Variation in Bird Eggs—Does Female Factor, Season, and Laying Order Impact the Egg Size, Pigmentation, and Eggshell Thickness of the Eggs of Capercaillie?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flock Maintenance Conditions
2.2. Egg Evaluation
2.3. Samples Selection
2.4. Statistical Analyze
2.5. Ethical Note
3. Results
3.1. The Effect of Laying Order on Capercaillie Egg Characteristics
3.2. Variation of the Egg Characteristics in Subsequent Seasons
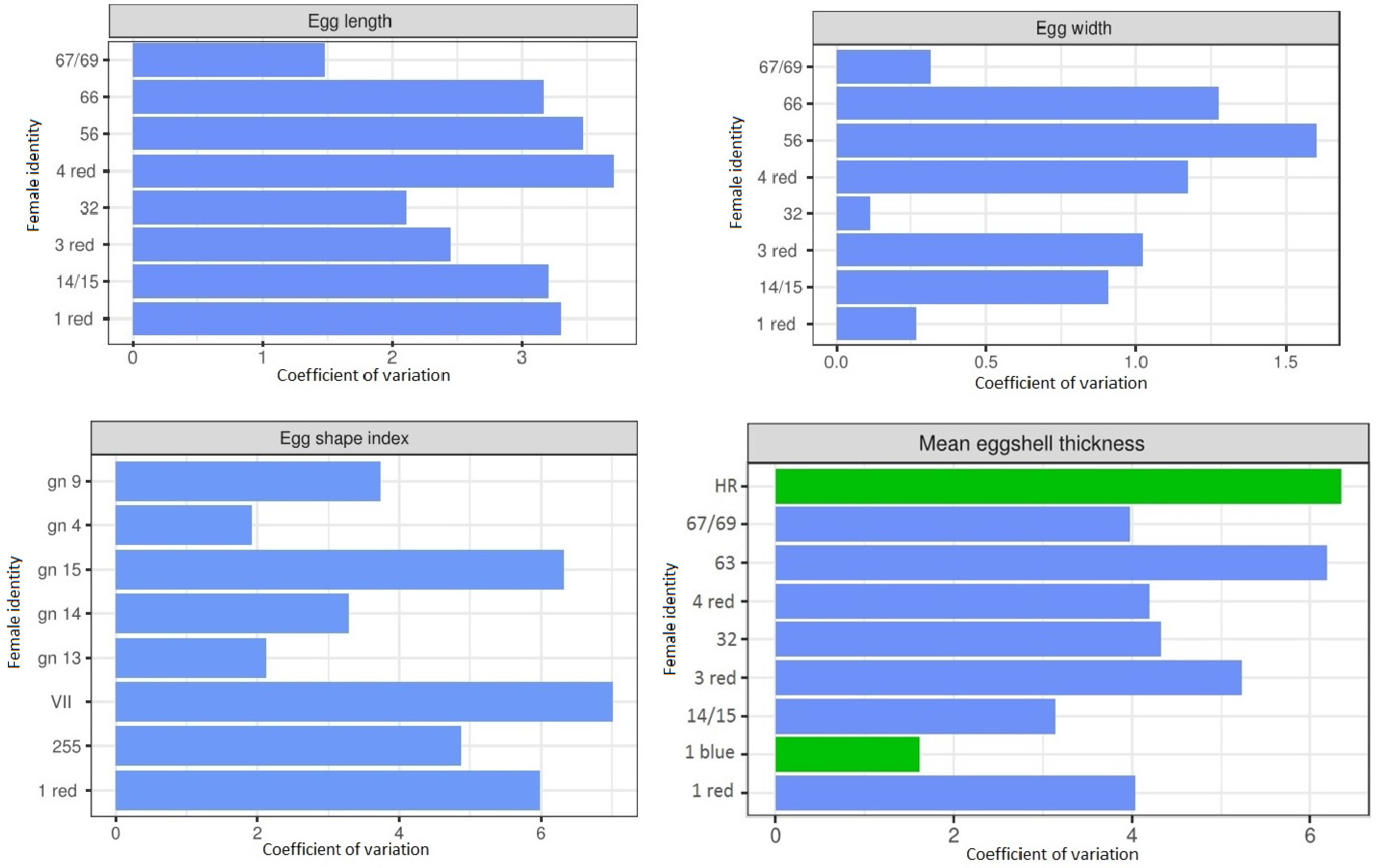
3.3. Eggshells Characteristics of Individual Females in Subsequent Seasons
3.4. Eggshell Characteristics from Different Breeding Centers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kilner, R.M. The Evolution of Egg Colour and Patterning in Birds. Biol. Rev. 2006, 81, 383. [Google Scholar] [CrossRef]
- Stoddard, M.C.; Yong, E.H.; Akkaynak, D.; Sheard, C.; Tobias, J.A.; Mahadevan, L. Avian Egg Shape: Form, Function, and Evolution. Science 2017, 356, 1249–1254. [Google Scholar] [CrossRef]
- Prager, E.; Wilson, A.; Arnheim, N. Widespread Distribution of Lysozyme g in Egg White of Birds. J. Biol. Chem. 1974, 249, 7295–7297. [Google Scholar] [CrossRef]
- Dauphin, Y.; Luquet, G.; Perez-Huerta, A.; Salomé, M. Biomineralization in Modern Avian Calcified Eggshells: Similarity versus Diversity. Connect. Tissue Res. 2018, 59, 67–73. [Google Scholar] [CrossRef]
- Kusuda, S.; Iwasawa, A.; Doi, O.; Ohya, Y.; Yoshizaki, N. Diversity of the Cuticle Layer of Avian Eggshells. J. Poult. Sci. 2011, 48, 119–124. [Google Scholar] [CrossRef]
- Cassey, P.; Thomas, G.H.; Portugal, S.J.; Maurer, G.; Hauber, M.E.; Grim, T.; Lovell, P.G.; Mikšík, I. Why Are Birds’ Eggs Colourful? Eggshell Pigments Co-Vary with Life-History and Nesting Ecology among British Breeding Non-Passerine Birds. Biol. J. Linn. Soc. 2012, 106, 657–672. [Google Scholar] [CrossRef]
- Krist, M. Egg Size and Offspring Quality: A Meta-Analysis in Birds. Biol. Rev. 2011, 86, 692–716. [Google Scholar] [CrossRef] [PubMed]
- Brooker, M.G.; Brooker, L.C. Eggshell Strength in Cuckoos and Cowbirds. IBIS 2008, 133, 406–413. [Google Scholar] [CrossRef]
- Soler, M.; Rodríguez-Navarro, A.B.; Pérez-Contreras, T.; García-Ruiz, J.M.; Soler, J.J. Great Spotted Cuckoo Eggshell Microstructure Characteristics Can Make Eggs Stronger. J. Avian Biol. 2019, 50, jav.02252. [Google Scholar] [CrossRef]
