Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diets, and Growth Performance
2.2. Gastrointestinal Permeability, Oocyst Shedding, and Fecal Consistency
2.3. Sample Collection and Lesion Score
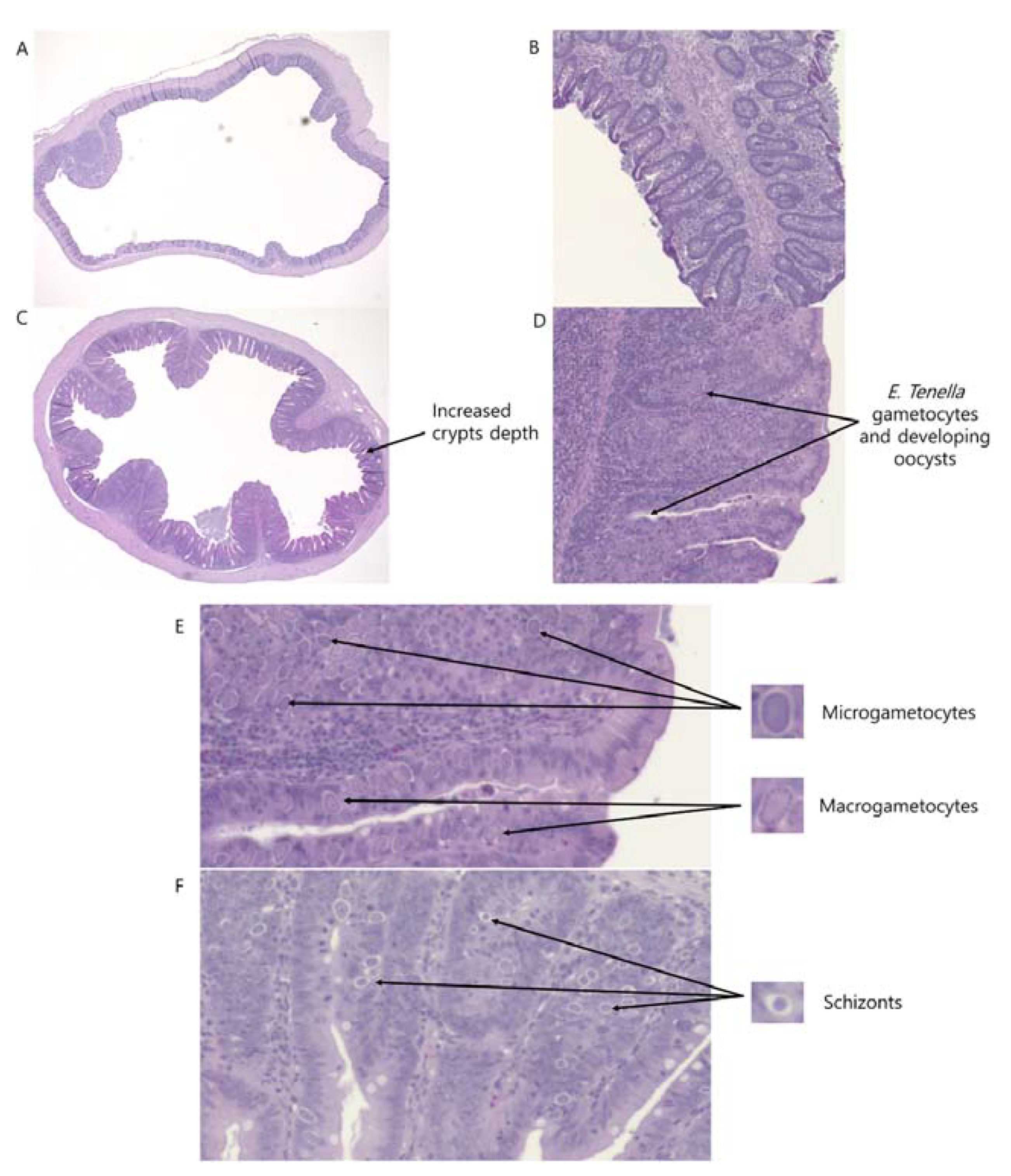
2.4. Intestinal Morphology
2.5. Apparent Ileal Digestibility
2.6. Liver Total Antioxidant Capacity (TAC)
2.7. VFA Concentrations in Cecal Digesta
2.8. Statistical Analyses
3. Results
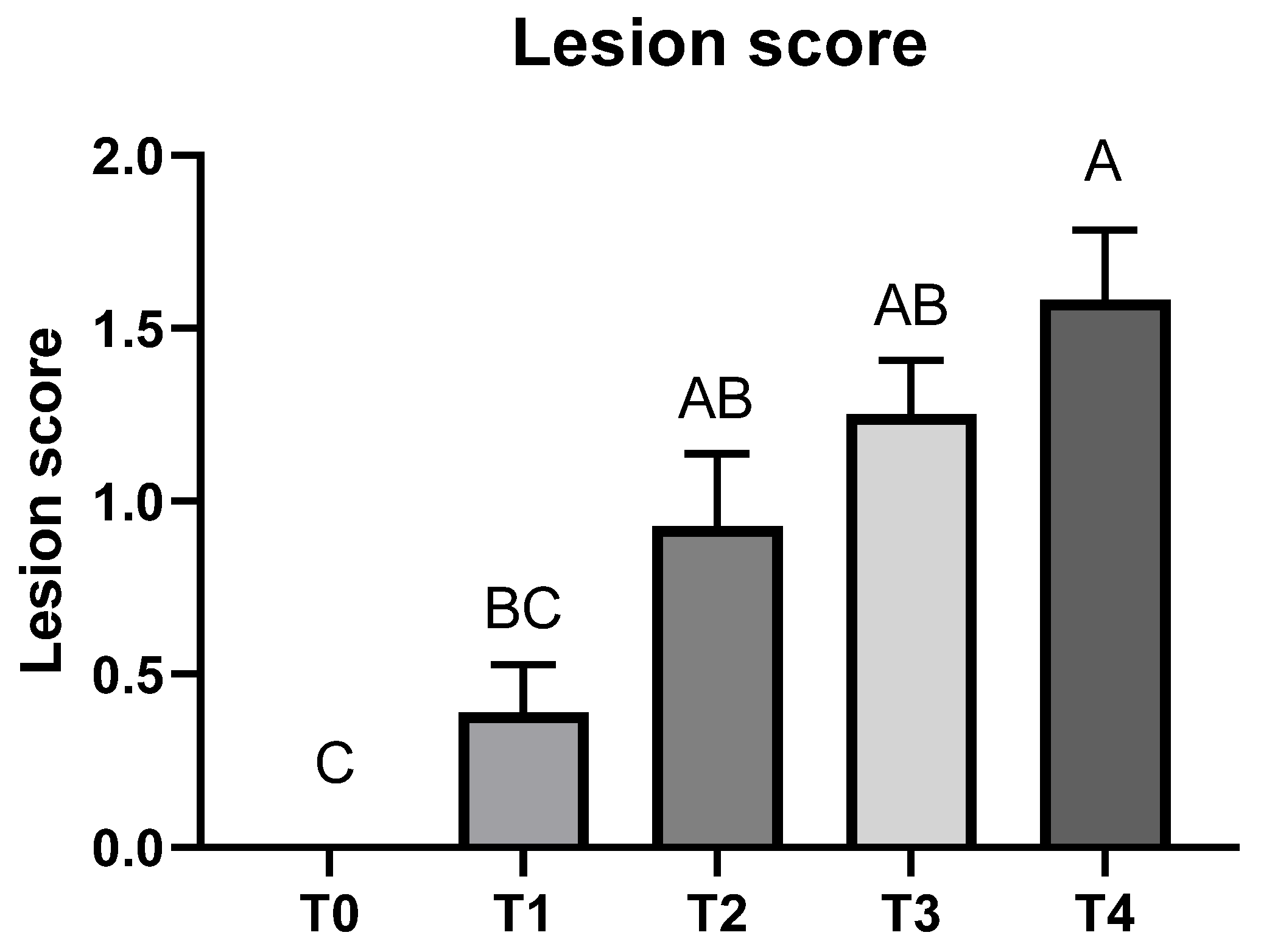
3.1. Growth Performance and Lesion Score
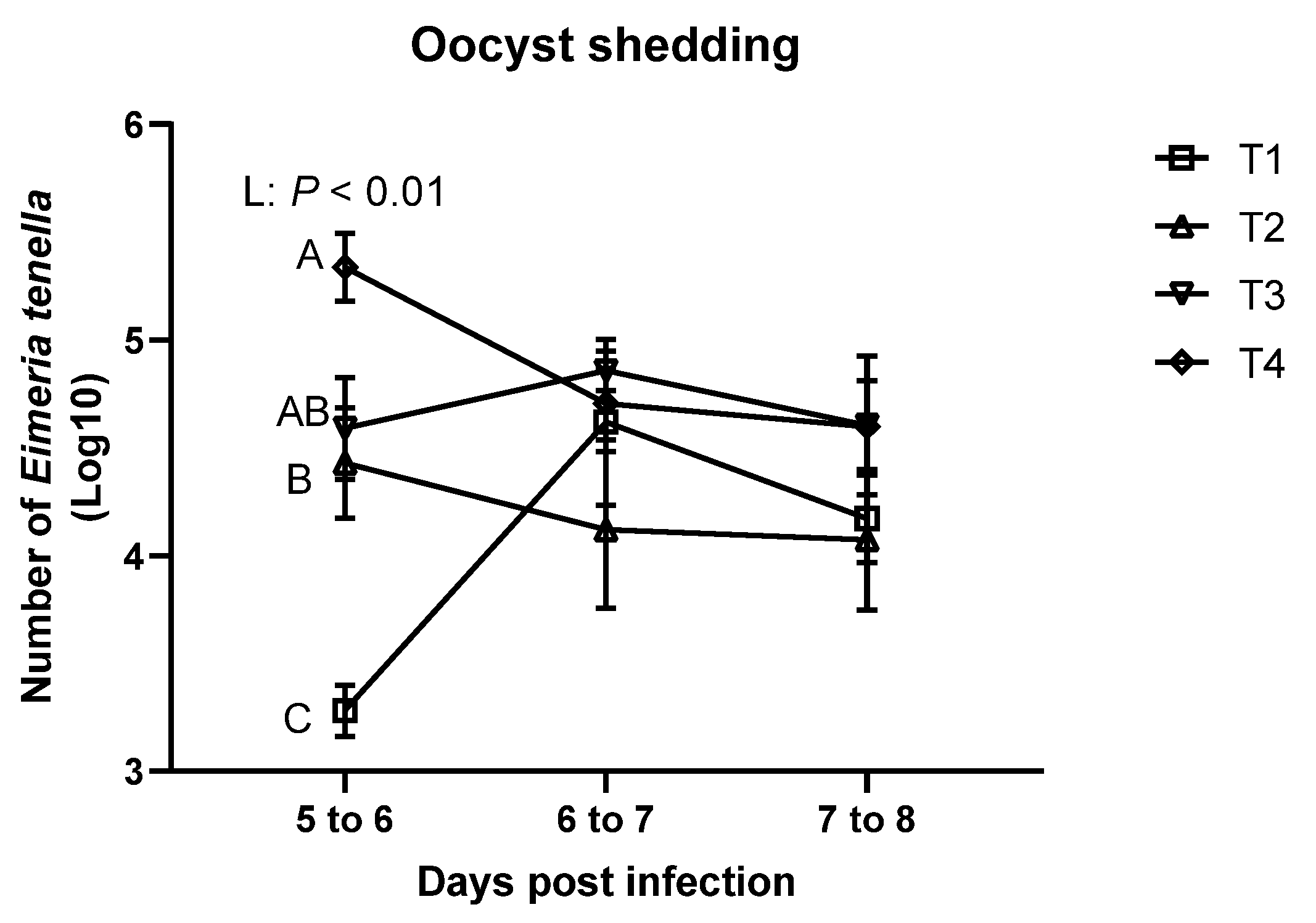
3.2. Oocyst Shedding and Fecal/ileal Moisture Content
3.3. Gastrointestinal Permeability
3.4. Intestinal Morphology and Apparent Ileal Digestibility
3.5. Liver Total Antioxidant Capacity (TAC)
3.6. VFA Concentrations in Cecal Digesta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W.K. Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: A review. Animals 2020, 10, 2389. [Google Scholar] [CrossRef]
