Characterization of Oviduct Lining, with Emphasis on the Sperm Storage Tubule Region (Uterovaginal Junction), Correlated with Fertility in Mature and Old Thai Native Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Feeding
2.2. Experimental Design
2.2.1. Experiment 1: Effect of Age on Morphological Characteristics of Reproductive Organs and Histological Characteristics of UVJ Tissues
2.2.2. Experiment 2: Effects of Ages on Resident Sperm in the Uterovaginal Junction after Artificial Insemination (AI)
2.2.3. Experiment 3: Effects of Age on Fertility
2.3. Morphological Characteristics of Reproductive Organs and Histological Characteristics of UVJ Tissues
2.4. Semen Collection, Evaluation, and AI
2.5. Sperm Recovery
2.6. Percentages of SSTs Containing Sperm
2.7. Fertility Test
2.8. Statistical Analysis
3. Results
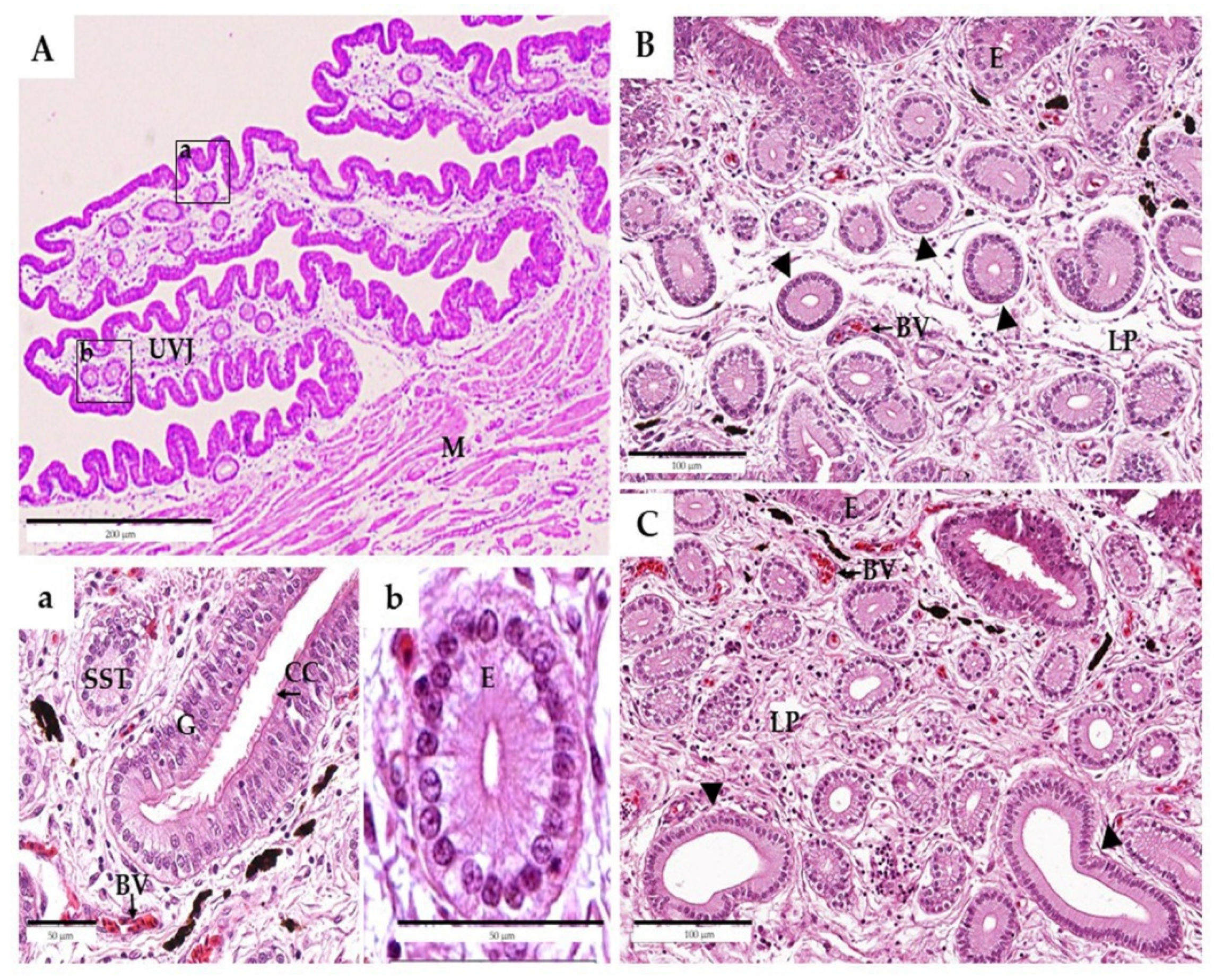
3.1. Experiment 1: Effect of Age on Morphological Characteristics of Reproductive Organs and Histological Characteristics of UVJ Tissues
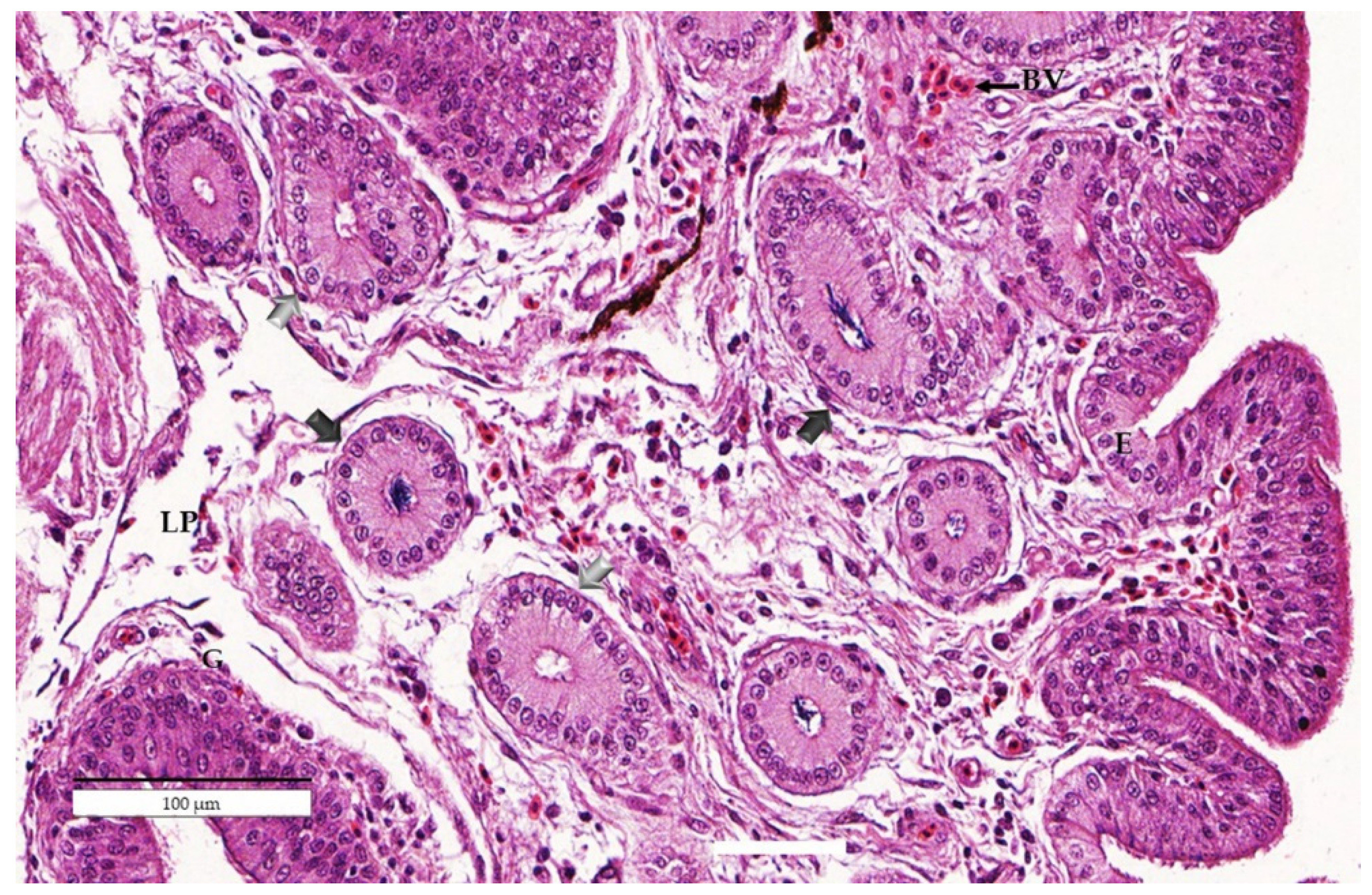
3.2. Experiment 2: Effects of Age on Resident Sperm in the Uterovaginal Junction after Artificial Insemination (AI)
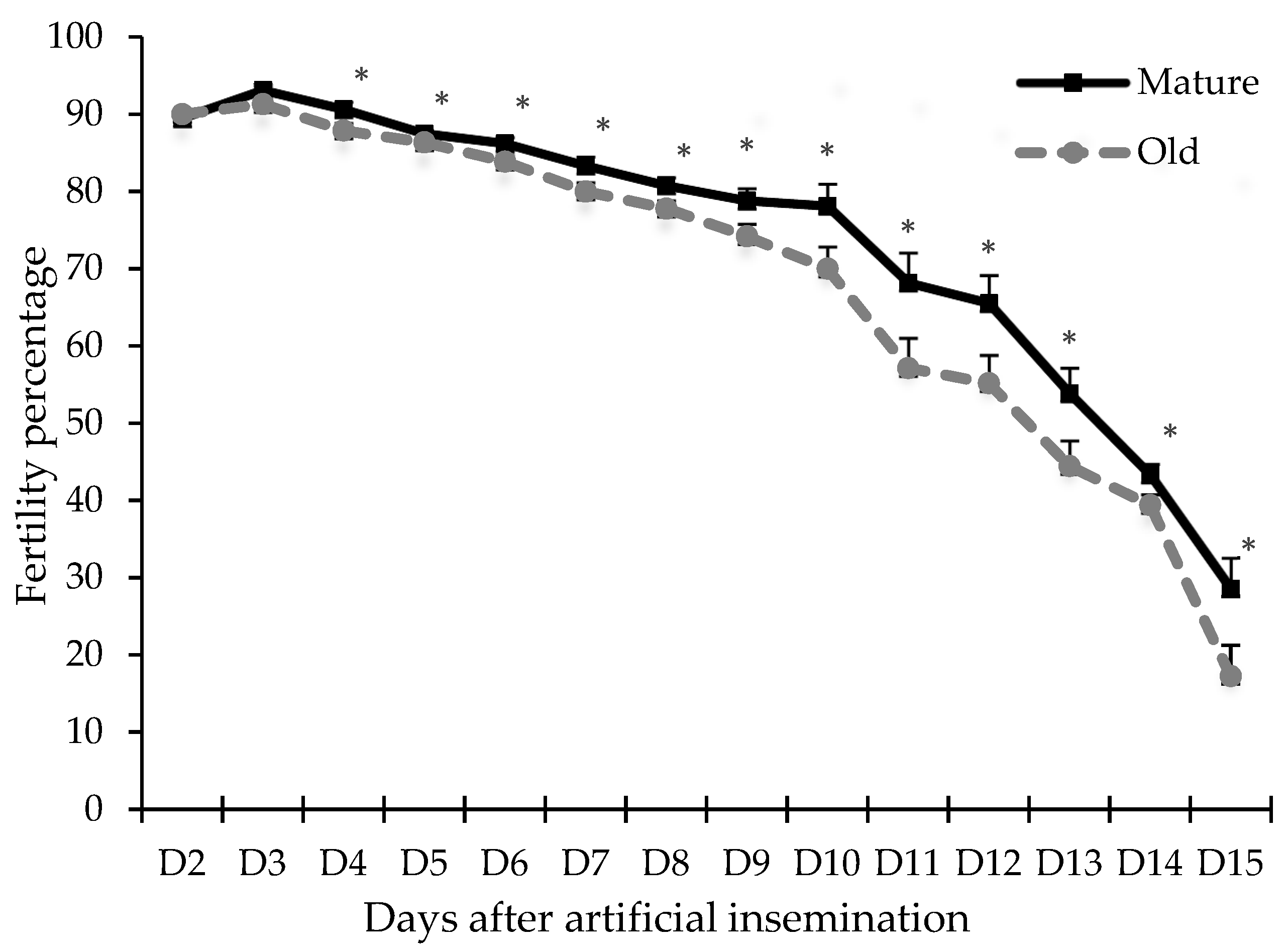
3.3. Experiment 3: Effects of Age on Fertility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, J.; Wilson, H.R.; Miles, R.D.; Butcher, G.D.; Mather, F.B. Factors Affecting Egg Production in Backyard Chicken. 2014. Available online: https://edis.ifas.ufl.edu/publication/PS029 (accessed on 15 September 2021).
- Kirk, S.; Emmans, G.C.; McDonald, R.; Arnot, D. Factors affecting the hatchability of eggs from broiler breeders. Br. Poult. Sci. 1980, 21, 37–53. [Google Scholar] [CrossRef]
- Lake, P.E. Recent progress in poultry production. World’s Poult. Sci. J. 1989, 45, 53–59. [Google Scholar] [CrossRef]