- Igic, B.; Braganza, K.; Hyland, M.M.; Silyn-Roberts, H.; Cassey, P.; Grim, T.; Rutila, J.; Moskát, C.; Hauber, M.E. Alternative Mechanisms of Increased Eggshell Hardness of Avian Brood Parasites Relative to Host Species. J. R. Soc. Interface 2011, 8, 1654–1664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solis, J.C.; de Lope, F. Nest and Egg Crypsis in the Ground-Nesting Stone Curlew Burhinus oedicnemus. J. Avian Biol. 1995, 26, 135. [Google Scholar] [CrossRef]
- Castilla, A.M.; Dhondt, A.A.; Díaz-Uriarte, R.; Westmoreland, D. Predation in Ground-Nesting Birds: An experimental Study Using Natural Egg Color Variation. Avian Conserv. Ecol. 2007, 2, 3412. [Google Scholar] [CrossRef][Green Version]
- Blanco, G.; Bertellotti, M. Differential Predation by Mammals and Birds: Implications for Egg-Colour Polymorphism in a Nomadic Breeding Seabird. Biol. J. Linn. Soc. 2002, 75, 137–146. [Google Scholar] [CrossRef]
- Birkhead, T.R. Behavioural Adaptations to High Density Nesting in the Common Guillemot Uria aalge. Anim. Behav. 1978, 26, 321–331. [Google Scholar] [CrossRef]
- Cherry, M.I.; Bennett, A.T.D.; Moskát, C. Host Intra-Clutch Variation, Cuckoo Egg Matching and Egg Rejection by Great Reed Warblers. Naturwissenschaften 2007, 94, 441–447. [Google Scholar] [CrossRef] [PubMed]
- López-de-Hierro, M.D.G.; Moreno-Rueda, G. Egg-Spot Pattern Rather than Egg Colour Affects Conspecific Egg Rejection in the House Sparrow (Passer domesticus). Behav. Ecol. Sociobiol. 2010, 64, 317–324. [Google Scholar] [CrossRef]
- Birkhead, T.R.; Thompson, J.E.; Jackson, D.; Biggins, J.D. The Point of a Guillemot’s Egg. IBIS 2017, 159, 255–265. [Google Scholar] [CrossRef]
- Birkhead, T.R.; Thompson, J.E.; Montgomerie, R. The Pyriform Egg of the Common Murre (Uria aalge) Is More Stable on Sloping Surfaces. Auk 2018, 135, 1020–1032. [Google Scholar] [CrossRef]
- Massaro, M.; Davis, L.S. The Influence of Laying Date and Maternal Age on Eggshell Thickness and Pore Density in Yellow-Eyed Penguins. Condor 2004, 106, 496–505. [Google Scholar] [CrossRef]
- Coulson, J.C. Egg Size and Shape in the Kittiwake (Rissa tridactyla) and Thair Use in Estimating Age Composition of Populations. Proc. Zool. Soc. Lond. 2009, 140, 211–226. [Google Scholar] [CrossRef]
- Chylarecki, P.; Kuczynski, L.; Vogrin, M.; Tryjanowski, P. Geographic Variation in Egg Measurements of the Lapwing (Vanellus vanellus). Acta Ornithol. 1997, 2, 137–148. [Google Scholar]
