
| Cheetah | ||||
|---|---|---|---|---|
| Category | Sample Dates | Observation Count (B10183) | Observation Count (B10184) | |
| Transition Period 1 | Open * | 2/11/2020–3/15/2020 | 3 | 2 |
| Closed (A) | 3/16/2020–4/4/2020 A | 8 | 8 | |
| Transition Period 2 | Closed (B) | 6/16/2020–7/12/2020 A | 14 | 14 |
| Reopen | 7/13/2020–8/8/2020 | 13 | 13 | |
| Giraffe | ||||
| Category | Sample Dates | Observation Count (B20186) | Observation Count (B60180) | |
| Transition Period 1 | Open * | 1/18/2020–3/15/2020 | 4 | 4 |
| Closed (A) | 3/16/2020–4/12/2020 A | 8 | 8 | |
| Transition Period 2 | Closed (B) | 6/9/2020–7/12/2020 A | 16 | 16 |
| Reopen | 7/13/2020–8/8/2020 | 12 | 12 | |
| (I) Trial | (II) Trial | Mean Difference (A-B) (ng/g) | Sig a | |
|---|---|---|---|---|
| Cheetah | ||||
| Transition Period 1 | Open | Closed (A) | −6.06 | 1.00 |
| Closed (B) * | −87.46 * | 0.008 * | ||
| Reopen * | −79.63 * | <0.001 * | ||
| Closed (A) | Open | 6.06 | 1.00 | |
| Closed (B) * Reopen * | −81.40 * −73.56 * | <0.001 *0.002 * | ||
| Transition Period 2 | Closed (B) | Open * | 87.46 * | 0.008 * |
| Closed (A) * | 81.40 * | <0.001 * | ||
| Reopen | 7.83 | 1.00 | ||
| Reopen | Open * | 79.63 * | <0.001 * | |
| Closed (A) * | 73.56 * | 0.002 * | ||
| Closed (B) | −7.83 | 1.00 | ||
| Giraffe | ||||
| Transition Period 1 | Open | Closed (A) | 11.13 | 0.053 |
| Closed (B) | 0.00 | 1.000 | ||
| Reopen | −11.57 | 1.000 | ||
| Closed (A) | Open | −11.13 | 0.053 | |
| Closed (B) | −11.13 | 0.262 | ||
| Reopen | −22.70 * | 0.016 * | ||
| Transition Period 2 | Closed (B) | Open | 0.00 | 1.000 |
| Closed (A) | 11.13 | 0.262 | ||
| Reopen | −11.57 | 1.000 | ||
| Reopen | Open | 11.57 | 1.000 | |
| Closed (A) | 22.70 * | 0.016 * | ||
| Closed (B) | 11.57 | 1.000 | ||
| Cheetah B10183 | ||||||
|---|---|---|---|---|---|---|
| Behavior | Treatment (I) | Treatment (II) | Mean Rank (I) | Mean Rank (II) | Direction of Change | Adj. p-Value * |
| not visible | Open | Reopen | 10.00 | 28.27 | ↑ | 0.034 |
| Closed (A) | Reopen | 15.25 | 28.27 | ↑ | 0.030 | |
| Closed (B) | Reopen | 15.82 | 28.27 | ↑ | 0.010 | |
| stationary | Open | Reopen | 30.50 | 12.31 | ↓ | 0.043 |
| Giraffe B20186 | ||||||
|---|---|---|---|---|---|---|
| Behavior | Treatment (I) | Treatment (II) | Mean Rank (I) | Mean Rank (II) | Direction of Change | Adj. p-Value * |
| vigilant | Closed (A) | Reopen | 29.63 | 15.79 | ↓ | 0.039 |
| Giraffe B60180 | ||||||
| environmental interaction | Closed (A) | Closed (B) | 8.81 | 23.97 | ↑ | 0.013 |
| Closed (A) | Reopen | 8.81 | 23.42 | ↑ | 0.030 | |
| stationary | Open | Closed (B) | 34.88 | 17.81 | ↓ | 0.016 |
| Open | Reopen | 34.88 | 16.29 | ↓ | 0.009 | |
| vigilant | Closed (A) | Closed (B) | 32.81 | 17.59 | ↓ | 0.003 |
| Closed (A) | Reopen | 32.81 | 14.67 | ↓ | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, L.B.; Scarlata, C.D.; VanBeek, B.; Bodner, T.E.; Wielebnowski, N.C. Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. jubatus) and Giraffe (G. c. reticulata and G. c. tippelskirchii). Animals 2021, 11, 3526. https://doi.org/10.3390/ani11123526
Fink LB, Scarlata CD, VanBeek B, Bodner TE, Wielebnowski NC. Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. jubatus) and Giraffe (G. c. reticulata and G. c. tippelskirchii). Animals. 2021; 11(12):3526. https://doi.org/10.3390/ani11123526
Chicago/Turabian StyleFink, Laurel B., Candace D. Scarlata, Becca VanBeek, Todd E. Bodner, and Nadja C. Wielebnowski. 2021. "Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. jubatus) and Giraffe (G. c. reticulata and G. c. tippelskirchii)" Animals 11, no. 12: 3526. https://doi.org/10.3390/ani11123526
APA StyleFink, L. B., Scarlata, C. D., VanBeek, B., Bodner, T. E., & Wielebnowski, N. C. (2021). Applying Behavioral and Physiological Measures to Assess the Relative Impact of the Prolonged COVID-19 Pandemic Closure on Two Mammal Species at the Oregon Zoo: Cheetah (A. jubatus) and Giraffe (G. c. reticulata and G. c. tippelskirchii). Animals, 11(12), 3526. https://doi.org/10.3390/ani11123526





