Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Holding
2.2. Experiment I
2.3. Experiment II
2.4. Protocols of Metabolic Rates
2.5. Na+-K+-ATPase Activity
2.6. Gill Microscopy
2.7. Statistical Analyses
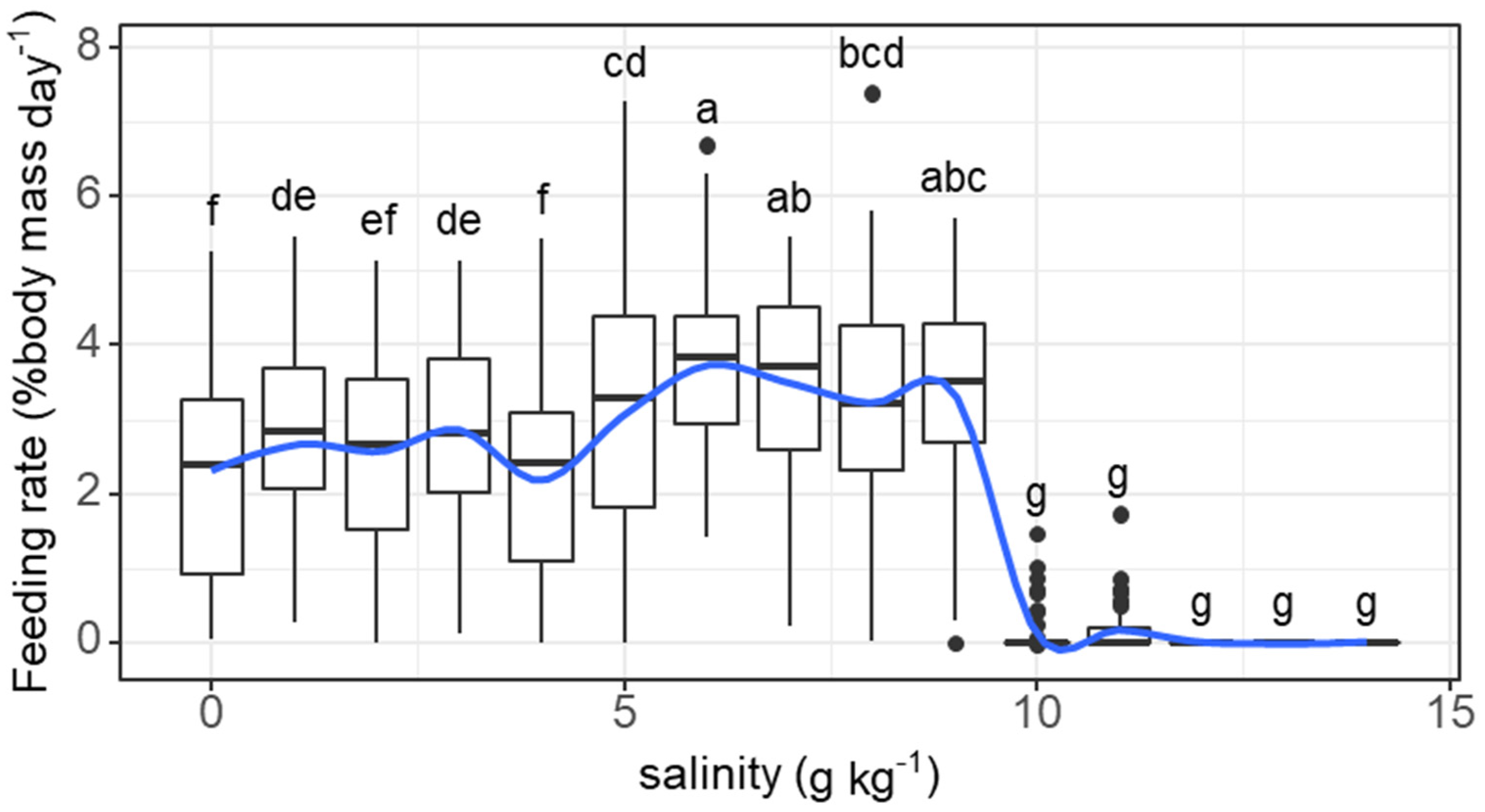
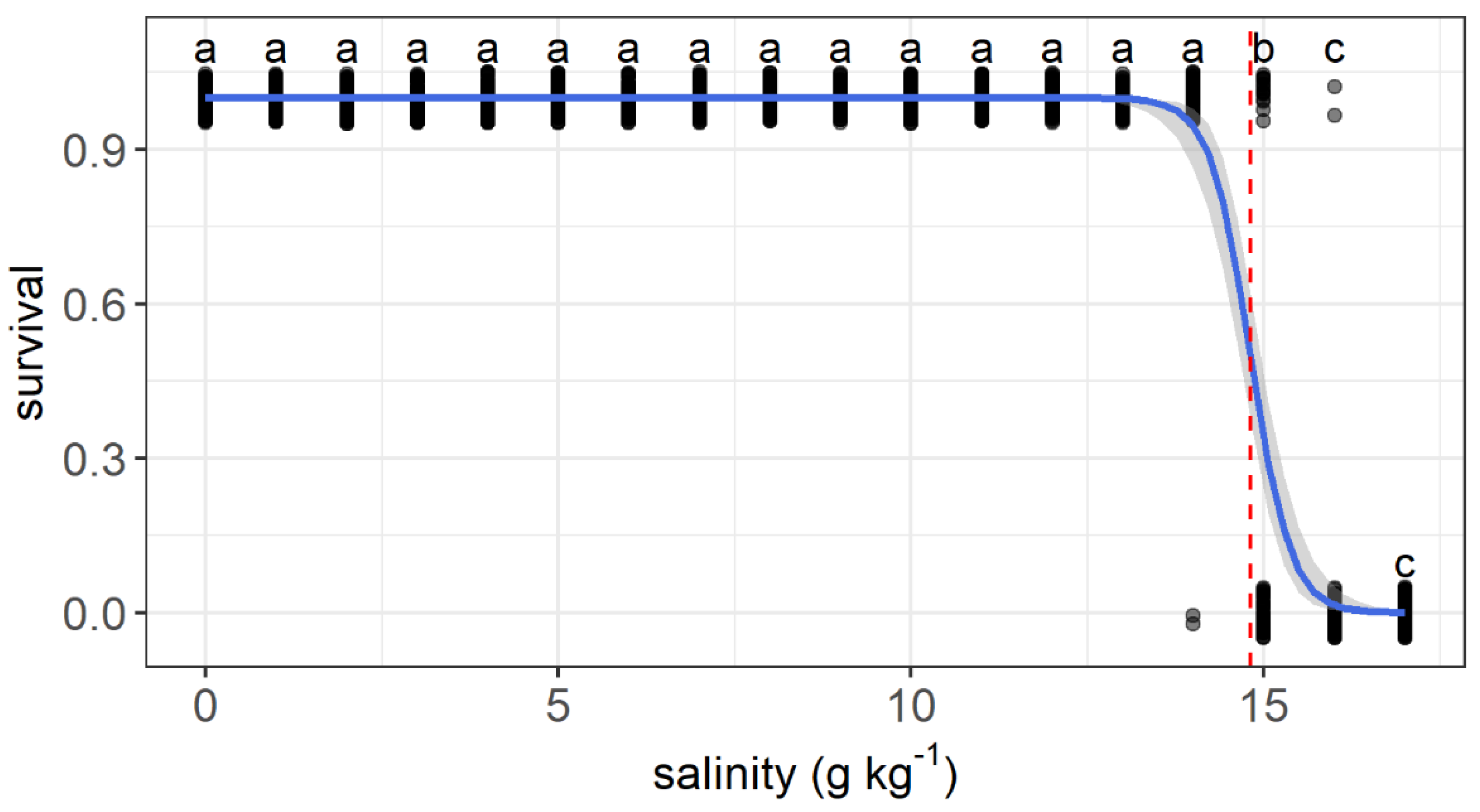
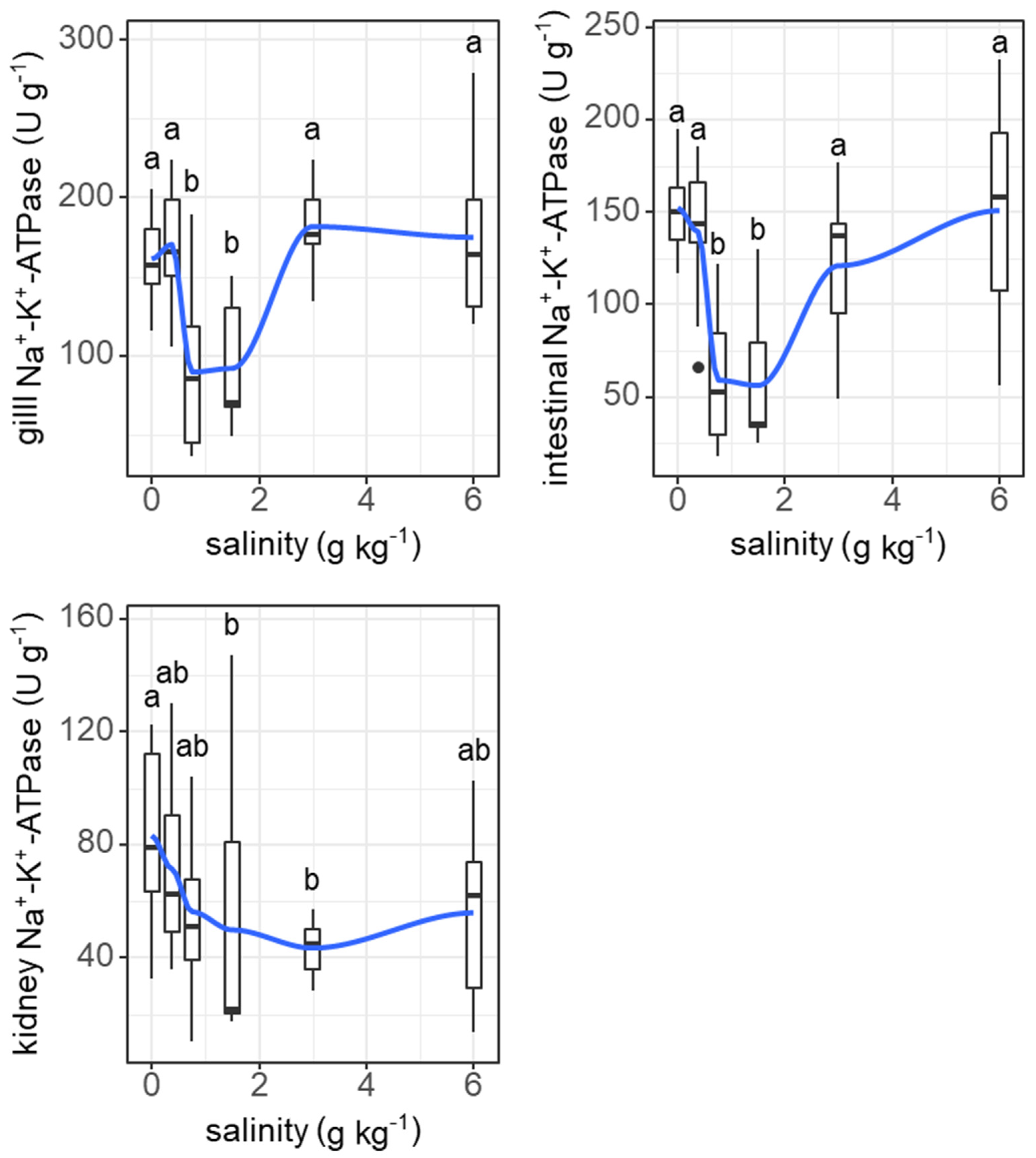
3. Results
3.1. Experiment I
3.2. Experiment II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadi, N.; Baroiller, J.F.; D’Cotta, H.; Morillon, R. Adaptation à la salinité. In Changement Climatique et Agricultures du Monde; Torquebiau, E., Ed.; Editions Quae: Versailles, France, 2015; pp. 50–62. [Google Scholar]
- Bassel, M. Conséquence Durable de Deux Décennies de Décheresse: L’hypersalinisation de la Casamance Entre 1987 et 1992; UCAD: Dakar, Senegal, 1993. [Google Scholar]
- Niang, I. Etude de vulnérabilité des zones côtières sénégalaises aux changements climatiques: Le cas des pays africains côtiers. Bull. Afr. 1998, 10, 25–37. [Google Scholar]
- Iwama, G.K.; Pickering, A.D.; Sumpter, J.P.; Schreck, C.B. Fish Stress and Health in Aquaculture; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Bentley, P.J. Endocrines and Osmoregulation: A Comparative Account in Vertebrates, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Wolter, C.; Arlinghaus, R. Navigation impacts on freshwater fish assemblages: The ecological relevance of swimming performance. Rev. Fish. Biol. Fish. 2003, 13, 63–89. [Google Scholar] [CrossRef]
- Nordlie, F.G. Environmental influences on regulation of blood plasma/serum components in teleost fishes: A review. Rev. Fish. Biol. Fish. 2009, 19, 481–564. [Google Scholar] [CrossRef]