- Walsh, T.J.; Brake, J. The effect of nutrient intake during rearing of broiler breeder females on subsequent fertility. Poult. Sci. 1997, 76, 297–305. [Google Scholar] [CrossRef]
- Wolc, A.; White, I.M.; Olori, V.E.; Hill, W.G. Inheritance of fertility in broiler chickens. Genet. Sel. Evol. 2009, 41, 47. [Google Scholar] [CrossRef] [PubMed]
- Brillard, J.P.; McDaniel, G.R. Influence of spermatozoa numbers and insemination frequency on fertility in dwarf broiler breeder hens. Poult. Sci. 1986, 65, 2330–2334. [Google Scholar] [CrossRef]
- Bramwell, R.K.; McDaniel, C.D.; Wilson, J.L.; Howarth, B. Age effect of male and female broiler breeders on sperm penetration of the perivitelline layer overlying the germinal disc. Poult. Sci. 1996, 75, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Gumułka, M.; Kapkowska, E. Age effect of broiler breeders on fertility and sperm penetration of the perivitelline layer of the ovum. Anim. Reprod. Sci. 2005, 90, 135–148. [Google Scholar] [CrossRef]
- Orr, T.J.; Zuk, M. Sperm storage. Curr. Biol. 2012, 22, 8–10. [Google Scholar] [CrossRef]
- Fujii, S.; Tamamura, T. Location of Sperms in the Oviduct of the Domestic Fowl with Special Reference to Storage of Sperm in the Vaginal Gland. J. Fac. Fish. Hiroshima Univ. 1963, 5, 145–163. [Google Scholar]
- Bakst, M.R. Structure of the Avian Oviduct with Emphasis on Sperm Storage in Poultry. J. Exp. Zool. 1998, 282, 618–626. [Google Scholar] [CrossRef]
- Bréque, C.; Surai, P.; Brillard, J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003, 66, 314–323. [Google Scholar] [CrossRef]
- Holt, W.V.; Fazeli, A. Sperm Storage in the Female Reproductive. Annu. Rev. Anim. Biosci. 2016, 4, 291–310. [Google Scholar] [CrossRef]