- Morales, J.; Ruuskanen, S.; Laaksonen, T.; Eeva, T.; Mateo, R.; Belskii, E.; Ivankina, E.V.; Järvinen, A.; Kerimov, A.; Korpimäki, E.; et al. Variation in Eggshell Traits between Geographically Distant Populations of Pied Flycatchers Ficedula hypoleuca. J. Avian Biol. 2013, 44, 111–120. [Google Scholar] [CrossRef]
- Avilés, J.M.; Stokke, B.G.; Moksnes, A.; Røskaft, E.; Møller, A.P. Environmental Conditions Influence Egg Color of Reed Warblers Acrocephalus scirpaceus and Their Parasite, the Common Cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 2006, 61, 475–485. [Google Scholar] [CrossRef]
- Lahti, D.C. Population Differentiation and Rapid Evolution of Egg Color in Accordance with Solar Radiation. Auk 2008, 125, 796–802. [Google Scholar] [CrossRef]
- Huo, J.; Su, T.; Niu, N.; Yang, C.; Liang, W. Last but Not the Least: Effects of Laying Sequence on Egg Color Variation and Embryonic Development of Russet Sparrow (Passer cinnamomeus). Avian Res. 2018, 9, 21. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Merino, S.; Moreno, J.; Tomás, G.; Morales, J.; Lobato, E.; García-Fraile, S.; Martínez, J. Are Eggshell Spottiness and Colour Indicators of Health and Condition in Blue Tits Cyanistes caeruleus? J. Avian Biol. 2007, 38, 377–384. [Google Scholar] [CrossRef]
- Wendeln, H. Allocation of Parental Duties and Foraging Behavior Influence Body Condition of Adult Common Terns, Sterna hirundo. Bird Behav. 1997, 12, 47–54. [Google Scholar] [CrossRef]
- Rabsztyn, A.; Andres, K.; Dudek, M. Variability, Heritability and Correlations of Egg Shape in the Zatorska Goose. J. Cent. Eur. Agric. 2010, 11, 433–436. [Google Scholar] [CrossRef]
- Rosenberger, J.; Łukaszewicz, E.; Kowalczyk, A.; Rzońca, Z. Capercaillie (Tetrao urogallus) Eggshell Pigmentation, Maculation and Thickness. Ornis. Fenn. 2018, 95, 160–170. [Google Scholar]
- Romanoff, A.L.; Romanoff, A.J. (Eds.) The Avian Egg; Wiley: New York, NY, USA, 1949. [Google Scholar]
- Birkhead, T.R.; Nettleship, D.N. Egg Size, Composition and Offspring Quality in Some Alcidae (Aves: Charadriiformes). J. Zool. 2009, 202, 177–194. [Google Scholar] [CrossRef]
- Kendeigh, S.C.; Hamerstrom, F. Variations in Egg Characteristics of the House Wren. Auk 1956, 73, 42–65. [Google Scholar] [CrossRef]
- Preston, F.W.; Preston, E.J. Variation of the Shapes of Birds’ Eggs within the Clutch. Ann. Carnegie Mus. 1953, 11, 139. [Google Scholar]