- Kapoor, A.; Mehta, N.; Esper, F.; Poljsak-Prijatelj, M.; Quan, P.L.; Qaisar, N.; Delwart, E.; Lipkin, W.I. Identification and characterization of a new bocavirus species in Gorillas. PLoS ONE 2010, 5, e11948. [Google Scholar] [CrossRef] [Green Version]
- Uliano, E.; Cataldi, M.; Carella, F.; Migliaccio, O.; Iaccarino, D.; Agnisola, C. Effects of acute changes in salinity and temperature on routine metabolism and nitrogen excretion in gambusia (Gambusia affinis) and zebrafish (Danio rerio). Comp. Biochem. Physiol. Part A 2010, 157, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Palmer, B.D. Climate change, multiple stressors, and the decline of the ectotherms. Conserv. Biol. 2013, 27, 741–751. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Campbell, N.; Reece, J. Biologie, 7th ed.; Pearson Education: Hoboken, NJ, USA, 2007; pp. 958–959. [Google Scholar]
- Christensen, E.A.F.; Grosell, M.; Steffensen, J.F. Maximum salinity tolerance and osmoregulatory capabilities of European perch Perca fluviatilis populations originating from different salinity habitats. Conserv. Physiol. 2019, 7, coz004. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.A.F.; Stieglitz, J.D.; Grosell, M.; Steffensen, J.F. Intra-specific difference in the effect of salinity on physiological performance in European perch (Perca fluviatilis) and its ecological importance for fish in estuaries. Biology 2019, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, N.B.; Taylor, A.C.; Thorpe, J.E. Metabolic rate, social status and life history strategies in Atlantic salmon. Anim. Behav. 1995, 49, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Cutts, C.J.; Metcalfe, N.B.; Taylor, A.C. Juvenile Atlantic salmon (Salmo salar) with relatively high standard metabolic rates have small metabolic scopes. Funct. Ecol. 2002, 16, 73–78. [Google Scholar] [CrossRef]