- Bakst, M.; Wishart, R.G.; Brillard, J.P. Oviductal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 1994, 5, 117–143. [Google Scholar]
- Brillard, J.P. Factors affecting oviductal sperm storage in the domestic fowl following artificial insemination. Anim. Reprod. Sci. 1992, 27, 247–256. [Google Scholar] [CrossRef]
- Ebeid, T.; Eid, Y.; Saleh, A.; Abd El-Hamid, H. Ovarian follicular development, lipid peroxidation, antioxidative status and immune response in laying hens fed fish oil-supplemented diets to produce n-3-enriched eggs. Animal 2008, 2, 84–91. [Google Scholar] [CrossRef]
- Das, S.; Das, C.; Nagasak, N.; Yoshimura, Y. Effects of Repeated Artificial Insemination on the Structure and Function of Oviducal Sperm Storage Tubules in Hens. Poult. Sci. 2005, 42, 39–47. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. The Collection of Spermatozoa from the Domestic Fowl and Turkey. Poult. Sci. 1937, 16, 19–24. [Google Scholar] [CrossRef]
- Łukaszewicz, E. Effects of semen filtration and dilution rate on morphology and fertility of frozen gander spermatozoa. Theriogenology 2000, 55, 1819–1829. [Google Scholar] [CrossRef]
- Chauychu-Noo, N.; Thananurak, P.; Boonkum, W.; Vongpralub, T.; Chankitisakul, V. Effect of organic selenium dietary supplementation on quality and fertility of cryopreserved chicken sperm. Cryobiology 2021, 98, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Brillard, J.P.; Bakst, M.R. Quantification of spermatozoa in the sperm-storage tubules of turkey hens and the relation to sperm numbers in the perivitelline layer of eggs. Biol. Reprod. 1990, 43, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Brillard, J.P.; Beaumont, C.; Scheller, M.F. Physiological responses of hens divergently selected on the basis of the number of chicks obtained from a single insemination. J. Reprod. Fertil. 1998, 114, 111–117. [Google Scholar] [CrossRef][Green Version]