- Gemperle, M.; Preston, F.W. Variation of Shape in the Eggs of the Common Tern in the Clutch-Sequence. Auk 1955, 72, 184–198. [Google Scholar] [CrossRef]
- Cabezas-Diaz, S.A.R.A.; Virgos, E.; Villafuerte, R. Reproductive performance changes with age and laying experience in the Red-legged Partridge Alectoris rufa. IBIS 2005, 147, 316–323. [Google Scholar] [CrossRef]
- Amat, J.A.; Fraga, R.M.; Arroyo, G.M. Intraclutch egg size variation and offspring survival in the Kentish Plover Charadrius alexandrinus. IBIS 2001, 143, 17–27. [Google Scholar] [CrossRef]
- Svagelj, W.S.; Quintana, F. Egg-size variation in the Imperial Cormorant: On the importance of individual effects. Condor 2011, 113, 528–537. [Google Scholar] [CrossRef]
- Rosenberger, J.; Kowalczyk, A.; Łukaszewicz, E.; Strzała, T. Female-Male and Female-Female Social Interactions of Captive Kept Capercaillie (Tetraoc urogallus) and Its Consequences in Planning Breeding Programs. Animals 2020, 10, 583. [Google Scholar] [CrossRef]
- Dolenec, Z. Relationship between laying order and egg dimensions in the Blackcap Sylvia atricapilla. Acta Ornithol. 2004, 39, 176–179. [Google Scholar] [CrossRef]
- Summers, R.W.; Green, R.E.; Proctor, R.; Dugan, D.; Lambie, D.; Moncrieff, R.; Moss, R.; Baines, D. An Experimental Study of the Effects of Predation on the Breeding Productivity of Capercaillie and Black Grouse. J. Appl. Ecol. 2004, 41, 513–525. [Google Scholar] [CrossRef]
- Montevecchi, W.A. Egg Size and the Egg Predatory Behaviour of Crows. Behavior 1976, 57, 307–320. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Eggshell Structure, Measurements, and Quality-Affecting Factors in Laying Hens: A Review. Czech J. Anim. Sci. 2016, 299–309. [Google Scholar] [CrossRef]
- Christians, J.K. Avian egg size: Variation within species and inflexibility within individuals. Biol. Rev. 2002, 77, 1–26. [Google Scholar] [CrossRef] [PubMed]
- de Juana, E. Family Tetraonidae (Grouse). In Handbook of the Birds of the World Vol 2, 1st ed.; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1994. [Google Scholar]
- Clements, J.; Schulenberg, T.; Iliff, M.; Roberson, D.; Fredericks, T.; Sullivan, B.; Wood, C. The EBird/Clements Checklist of Birds of the World: V2019. 2019. Available online: http://www.Birds.Cornell.Edu/Clementschecklist/Download/ (accessed on 5 October 2021).