- Auer, S.K.; Killen, S.S.; Rezende, E.L. Resting vs. active: A meta-analysis of the intra- and inter-specific associations between minimum, sustained, and maximum metabolic rates in vertebrates. Funct. Ecol. 2017, 31, 1728–1738. [Google Scholar] [CrossRef]
- Giacomin, M.; Bryant, H.J.; Adalberto, L.V.; Schulte, P.M.; Wood, C.M. The osmorespiratory compromise: Physiological responses and tolerance to hypoxia are affected by salinity acclimation in the euryhaline Atlantic killifish (Fundulus heteroclitus). J. Exp. Biol. 2019, 222, jeb206599. [Google Scholar] [CrossRef]
- Randall, D.J.; Baumgarten, D.; Malyusz, M. The relationship between gas and ion transfer across the gills of fishes. Comp. Biochem. Physiol. A 1972, 41, 629–637. [Google Scholar] [CrossRef]
- Rooij, V.J.; Videler, J.J. Estimating oxygen uptake rate from ventilation frequency in the reef fish Sparisoma viride. Mar. Ecol. Prog. Ser. 1996, 132, 31–41. [Google Scholar] [CrossRef]
- Frisk, M.; Skov, P.V.; Steffensen, J.F. Thermal optimum for pikeperch (Sander lucioperca) and the use of ventilation frequency as a predictor of metabolic rate. Aquaculture 2012, 324, 151–157. [Google Scholar] [CrossRef]
- Killen, S.S.; Glazier, D.S.; Rezende, E.L.; Clark, T.D.; Atkinson, D.; Willener, A.S.T.; Halsey, L.G. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 2016, 187, 592–606. [Google Scholar] [CrossRef] [Green Version]