- Brillard, J.P. Sperm Storage and Transport Following Natural Mating and Artificial Insemination. Poult. Sci. 1993, 72, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, F. Effect of age, sex, genotype and environment on fat deposition in broiler chickens-A review. World’s Poult. Sci. J. 1986, 42, 12–25. [Google Scholar] [CrossRef]
- Renema, R.A.; Robinson, F.E.; Proudman, J.A.; Newcombe, M.; Mackay, R.I. Effects of body weight and feed allocation during sexual maturation in broiler breeder hens. 2. Ovarian morphology and plasma hormone profile. Poult. Sci. 1999, 78, 629–639. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Yao, Y.; Cao, Z.F.; Gu, T.T.; Xu, Q.; Chen, G.H. Histological characteristics of follicles and reproductive hormone secretion during ovarian follicle development in laying geese. Poult. Sci. 2019, 98, 6063–6070. [Google Scholar] [CrossRef]
- Zakaria, A.H.; Miyaki, T.; Imai, K. The effect of aging on the ovarian follicular growth in laying hens. Poult. Sci. 1983, 62, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Boonkum, W.; Duangjinda, M.; Laopaiboon, B.; Vongpralub, T. Genetic evaluation and genetic curve for egg production in Thai native chickens (Pradu Hang Dam) using a random regression test-day model. KhonKaen Agri. J. 2012, 40, 69–78. [Google Scholar]
- Mookprom, S.; Boonkum, W.; Kunhareang, S.; Siripanya, S.; Duangjinda, M. Genetic evaluation of egg production curve in Thai native chickens by random regression and spline models. Poult. Sci. 2017, 96, 274–281. [Google Scholar] [CrossRef]
- Yu, M.W.; Robinson, F.E.; Charles, R.G.; Weingardt, R. Effect of feed allowance during rearing and breeding on female broiler breeders 2. Ovarian morphology and production. Poult. Sci. 1992, 71, 1750–1761. [Google Scholar] [CrossRef]
