Welfare of Farmed Crocodilians: Identification of Potential Animal-Based Measures Using Elicitation of Expert Opinion
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Crocodilian Farming Standards
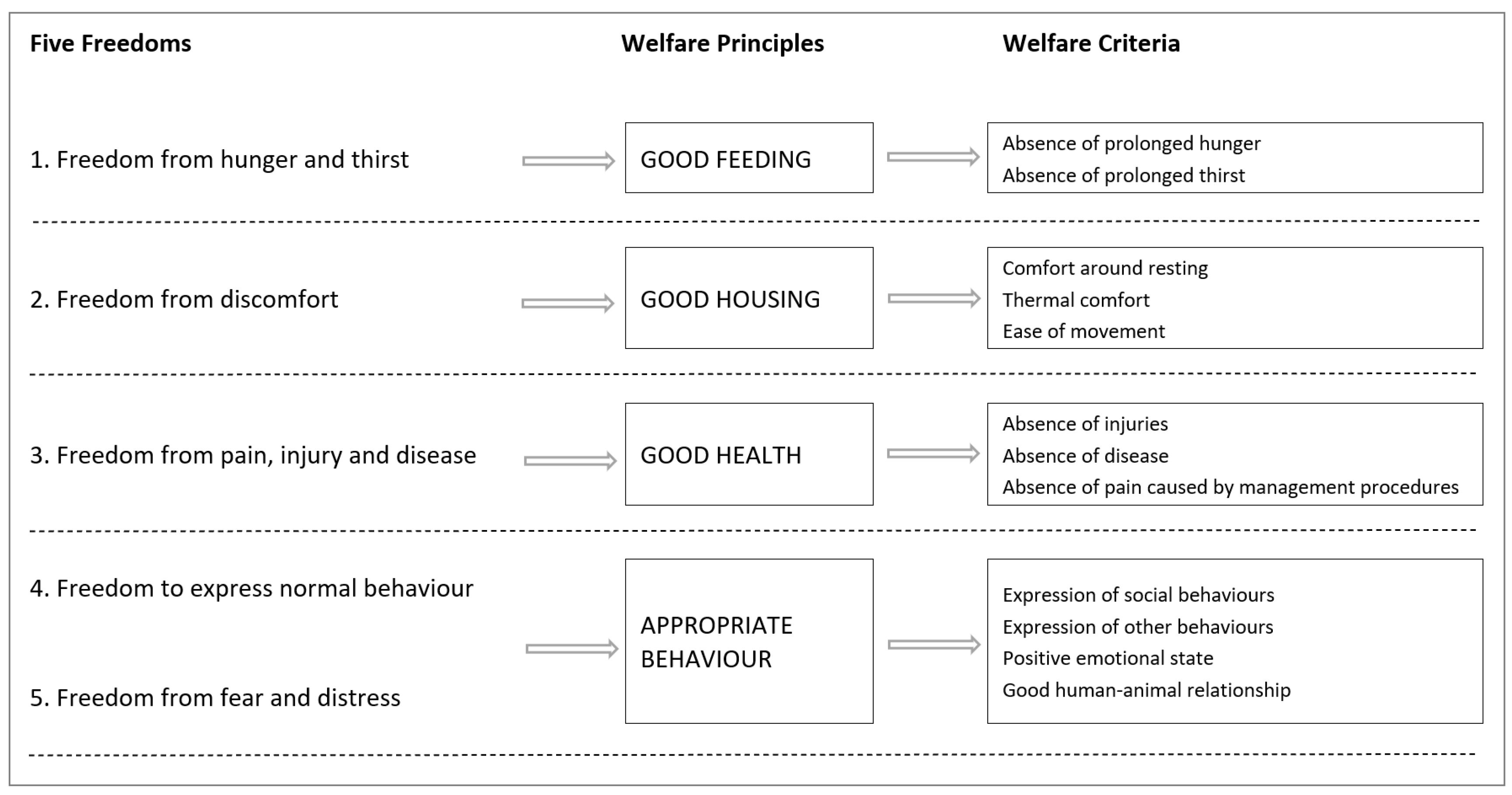
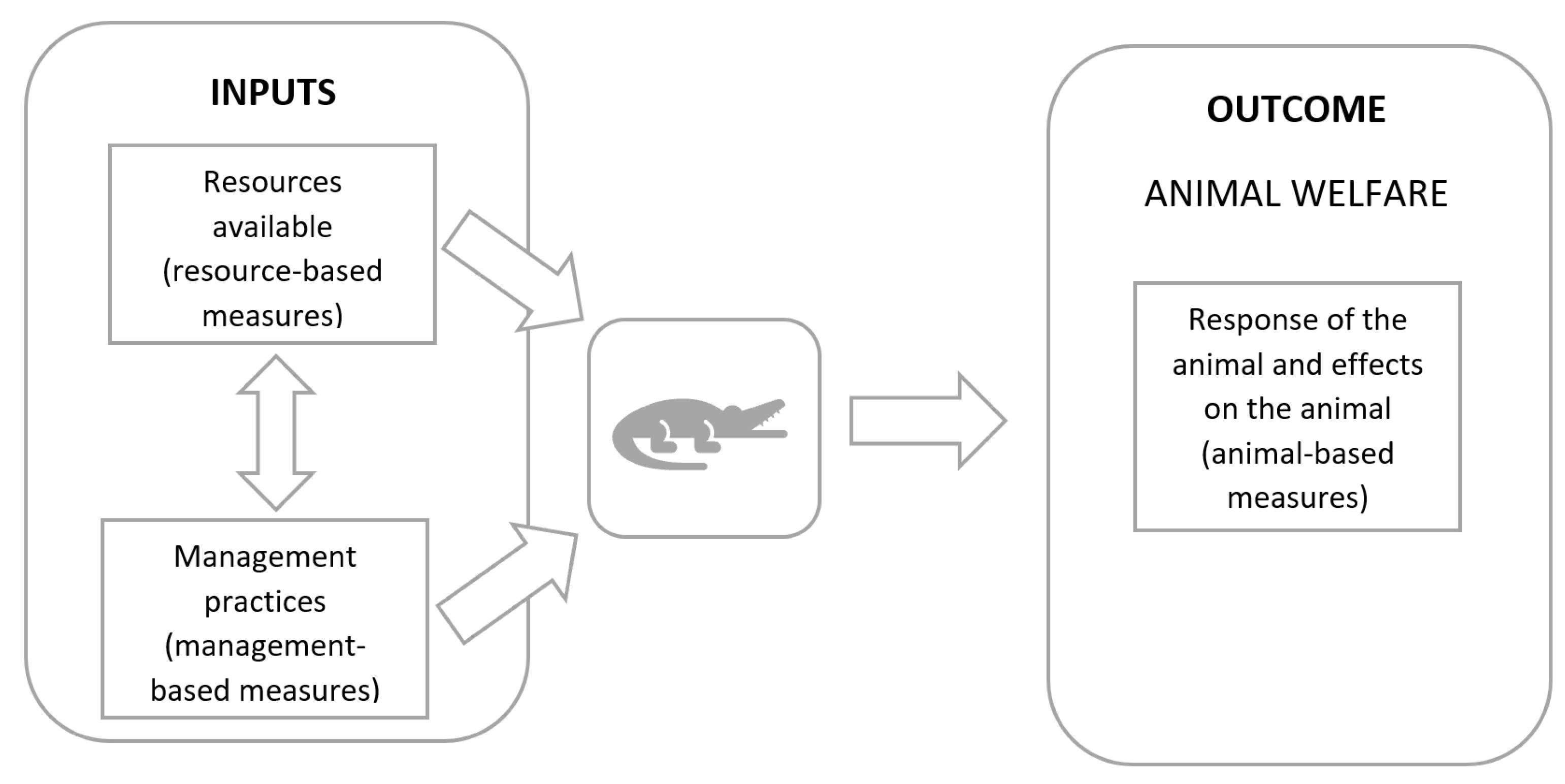
1.2. Animal Welfare Assessment
2. Materials and Methods
2.1. Preparation of Potential Measures
2.2. Elicitation of Expert Opinion
2.3. Analysis of the Data
3. Results
3.1. Response to the Exercise
3.2. Judgement and Scoring of Proposed Animal-Based Measures
4. Discussion
4.1. General Findings
4.2. Good Feeding
4.2.1. Absence of Prolonged Hunger
4.2.2. Absence of Prolonged Thirst
4.3. Good Housing
4.3.1. Physical Comfort around Resting
4.3.2. Thermal Comfort
4.3.3. Ease of Movement
4.4. Good Health
4.4.1. Absence of Injuries
4.4.2. Absence of Disease
4.4.3. Absence of Pain Induced by Management Procedures
4.5. Appropriate Behaviour
4.5.1. Expression of Social Behaviours
4.5.2. Expression of Other Behaviours
4.5.3. Positive Emotional State
4.5.4. Good Human–Animal Relationships
4.6. Toolbox of Animal Welfare Outcome Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICFA 1001 International Standard for Crocodilian Farming—Requirements. Available online: https://www.internationalcrocodilian.com/standard-development/ (accessed on 8 July 2021).