- Ern, R.; Houng, D.T.T.; Cong, N.V.; Bayley, M.; Wang, T. Effect of salinity on oxygen consumption in fishes: A review. J. Fish. Biol. 2014, 84, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Altinok, I.; Grizzle, J.M. Effects of low salinities on oxygen consumption of selected euryhaline and stenohaline freshwater fish. J. World Aquacult. Soc. 2003, 34, 113–117. [Google Scholar] [CrossRef]
- Morgan, J.D.; Iwama, G.K. Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 1991, 48, 2083–2094. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C 2001, 130, 411–423. [Google Scholar]
- Akin, S.; Neill, W. Routine metabolism of mosquitofish (Gambusia affinis) at three different salinities. Tex. J. Sci. 2003, 55, 255–262. [Google Scholar]
- Behrens, J.W.; Deurs, M.V.; Christensen, E.A.F. Evaluating dispersal potential of an invasive fish by the use of aerobic scope and osmoregulation capacity. PLoS ONE 2017, 12, e0176038. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.A.F.; Illing, B.; Iversenc, N.S.; Johansen, J.L.; Domenicie, P.; Steffensen, J.F. Effects of salinity on swimming performance and oxygen consumption rate of shiner perch Cymatogaster aggregate. J. Exp. Mar. Biol. Ecol. 2018, 204, 32–37. [Google Scholar] [CrossRef]
- Sui, Y.; Huang, X.; Kong, H.; Lu, W.; Wang, Y. Physiological responses to salinity increase in blood parrotfish (Cichlasoma synspilum ♀ × Cichlasoma citrinellum ♂). SpringerPlus 2016, 5, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, R.; Mattioli, C.C.; Bazzoli, N.; Corrêa Júnior, J.D.; Luz, R.K. The effects of salinity on growth, gill tissue and muscle cellularity in Lophiosilurus alexandri juvenile, a Neotropical freshwater catfish. Aquac. Res. 2021, 52, 4064–4075. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.S.; Nasif, O.; Van Doan, H.; Dawood, M.A.O. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Pike, T. Fish and shellfish introductions: Grass carp in south Africa. FAO Aquacult. Bull. 1977, 8, 20. [Google Scholar]
- Adams, B.M.; Bertrand, K.N.; Brown, M.L.; Auger, D. Genetic structure of grass carp populations in the Missouri and Mississippi River basins, USA. Praire Nat. 2011, 43, 84–91. [Google Scholar]
- Moses, B.S. Aquaculture development: Nigeria. Fish culture development in Nigeria. FAO Aquacult. Bull. 1972, 5, l2. [Google Scholar]
- Enayati, A.; Peyghan, R.; Papahn, A.A.; Khadjeh, G.H. Study on effect of salinity level of water on electrocardiogram and some of blood serum minerals in grass carp, Ctenopharyngodon idella. Vet. Res. Forum. 2013, 4, 49–53. [Google Scholar]
- Kaleem, O.; Sabi, A.F.B.S. Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquacult. Fish. 2020, 6, 535–547. [Google Scholar] [CrossRef]
- Yavuzcan-Yildiz, H.; Kirkaǧaç-Uzbilek, M. The evaluation of secondary stress response of grass carp (Ctenopharyngodon idella, Val. 1844) after exposing to the saline water. Fish. Physiol. Biochem. 2001, 25, 287–290. [Google Scholar] [CrossRef]
- Nico, L.G.; Fuller, P.L.; Schofield, P.J.; Neilson, M.E.; Benson, A.J.; Li, J. Ctenopharyngodon idella (Valenciennes in Cuvier and Valenciennes); Nonindigenous Aquatic Species Database; U.S. Geological Survey: Gainesville, FL, USA, 1844. Available online: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=514 (accessed on 5 June 2020).