- Oguntunji, A.O.; Alabi, O.M. Influence of high environmental temperature on egg production and shell quality: A review. World’s Poult. Sci. J. 2010, 66, 739–750. [Google Scholar] [CrossRef]
- Donoghue, A.M. Prospective approach to avoid flock fertility problems: Predictive assessment of sperm function traits in poultry. Poult. Sci. 1999, 78, 437–443. [Google Scholar] [CrossRef]
- Wentworth, B.C.; Mellen, W.J. Effects of spermatozoa1 antibodies and method of insemination on the fecundity of domestic hens. Br. Poult. Sci. 1964, 5, 59–65. [Google Scholar] [CrossRef]
- Saeki, Y.; Miyauchi, S.; Abe, T.; Hosoda, T.; Akita, T. Relationship between fertility of eggs from hens continuously inseminated for a long period and their agglutinin titers against cock spermatozoa. Jpn. Poult. Sci. 1965, 2, 109–144. [Google Scholar] [CrossRef]
- Holm, L.; Ekwall, H.; Wishart, G.J.; Ridderstrale, Y. Localisation of calcium and zinc in the sperm storage tubule of chicken, quail and turkey using X-ray microanalysis. J. Reprod. Fertil. 2000, 118, 331–336. [Google Scholar] [CrossRef]
- Zaniboni, L.; Bakst, M.B. Localization of aquaporins in the sperm storage tubules in the turkey oviduct. Poult. Sci. 2004, 83, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Long, E.L.; Sonstegard, T.S.; Long, J.A.; Tassell, C.P.V.; Zuelke, K.A. Serial analysis of gene expression in turkey sperm storage tubules in the presence and absence of resident sperm. Biol Reprod. 2004, 69, 469–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasanami, T.; Matsuzaki, M.; Mizushima, S.; Hiyama, G. Sperm storage in the female reproductive tract in birds. J. Reprod. Dev. 2013, 59, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.M.; Yeatman, J.B.; Schultz, C.D.; Zumwalt, C.D.; McDaniel, C.D. Use of a sperm analyzer for evaluating broiler breeder males. 