- International Organization for Standardization. ISO/IEC, 17007:2009, Conformity Assessment—Guidance for Drafting Normative Documents Suitable for Use For Conformity Assessment; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- World Organisation for Animal Health. Chapter 7.14 Killing of Reptiles For for Their Skins, Meat and Other Products; OIE Terrestrial Animal Health Code; Rue de Prony: Paris, France, 2019. [Google Scholar]
- Elsey, R.M.; Joanen, T.; McNease, L.; Lance, V. Growth rate and plasma corticosterone levels in juvenile alligators maintained at different stocking densities. J. Exp. Zool. 1990, 255, 30–36. [Google Scholar] [CrossRef]
- Finger, J.W.; Thomson, P.C.J.; Isberg, S.R. Unexpected lower testosterone in faster growing farmed saltwater crocodile (Crocodylus porosus) hatchlings. Gen. Comp. Endocrinol. 2016, 226, 1–4. [Google Scholar] [CrossRef]
- Finger, J.W.; Thomson, P.C.; Adams, A.L.; Benedict, S.; Moran, C.; Isberg, S.R. Reference levels for corticosterone and immune function in farmed saltwater crocodiles (Crocodylus porosus) hatchlings using current Code of Practice guidelines. Gen. Comp. Endocrinol. 2015, 212, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Benn, A.L.; McLelland, D.J.; Whittaker, A.L. A Review of Welfare Assessment Methods in Reptiles, and Preliminary Application of the Welfare Quality((R)) Protocol to the Pygmy Blue-Tongue Skink, Tiliqua adelaidensis, Using Animal-Based Measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.; Diez-Leon, M.; Mason, G. Animal Welfare Science: Recent Publication Trends and Future Research Priorities. Int. J. Comp. Psychol. 2014, 27, 80–100. [Google Scholar] [CrossRef]
- Pond, W.G.; Bazer, F.W.; Rollin, B.E. Animal Welfare in Animal Agriculture: Husbandry, Stewardship, and Sustainability in Animal Production; Taylor & Francis Group: Abingdon, VA, USA, 2011. [Google Scholar]
- Veissier, I.; Boissy, A. Stress and welfare: Two complementary concepts that are intrinsically related to the animal’s point of view. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Botreau, R.; Veissier, I.; Butterworth, A.; Bracke, M.B.; Keeling, L.J. Definition of criteria for overall assessment of animal welfare. Anim. Welf. 2007, 16, 225–228. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J. Extending the ′Five Domains′ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Mellor, D.J. Positive animal welfare states and reference standards for welfare assessment. N. Z. Vet. J. 2014, 63, 17–23. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW). Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar]
- Blokhuis, H.J.; Veissier, I.; Miele, M.; Jones, B.C. The Welfare Quality® project and beyond: Safeguarding farm animal well-being. Acta Agric. Scand. Sect. A—Anim. Sci. 2010, 60, 129–140. [Google Scholar] [CrossRef]
- Brambell, F. Report on the Technical Committee to Enquire into the Welfare of Livestock Kept under Intensive Conditions; Her Majesty’s Stationary Office: London, UK, 1965. [Google Scholar]
- Improving Farm Animal Welfare; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013.
- Welfare Quality Assessment Protocol for Cattle. Available online: http://www.welfarequalitynetwork.net (accessed on 15 November 2020).