- Cross, D.G. The tolerance of grass carp Ctenopharyngodon idella (Val.) to seawater. J. Fish. Biol. 1970, 2, 231–233. [Google Scholar] [CrossRef]
- Maceina, M.J.; Shireman, J.V. Grass carp: Effects of salinity on survival, weight loss, and muscle tissue water content. Prog. Fish. Cult. 1979, 41, 69–73. [Google Scholar]
- Versar, Inc. Risk Assessment of the Potential Effects of Stocking Triploid-Certified Grass Carp in the Potomac River Watershed on Submersed Aquatic Vegetation; Report Prepared for Chesapeake Bay Program Office; U.S. Environmental Protection Agency: Annapolis, MD, USA, 1999. [Google Scholar]
- Fu, S.J.; Fu, C.; Yan, G.J.; Cao, Z.D.; Zhang, A.J.; Pang, X. Interspecific variation in hypoxia tolerance, swimming performance and plasticity in cyprinids that prefer different habitats. J. Exp. Biol. 2014, 217, 590–597. [Google Scholar]
- Zhang, Y.; Huang, Q.; Liu, S.; He, D.; Wei, G.; Luo, Y. Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J. Comp. Physiol. B 2014, 184, 347–354. [Google Scholar] [CrossRef]
- Maceina, M.J.; Nordlie, F.G.; Shireman, J.V. The influence of salinity on oxygen consumption and plasma electrolytes in grass carp, Ctenopharyngodon idella Val. J. Fish. Biol. 1980, 16, 613–619. [Google Scholar] [CrossRef]
- Millidine, K.J.; Metcalfe, N.B.; Armstrong, J.D. The use of ventilation frequency as an accurate indicator of metabolic rate in juvenile Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2008, 65, 2081–2087. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Huang, Q.; Zhang, Y.; Luo, Y. Effect of meal size on the specific dynamic action of the juvenile snakehead (Channa argus). Comp. Biochem. Physiol. A 2012, 161, 401–405. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austira, 2020; Available online: https://www.R-project.org (accessed on 18 May 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chen, Z.; Snow, M.; Lawrence, C.S.; Church, A.R.; Narum, S.R.; Devlin, R.H.; Farrell, A.P. Selection for upper thermal tolerance in rainbow trout (Oncorhynchus mykiss Walbaum). J. Exp. Biol. 2015, 218, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millidine, K.J.; Armstrong, J.D.; Metcalfe, N.B. Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc. R. Soc. B. 2009, 276, 2103–2108. [Google Scholar] [CrossRef] [Green Version]
- Maceina, M.J.; Shireman, J.V. Effects of salinity on vegetation consumption and growth in grass carp. Prog. Fish. Cult. 1980, 42, 50–53. [Google Scholar]
- Kilambi, R.V. Food consumption, growth and survival of grass carp Ctenopharyngodon idella Val at four salinities. J. Fish. Biol. 1980, 17, 613–618. [Google Scholar] [CrossRef]
- Dendrinos, P.; Thorpe, J.P. Effects of reduced salinity on growth and body composition in the European. bass Dicentrarchus labrax L. Aquaculture 1985, 49, 333–358. [Google Scholar] [CrossRef]
- Gutt, J. The growth of juvenile flounders Platichthys flesus L. at salinities of 0, 5, 15 and 35‰. J. Appl. Ichthyol. 1985, 1, 17–26. [Google Scholar] [CrossRef]
- McCormick, S.D.; Saunders, R.L.; MacIntyre, A.D. The effect of salinity and ration level on growth rate and conversion efficiency of Atlantic salmon Salmo salar smolts. Aquaculture 1989, 82, 173–180. [Google Scholar] [CrossRef]