2. Selection of young broiler breeder roosters for the sperm quality index increases fertile egg production. Poult. Sci. 2000, 79, 771–777. [Google Scholar] [CrossRef]
| Parameters | Hen Groups | p-Value | |
|---|---|---|---|
| Mature | Old | ||
| Body weight (kg) | 1.8 ± 0.0 b | 2.1 ± 0.1 a | 0.03 |
| Ovary weight (g) | 36.3 ± 3.5 | 35.2 ± 2.1 | 0.53 |
| Oviductal weight (g) | 35.8 ± 3.0 | 38.4 ± 2.9 | 0.96 |
| Oviductal length (cm) | 67.1 ± 5.9 | 67.2 ± 2.7 | 0.15 |
| Parameters | Hen Groups | p-Value | |||
|---|---|---|---|---|---|
| Follicle Types | Mature | Old | |||
| Quantity (Number of follicles) | SWFs (1–3 mm) | 26.8 ± 1.9 a | 23.0 ± 1.8 b | 0.03 | |
| LWFs (3–5 mm) | 17.7 ± 1.9 a | 14.0 ± 1.7 b | 0.04 | ||
| SYFs (5–10 mm) | 11.2 ± 2.4 | 8.3 ± 2.7 | 0.10 | ||
| LYFs (>10 mm) | 2.5 ± 0.8 a | 1.3 ± 0.5 b | 0.01 | ||
| TF (1–10 mm) | 55.7 ± 4.2 a | 45.3 ± 4.1 b | 0.03 | ||
| Top five largest yellow follicle diameters | Major axis (mm) | F1 (mm) | 36.6 ± 3.5 | 35.6 ± 8.8 | 0.76 |
| F2 (mm) | 33.4 ± 2.5 | 30.0 ± 8.0 | 0.39 | ||
| F3 (mm) | 30.0 ± 3.0 | 23.9 ± 9.5 | 0.21 | ||
| F4 (mm) | 23.1 ± 3.7 | 16.3 ± 6.2 | 0.16 | ||
| F5 (mm) | 16.9 ± 4.7 a | 10.1 ± 1.8 b | 0.04 | ||
| Minor axis (mm) | F1 (mm) | 32.7 ± 2.5 | 31.1 ± 8.1 | 0.63 | |
| F2 (mm) | 28.9 ± 2.3 | 25.4 ± 7.1 | 0.20 | ||
| F3 (mm) | 24.6 ± 1.8 | 20.1 ± 5.7 | 0.21 | ||
| F4 (mm) | 20.2 ± 3.8 | 14.0 ± 5.7 | 0.16 | ||
| F5 (mm) | 14.9 ± 3.8 a | 8.8 ± 1.8 b | 0.36 | ||
| Parameters | Hen Groups | p-Value | |
|---|---|---|---|
| Mature | Old | ||
| Number of folds, n | 23.0 ± 1.1 | 23.8 ± 1.4 | 0.59 |
| Fold length (µm) | 463.6 ± 36.7 | 485.3 ± 42.4 | 0.79 |