- Costa, E.D.; Murray, L.; Dai, F.; Canali, E.; Minero, M. Equine on-farm welfare assessment: A review of animal-based indicators. Anim. Welf. 2014, 23, 323–341. [Google Scholar] [CrossRef]
- Costa, E.D.; Dai, F.; Lebelt, D.; Scholz, P.; Barbieri, S.; Canali, E.; Zanella, A.; Minero, M. Welfare assessment of horses: The AWIN approach. Anim. Welf. 2016, 25, 481–488. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Borger-Turner, J.L.; Eskelinen, H.C. C-Well: The development of a welfare assessment index for captive bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2015, 24, 267–282. [Google Scholar] [CrossRef]
- Mononen, J.; Møller, S.H.; Hansen, S.; Hovland, A.; Koistinen, T.; Lidfors, L.; Malmkvist, J.; Vinke, C.; Ahola, L. The development of on-farm welfare assessment protocols for foxes and mink: The WelFur project. Anim. Welf. 2012, 21, 363–371. [Google Scholar] [CrossRef]
- Polgar, Z.; Blackwell, E.J.; Rooney, N.J. Assessing the welfare of kenelled dogs—A review of animal-based measures. Appl. Anim. Behav. Sci. 2019, 213, 1–13. [Google Scholar] [CrossRef]
- Heath, C.; Lin, Y.-C.; Mullan, S.; Browne, W.; Main, D. Implementing Welfare Quality® in UK assurance schemes: Evaluating the challenges. Anim. Welf. 2014, 23, 95–107. [Google Scholar] [CrossRef]
- Five Freedoms. Available online: http://www.fawc.org.uk/freedoms.htm (accessed on 15 June 2020).
- EFSA. Guidance on Risk Assessment for Animal Welfare. EFSA J. 2012, 10, 2513. [Google Scholar] [CrossRef] [Green Version]
- Lambert, H.; Carder, G.; D’Cruze, N. Given the cold shoulder: A review of the scientific literature for evidence of reptil sentience. Animals 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA J. 2014, 12, 3734. [Google Scholar]
- Whaytt, H.R.; Main, D.C.J.; Greent, L.E.; Webster, A.J.F. Animal-based measures for the assessment of welfare state of diary cattle, pigs and laying hens: Consensus of expert opinion. Anim. Welf. 2003, 12, 205–217. [Google Scholar]
- Shilton, C.; Brown, G.P.; Chambers, L.; Benedict, S.; Davis, S.; Aumann, S.; Isberg, S. Pathology of runting in farmed saltwater crocodiles (Crocodylus porosus) in Australia. Vet. Pathol. 2014, 51, 1022–1034. [Google Scholar] [CrossRef] [Green Version]
- Ganswindt, S.B.; Myburgh, J.G.; Cameron, E.Z.; Ganswindt, A. Non-invasive assessment of adrenocortical function in captive Nile crocodiles (Crocodylus niloticus). Comp. Biochem. Physiol. A Mol. Integr Physiol. 2014, 177, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Webb, G.J.; Hollis, G.J.; Manolis, S.C. Feeding, Growth, and Food Conversion Rates of Wild Juvenile Saltwater Crocodiles (Crocodylus porosus). J. Herpetol. 1991, 25, 462–473. [Google Scholar] [CrossRef]
- Dunston-Clarke, E.J.; Willis, R.S.; Fleming, P.A.; Barnes, A.L.; Miller, D.W.; Collins, T. Developing an Animal Welfare Assessment Protocol for Livestock Transported by Sea. Animals 2020, 10, 705. [Google Scholar]
- Ojeda-Adame, R.A.; Hernández-Hurtado, H.; Ramírez-Martinez, M.M.; Iñiguez-Davalos, L.I. A Body Condition Score for Crocodilians. S. Am. J. Herpetol. 2020, 16, 10–15. [Google Scholar] [CrossRef]
- Nell, L.A.; Frederick, P.C.; Mazzotti, F.J.; Vliet, K.A.; Brandt, L.A. Presence of Breeding Birds Improves Body Condition for a Crocodilian Nest Protector. PLoS ONE 2016, 11, e0149572. [Google Scholar] [CrossRef] [PubMed]
- Somaweera, R.; Rhind, D.; Reynolds, S.; Eisemberg, C.; Sonneman, T.; Woods, D. Observations of mammalian feeding by Australian freshwater crocodiles (Crocodylus johnstoni) in the Kimberley region of Western Australia. Rec. West. Aust. Mus. 2018, 33, 103–107. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Terrestrial Animal Health Code; OIE Terrestrial Animal Health Code; Rue de Prony: Paris, France, 2019. [Google Scholar]
- Auer, U.; Kelemen, Z.; Engl, V.; Jenner, F. Activity Time Budgets-A Potential Tool to Monitor Equine Welfare? Animals 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Lang, J. Thermophilic Response of the American Alligator and the American Crocodile to Feeding. Copeia 1979, 1979, 48–59. [Google Scholar] [CrossRef]
- Price, E.R.; Sirsat, T.S.; Sirsat, S.K.; Kang, G.; Keereetaweep, J.; Aziz, M.; Chapman, K.D.; Dzialowski, E.M. Thermal acclimation in American alligators: Effects of temperature regime on growth rate, mitochondrial function, and membrane composition. J. Biol. 2017, 68, 45–54. [Google Scholar] [CrossRef]
- Kanui, T.; Mwendia, C.; Aulie, A.; Wanyoike, M. Effects of temperature on growth, food uptake and retention time of juvenile nile crocodiles (Crocodylus niloticus). Comp. Biochem. Physiol. Part A Physiol. 1991, 99, 453–456. [Google Scholar] [CrossRef]
- Friedrich, L.; Krieter, J.; Kemper, N.; Czycholl, I. Interobserver reliability of measures of the Welfare Quality® animal welfare assessment protocol for sows and piglets. Anim. Welf. 2020, 29, 323–337. [Google Scholar] [CrossRef]
- Biology and Evolution of Crocodylians; CSIRO Publishing: Clayton, Australia, 2015; p. 670.