- Lambert, Y.; Dutil, J.D.; Munro, J. Effects of intermediate and low salinity conditions on growth rate and food conversion of Atlantic cod Gadus morhua. Can. J. Fish. Aquat. Sci. 1994, 51, 1569–1576. [Google Scholar] [CrossRef]
- Conides, A.J.; Parpoura, A.R.; Fotis, G. Study on the effects of salinity on the fry of the euryhaline species gilthead sea bream Sparus aurata L. 1758. J. Trop. Aquacult. 1997, 12, 297–303. [Google Scholar]
- Perry, S.F. Respiratory responses to hypoxia in fishes. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Elsevier Inc.: San Diego, CA, USA, 2011; pp. 1751–1756. [Google Scholar]
- Gonzalez, R.J. The physiology of hyper-salinity tolerance in teleost fish: A review. J. Comp. Physiol. B 2012, 182, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, L.B. Energetics of osmoregulation in fresh water vertebrates. J. Exp. Zool. 1995, 271, 243–252. [Google Scholar] [CrossRef]
- Morgan, J.D.; Sakamoto, T.; Grau, E.G.; Iwama, G.K. Physiological and respiratory responses of the Mozambique tilapia (Oreochromis mossambicus) to salinity acclimation. Comp. Biochem. Physiol. A 1997, 117, 391–398. [Google Scholar] [CrossRef]
- LeBlanc, D.M.; Wood, C.M.; Fudge, D.S.; Wright, P.A. A fish out of water: Gill and skin remodeling promotes osmo- and ionoregulation in the mangrove killifish Kryptolebias marmoratus. Physiol. Biochem. Zool. 2010, 83, 932–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, S.D.; Matheson, D.; He, Y.; Goss, G.G. Reduced salinity tolerance in the Arctic grayling (Thymallus arcticus) is associated with rapid development of a gill interlamellar cell mass: Implications of high-saline spills on native freshwater salmonids. Conserv. Physiol. 2016, 4, cow010. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, T.C.; McBryan, T.L.; Schulte, P.M. Interactive effects of salinity and temperature acclimation on gill morphology and gene expression in threespine stickleback. Comp. Biochem. Physiol. A 2018, 221, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sardella, B.A.; Brauner, C.J. The effect of elevated salinity on ‘California’ Mozambique tilapia (Oreochromis mossambicus × O. urolepis hornorum) metabolism. Comp. Biochem. Physiol. C 2008, 148, 430–436. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djiba, P.K.; Zhang, J.; Xu, Y.; Zhang, P.; Zhou, J.; Zhang, Y.; Luo, Y. Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella). Animals 2021, 11, 3445. https://doi.org/10.3390/ani11123445
Djiba PK, Zhang J, Xu Y, Zhang P, Zhou J, Zhang Y, Luo Y. Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella). Animals. 2021; 11(12):3445. https://doi.org/10.3390/ani11123445
Chicago/Turabian StyleDjiba, Pathe Karim, Jianghui Zhang, Yuan Xu, Pan Zhang, Jing Zhou, Yan Zhang, and Yiping Luo. 2021. "Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella)" Animals 11, no. 12: 3445. https://doi.org/10.3390/ani11123445
APA StyleDjiba, P. K., Zhang, J., Xu, Y., Zhang, P., Zhou, J., Zhang, Y., & Luo, Y. (2021). Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella). Animals, 11(12), 3445. https://doi.org/10.3390/ani11123445