- Rodríguez-Muñoz, R.; Mirol, P.M.; Segelbacher, G.; Fernández, A.; Tregenza, T. Genetic Differentiation of an Endangered Capercaillie (Tetrao urogallus) Population at the Southern Edge of the Species Range. Conserv. Genet. 2007, 8, 659–670. [Google Scholar] [CrossRef]
- Gotzman, J.; Jabloński, B.; Desselberger, J. Gniazda Naszych Ptaków, 1st ed.; Państwowe Zakłady Wydawnictw Szkolnych: Warszawa, Poland, 1972. [Google Scholar]
- Buła, E. Materiały do rozmieszczenia i biologii głuszca (Tetrao urogallus) w województwie wrocławskim. Prz. Zool. 1969, 13, 212–223. [Google Scholar]
- Potapov, R.; Flint, V. Handbuch der Vögel der Sowjetunion. Galliformes, Gruiformes, 1st ed.; Dmitrievich, I., Potapov, R., Eds.; Aula: Wittenberg, Germany, 1989. [Google Scholar]
- Storch, I.; Segelbacher, G. Two Grouse Clutches in the Same Nest: Evidence for Nest Site Adoption in Capercaillie (Tetrao urogallus). J. Ornithol. 2005, 146, 85–88. [Google Scholar] [CrossRef]
| Female ID | Season/Year | |||
|---|---|---|---|---|
| L * in 2018 | L * in 2019 | L * in 2020 | p-Value | |
| 6 red | 69.768 (n = 4) | 67.043 (n = 5) | 72.1 (n = 3) | 0.9832 (K-W) |
| 2 green | * | 72.944 (n = 3) | 73.8 (n = 7) | 0.296 (T) |
| * | 1.19 | 2.471 | ||
| 8 blue | * | 69.63 (n = 4) | 69.735 (n = 9) | 0.467 (T) |
| * | 1.758 | 2.084 | ||
| 25 green | 68.439 (n = 5) | * | 66.5 (n = 3) | 0.157 (T) |
| 2.19 | * | 2.798 | ||
| 56 | 71.062 (n = 5) | 72.639 (n = 7) | * | 0.053 (T) |
| 1.202 | 1.691 | * | ||
| 73 | * | 67.692 (n = 3) | 67.86 (n = 5) | 0.449 (T) |
| * | 2.189 | 1.434 | ||
| 60 | * | 64.31(n = 3) | 67.701 (n = 5) | 0.058 (T) |
| * | 2.796 | 2.382 | ||
| 23 | 74.236 (n = 4) | * | 70.938 (n = 8) | 0.07 (T) |
| 2.579 | * | 3.631 | ||
| Measured Trait | Average | SD | Min | Max |
|---|---|---|---|---|
| Egg length (mm) | WF 55.421 | WF 2.749 | WF 49.330 | WF 61.260 |
| LF 56.766 | LF 2.892 | LF 44.660 | LF 62.230 | |
| Egg width (mm) | WF 41.286 | WF 1.059 | WF 38.790 | WF 44.300 |
| LF 41.281 | LF 0.960 | LF 36.430 | LF 43.130 | |
| Egg shape | WF 1.342 | WF 0.049 | WF 1.255 | WF 1.444 |
| LF 1.375 | LF 0.068 | LF 1.226 | LF 1.536 | |
| Eggshell lightness | WF 70.364 | WF 4.357 | WF 57.573 | WF 79.760 |
| LF 70.166 | LF 4.302 | LF 54.523 | LF 78.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenbeger, J.; Pytlak, K.; Łukaszewicz, E.; Kowalczyk, A. Variation in Bird Eggs—Does Female Factor, Season, and Laying Order Impact the Egg Size, Pigmentation, and Eggshell Thickness of the Eggs of Capercaillie? Animals 2021, 11, 3454. https://doi.org/10.3390/ani11123454
Rosenbeger J, Pytlak K, Łukaszewicz E, Kowalczyk A. Variation in Bird Eggs—Does Female Factor, Season, and Laying Order Impact the Egg Size, Pigmentation, and Eggshell Thickness of the Eggs of Capercaillie? Animals. 2021; 11(12):3454. https://doi.org/10.3390/ani11123454
Chicago/Turabian StyleRosenbeger, Joanna, Kamil Pytlak, Ewa Łukaszewicz, and Artur Kowalczyk. 2021. "Variation in Bird Eggs—Does Female Factor, Season, and Laying Order Impact the Egg Size, Pigmentation, and Eggshell Thickness of the Eggs of Capercaillie?" Animals 11, no. 12: 3454. https://doi.org/10.3390/ani11123454
APA StyleRosenbeger, J., Pytlak, K., Łukaszewicz, E., & Kowalczyk, A. (2021). Variation in Bird Eggs—Does Female Factor, Season, and Laying Order Impact the Egg Size, Pigmentation, and Eggshell Thickness of the Eggs of Capercaillie? Animals, 11(12), 3454. https://doi.org/10.3390/ani11123454