- Roberts, K.; Hemmings, A.J.; McBride, S.D.; Parker, M.O. Causal factors of oral versus locomotor stereotypy in the horse. J. Vet. Behav. 2017, 20, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Clubb, R.; Vickery, S. Locomotory Stereotypies in Carnivores: Does Pacing Stem from Hunting, Ranging or Frustrated Escape? In Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare, 2nd ed.; CABI Wallingford: Oxfordshire, UK, 2006; pp. 58–85. [Google Scholar]
- Isberg, S.R.; Shilton, C.M. Stress in Farmed Saltwater Crocodiles (Crocodylus porosus): No Difference between Individually- and Communally-Housed Animals. SpringerPlus 2013, 2, 381. [Google Scholar] [CrossRef] [Green Version]
- Broom, D. Welfare of Animals: Behavior as a Basis for Decisions. In Encyclopedia of Animal Behavior; Breed, M.D., Moore, J., Eds.; Academic Press: Oxford, UK, 2010; Volume 3, pp. 580–584. [Google Scholar]
- Brien, M.L.; Webb, G.J.; McGuinness, K.; Christian, K.A. The relationship between early growth and survival of hatchling saltwater crocodiles (Crocodylus porosus) in captivity. PLoS ONE 2014, 9, e100276. [Google Scholar] [CrossRef]
- Brien, M.L.; Lang, J.W.; Webb, G.J.; Stevenson, C.; Christian, K. The good, the bad, and the ugly: Agonistic behaviour in juvenile crocodilians. PLoS ONE 2013, 8, e80872. [Google Scholar]
- Kelly, M.L.; Peters, R.; Tisdale, R.K.; Lesku, J.A. Unihemispheric sleep in crocodilians? J. Exp. Biol. 2015, 218, 3175–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foris, B.; Zebunke, M.; Langbein, J.; Melzer, N. Comprehensive analysis of affiliative and agonistic social networks in lactating dairy cattle groups. Appl. Anim. Behav. Sci. 2018, 210, 60–67. [Google Scholar] [CrossRef]
- Goumon, S.; Illmann, G.; Leszkowová, I.; Dostalová, A.; Cantor, M. Dyadic affiliative preferences in a stable group of domestic pigs. Appl. Anim. Behav. Sci. 2020, 230, 105045. [Google Scholar] [CrossRef]
- Jasso del Toro, C.; Nekaris, K.A.-I. Affiliative Behaviors. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–6. [Google Scholar]
- Harvey, B.S.; Dudzinski, K.M.; Kuczaj, S.A. Associations and the role of affiliative, agonistic, and socio-sexual behaviors among common bottlenose dolphins (Tursiops truncatus). Behav. Process. 2017, 135, 145–156. [Google Scholar] [CrossRef]
- Dinets, V. Apparent coordination and collaboration in cooperatively hunting crocodilians. Ethol. Ecol. Evol. 2014, 27, 244–250. [Google Scholar] [CrossRef]
- Cedeño-Vázquez, J. Crocodylus moreletii. Cannibalism. Mesoam. Herpetol. 2016, 3, 470–472. [Google Scholar]
- Delany, M.F.; Woodward, A.R.; A Kiltie, R.; Moore, C.T. Mortality of American Alligators Attributed to Cannibalism. Herpetologica 2011, 67, 174–185. [Google Scholar] [CrossRef]
- Rushen, J.; Mason, G. A decade-or-more’s progress in understanding stereotypic behaviour. In Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare; Mason, G., Rushen, J., Eds.; CAB International: Wallingford, UK, 2008; pp. 1–18. [Google Scholar]