| SSTs epithelium height (µm) | 16.3 + 1.3 a | 13.6 ± 2.6 b | 0.04 |
| Outer diameter of the SSTs (µm) | 30.6 ± 5.8 a | 55.5 ± 8.9 b | 0.04 |
| Inner diameter of the SSTs (µm) | 6.9 ± 1.7 a | 27.5 ± 3.7 b | 0.03 |
| SSTs per fold, n | 338.0 ± 68.6 | 306.7 ± 64.0 | 0.90 |
| Parameters | Hen Groups | p-Value | |
|---|---|---|---|
| Mature | Old | ||
| Spermatozoa (×104) | 143.2 ± 14.0 a | 114.6 ± 17.1 b | 0.03 |
| SSTs containing with sperm (%) | 26.8 ± 3.4 a | 6.8 ± 1.7 b | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheawkanha, T.; Boonkum, W.; Vongpralub, T.; Chankitisakul, V. Characterization of Oviduct Lining, with Emphasis on the Sperm Storage Tubule Region (Uterovaginal Junction), Correlated with Fertility in Mature and Old Thai Native Hens. Animals 2021, 11, 3446. https://doi.org/10.3390/ani11123446
Kheawkanha T, Boonkum W, Vongpralub T, Chankitisakul V. Characterization of Oviduct Lining, with Emphasis on the Sperm Storage Tubule Region (Uterovaginal Junction), Correlated with Fertility in Mature and Old Thai Native Hens. Animals. 2021; 11(12):3446. https://doi.org/10.3390/ani11123446
Chicago/Turabian StyleKheawkanha, Theerapat, Wuttigrai Boonkum, Thevin Vongpralub, and Vibuntita Chankitisakul. 2021. "Characterization of Oviduct Lining, with Emphasis on the Sperm Storage Tubule Region (Uterovaginal Junction), Correlated with Fertility in Mature and Old Thai Native Hens" Animals 11, no. 12: 3446. https://doi.org/10.3390/ani11123446
APA StyleKheawkanha, T., Boonkum, W., Vongpralub, T., & Chankitisakul, V. (2021). Characterization of Oviduct Lining, with Emphasis on the Sperm Storage Tubule Region (Uterovaginal Junction), Correlated with Fertility in Mature and Old Thai Native Hens. Animals, 11(12), 3446. https://doi.org/10.3390/ani11123446