- Hill, S.P.; Broom, D.M. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral Diversity as a Potential Indicator of Positive Animal Welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Miller, L.; Pisacane, C.; Vicino, G. Relationship between behavioural diversity and faecal glucocorticoid metabolites: A case study with cheetahs (Acinonyx jubatus). Anim. Welf. 2016, 25, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.J.; Lauderdale, L.K.; Bryant, J.L.; Mellen, J.D.; Walsh, M.T.; Granger, D.A. Behavioral diversity as a potential positive indicator of animal welfare in bottlenose dolphins. PLoS ONE 2021, 16, e0253113. [Google Scholar] [CrossRef]
- Hemsworth, H.; Barnett, J.L. Human–animal interactions and animal stress. In The Biology of Animal Stress—Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: Oxon, UK, 2001; pp. 309–336. [Google Scholar]
- Bracke, M.B.M. Animal-Based Parameters are no Panacea for On-Farm. Monitoring of Animal Welfare. Anim. Welf. 2007, 16, 229–231. [Google Scholar]
| Welfare Principle | Welfare Criteria | Proposed Measures 1 |
|---|---|---|
| Good feeding | Absence of prolonged hunger | Body condition score, visual weight estimate, food seeking behaviour, feed intake, stomach contents, growth rate, normal faecal mass |
| Absence of prolonged thirst | Visible indicators of dehydration, biomarkers indicative of dehydration | |
| Good housing | Physical comfort around resting | Posture and orientation, behavioural indicators, skin quality, stress biomarkers, |
| Thermal comfort | Posture and orientation, behaviour (signs of overheating or chilling), skin quality, growth rate, body temperature | |
| Ease of movement | Behavioural indicators (caused by a restrictive environment), locomotor stereotypies | |
| Good health | Absence of injuries | Wounds, skin quality, lameness, abrasions |
| Absence of disease | Mortalities, deformities, presence of ocular or nasal discharge, behavioural indicators, skin quality, presence of parasites, respiration rate, runting, body position | |
| Absence of pain induced by management procedures | Physical damage, behavioural signs of ineffective stunning and killing, physical movement, stress biomarkers | |
| Appropriate behaviour | Expression of social behaviours | Affiliative behaviour (social cohesion), co-occupant aggression |
| Good animal–human relations | Human-directed aggression, behavioural indicators (reflective of human–animal interaction) | |
| Positive emotional state | Stress biomarkers, behavioural indicators, obesity and emaciation | |
| Expression of other behaviours | Absence of abnormal behaviours |
| Welfare Principle and Criteria | Animal-Based Measure | Validity | Feasibility | Overall %MPS | ||
|---|---|---|---|---|---|---|
| Mean | % MPS | Mean | % MPS | |||
| Good feeding: Absence from prolonged hunger | Body condition score | 4.4 ± 0.68 | 89 a | 4.0 ± 1.15 | 81 a | 85 a |
| Feed intake | 4.3 ± 0.76 | 86 a | 4.2 ± 0.89 | 84 a | 85 a | |
| Growth rate | 4.2 ± 0.87 | 85 a | 4.0 ± 1.12 | 79 b | 82 a | |
| Visual weight estimate | 3.4 ± 0.94 | 69 | 3.3 ± 1.28 | 66 | 68 | |
| Food seeking behaviour | 2.8 ± 1.31 | 56 | 2.3 ± 1.14 | 46 | 51 | |
| Stomach contents | 1.9 ± 1.61 | 38 | 2.2 ± 1.88 | 44 | 41 | |
| Faecal mass | 2.5 ± 1.27 | 50 | 1.5 ± 1.43 | 30 | 40 | |
| Good feeding: Absence from prolonged thirst | Visible signs dehydration | 3.5 ± 1.32 | 69 | 2.8 ± 1.31 | 56 | 63 |
| Biomarkers of dehydration | 3.6 ± 1.23 | 73 | 2.4 ± 1.49 | 47 | 60 | |
| Good housing: Physical comfort around resting | Posture and orientation | 3.9 ± 1.24 | 78 ᵇ | 4.4 ± 0.94 | 88 ᵃ | 83 ᵃ |
| Behavioural repertoire | 3.9 ± 1.13 | 77 ᵇ | 3.6 ± 1.35 | 72 | 75 ᵇ | |
| Skin quality | 3.3 ± 1.15 | 65 | 3.6 ± 1.27 | 71 | 68 | |
| Stress biomarkers | 3.3 ± 1.06 | 65 | 2.8 ± 1.51 | 56 | 61 | |
| Good housing: Thermal comfort | Growth rate | 4.0 ± 0.98 | 79 ᵇ | 3.9 ± 1.16 | 77 ᵇ | 78 ᵇ |
| Behaviour-overheating | 3.8 ± 1.05 | 76 | 3.3 ± 0.99 | 65 | 70 | |
| Skin quality | 3.2 ± 1.40 | 64 | 3.7 ± 1.13 | 74 | 69 | |
| Behaviour-chilling | 3.5 ± 1.43 | 69 | 2.9 ± 1.19 | 57 | 63 | |
| Body temperature | 3.1 ± 1.46 | 63 | 2.4 ± 1.47 | 49 | 56 | |
| Good housing: Ease of movement | Behavioural repertoire | 4.1 ± 1.22 | 81 ᵃ | 3.9 ± 1.44 | 74 ᵇ | 78 ᵇ |
| Locomotor stereotypies | 3.0 ± 1.55 | 61 | 2.5 ± 1.72 | 50 | 55 | |
| Good health: Absence of injuries | Wounds | 4.3 ± 0.76 | 86 ᵃ | 3.9 ± 1.06 | 77 ᵇ | 82 a |
| Lameness | 4.0 ± 1.02 | 81 ᵃ | 4.0 ± 0.82 | 81 ᵃ | 81 a | |
| Skin quality | 4.1 ± 0.80 | 81 ᵃ | 4.0 ± 0.87 | 79 ᵇ | 80 a | |
| Abrasions | 4.1 ± 0.91 | 83 ᵃ | 3.8 ± 0.95 | 75 ᵇ | 79 ᵇ | |
| Good health: Absence of disease | Mortality | 4.8 ± 0.38 | 96 ᵃ | 4.6 ± 0.56 | 92 ᵃ | 94 ᵃ |
| Ocular/nasal discharge | 4.4 ± 0.61 | 87 ᵃ | 4.1 ± 0.92 | 81 ᵃ | 84 ᵃ | |
| Behaviour | 4.5 ± 0.57 | 89 ᵃ | 3.8 ± 1.18 | 76 ᵇ | 83 ᵃ | |
| Skin quality | 4.0 ± 0.98 | 81 ᵃ | 4.1 ± 0.90 | 82 ᵃ | 81 ᵃ | |
| Runting | 3.4 ± 1.02 | 69 | 4.6 ± 0.56 | 92 ᵃ | 80 ᵃ | |
| Deformities | 3.5 ± 1.30 | 70 | 4.2 ± 1.11 | 84 ᵃ | 77 ᵇ | |
| Position of the body | 2.6 ± 1.05 | 52 | 3.5 ± 1.16 | 70 | 61 | |
| Presence of parasites | 3.0 ± 1.22 | 6 | 2.7 ± 1.26 | 54 | 57 | |
| Respiration rate | 3.1 ± 1.57 | 62 | 2.3 ± 1.73 | 46 | 54 | |
| Good health: Absence of pain induced by management procedures | Signs of an effective stun/kill | 4.8 ± 0.66 | 96 ᵃ | 4.3 ± 0.93 | 86 ᵃ | 91 ᵃ |
| Physical damage | 4.4 ± 0.86 | 89 ᵃ | 4.0 ± 0.91 | 79 ᵇ | 84 ᵃ | |
| Physical movement | 3.8 ± 1.01 | 76 ᵇ | 4.0 ± 1.13 | 80 ᵃ | 78 ᵇ | |
| Stress biomarkers | 3.6 ± 1.15 | 71 | 2.5 ± 1.45 | 51 | 61 | |
| Vocalisation | 2.7 ± 1.44 | 54 | 3.1 ± 1.75 | 61 | 58 | |
| Appropriate behaviour: Expression ofsocial behaviours | Co-occupant aggression | 3.8 ± 0.86 | 76 ᵇ | 3.5 ± 1.17 | 70 | 73 |
| Affiliative behaviour | 3.5 ± 0.96 | 71 | 3.6 ± 0.91 | 72 | 71 | |
| Appropriate behaviour: Good animal–human relation | Behaviour | 3.7 ± 1.07 | 74 | 3.8 ± 1.09 | 75 ᵇ | 74 |
| Human-directed aggression | 3.4 ± 1.49 | 67 | 3.8 ± 1.32 | 76 | 71 | |
| Appropriate behaviour: Positiveemotional state | Obesity/emaciation | 4.1 ± 0.94 | 82 ᵃ | 4.3 ± 0.80 | 86 ᵃ | 84 ᵃ |
| Behavioural repertoire | 4.0 ± 1.20 | 81 ᵃ | 3.4 ± 1.31 | 69 | 75 ᵇ | |
| Stress biomarkers | 3.6 ± 1.21 | 72 | 2.6 ± 1.47 | 52 | 62 | |
| Appropriate behaviour: Other behaviours | Abnormal behaviours | 4.0 ± 1.16 | 80 ᵃ | 3.8 ± 1.21 | 76 ᵇ | 78 ᵇ |
| Welfare Principle | Animal-Based Measures | Resource-Based Measures | Management-Based Measures |
|---|---|---|---|
| Good feeding | Postmortem examination, post-feeding behaviour, skin quality (presence of wrinkles), competition for feed, loose skin | Composition of feed, feed quality, water quality, availability of water (of appropriate quality for drinking), feed deck space and arrangement | Feeding regimen, feed preparation protocol |
| Good housing | Faecal consistency, pressure sores and abrasions, social dominance | Space allowance, physical characteristics of the pen (e.g., provision of hides, size of the enclosure), environmental measures (temperature and humidity) | Cleanliness and maintenance of the enclosure, adaptation period |
| Good health | Feed intake, time taken to return to feeding | Access to and use of veterinary treatment, antibiotic used, use of anaesthetic and analgesia for husbandry procedures | |
| Appropriate behaviour | Skin quality | CCTV use, enrichment provision, space allowance, feed deck space and arrangement, group size | Husbandry and handling methods, training and competency, attitude of stockperson |
| Welfare Principle | Welfare Criteria | Suggested Animal-Based Measure | Supporting Resource-Based Measure |
|---|---|---|---|
| Good feeding | Absence of prolonged hunger | Body condition score, feed intake | Distribution of feed, feeding frequency |
| Appropriate diet | Growth rate, feed intake | Feed composition and quality | |
| Absence of prolonged thirst | Lack of animal-based measure | Access to drinking water | |
| Good housing | Physical comfort when resting | Posture and orientation, behavioural indicators | Space allowance, pen design |
| Thermal comfort | Posture and orientation, behavioural repertoire | Provision of appropriate thermoregulatory resources, air quality | |
| Ease of movement | Behavioural indicators | Space allowance, pen design | |
| Good health | Absence of injuries | Wounds, skin quality | Veterinary treatment records |
| Absence of disease | Mortality, ocular/nasal discharge, skin quality, behaviour | Veterinary treatment, antibiotic use | |
| Absence of pain induced by management procedures | Physical damage, signs of an effective stun/kill | Pain management, operator competency | |
| Appropriate behaviour | Expression of social behaviours | Lack of animal-based measures | Access to resources, appropriate grouping for animal type |
| Good human–animal relationships | Lack of animal-based measures | Competency of handler | |
| Positive emotional state | Behavioural repertoire, obesity/emaciation | Access to resources | |
| Expression of other behaviours | Absence of abnormal behaviours | Access to resources |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewitt, L.; Small, A. Welfare of Farmed Crocodilians: Identification of Potential Animal-Based Measures Using Elicitation of Expert Opinion. Animals 2021, 11, 3450. https://doi.org/10.3390/ani11123450
Hewitt L, Small A. Welfare of Farmed Crocodilians: Identification of Potential Animal-Based Measures Using Elicitation of Expert Opinion. Animals. 2021; 11(12):3450. https://doi.org/10.3390/ani11123450
Chicago/Turabian StyleHewitt, Leisha, and Alison Small. 2021. "Welfare of Farmed Crocodilians: Identification of Potential Animal-Based Measures Using Elicitation of Expert Opinion" Animals 11, no. 12: 3450. https://doi.org/10.3390/ani11123450
APA StyleHewitt, L., & Small, A. (2021). Welfare of Farmed Crocodilians: Identification of Potential Animal-Based Measures Using Elicitation of Expert Opinion. Animals, 11(12), 3450. https://doi.org/10.3390/ani11123450