- Vermeulen, A.; Schaap, D.; Schetters, T.P. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001, 100, 13–20. [Google Scholar] [CrossRef]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Yadav, S.; Shi, H.; Kim, W.K. Evaluating endogenous loss and standard ileal digestibility of amino acids in response to the graded severity levels of E. maxima infection. Poult. Sci. 2021, 100, 101426. [Google Scholar] [CrossRef]
- Gaboriaud, P.; Sadrin, G.; Guitton, E.; Fort, G.; Niepceron, A.; Lallier, N.; Rossignol, C.; Larcher, T.; Sausset, A.; Guabiraba, R. The absence of gut microbiota alters the development of the apicomplexan parasite Eimeria tenella. Front. Cell. Infect. Microbiol. 2020, 10, 926. [Google Scholar] [CrossRef]
- Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.N. Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology 2012, 139, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cason, J.; Hinton, A., Jr.; Northcutt, J.; Buhr, R.; Ingram, K.; Smith, D.; Cox, N. Partitioning of external and internal bacteria carried by broiler chickens before processing. J. Food Prot. 2007, 70, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken gut microbiota: Importance and detection technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Martin, S. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Sun, Y.-S.; Zhao, L.-Q.; Chen, T.-T.; Fan, M.-N.; Jiao, H.-C.; Zhao, J.-P.; Wang, X.-J.; Li, F.-C.; Li, H.-F. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef] [Green Version]
- Broom, L.J. Evidence-based consideration of dietary ‘alternatives’ to anticoccidial drugs to help control poultry coccidial infections. Worlds Poult. Sci. J. 2021, 77, 43–54. [Google Scholar] [CrossRef]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, D.P.; Tomley, F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.; Spais, A. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef]
- Memon, F.; Yang, Y.; Lv, F.; Soliman, A.; Chen, Y.; Sun, J.; Wang, Y.; Zhang, G.; Li, Z.; Xu, B. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J. Appl. Microbiol. 2021, 131, 425–434. [Google Scholar] [CrossRef]
- Zhou, Z.; Nie, K.; Huang, Q.; Li, K.; Sun, Y.; Zhou, R.; Wang, Z.; Hu, S. Changes of cecal microflora in chickens following Eimeria tenella challenge and regulating effect of coated sodium butyrate. Exp. Parasitol. 2017, 177, 73–81. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Chen, S.; Jia, R.; Huang, Y.; Zou, Y.; Li, L.; Zhao, X.; Yin, Z. Anticoccidial effect of herbal powder “Shi Ying Zi” in chickens infected with Eimeria tenella. Animals 2020, 10, 1484. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Lin, Y.; Olukosi, O.A. Qualitative and quantitative profiles of jejunal oligosaccharides and cecal short-chain fatty acids in broiler chickens receiving different dietary levels of fiber, protein and exogenous enzymes. J. Sci. Food Agric. 2021, 101, 5190–5201. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.M.; Kieran, T.J.; Seidel, D.S.; Glenn, T.C.; Silveira, M.F.D.; Callaway, T.R.; Stewart, R.L., Jr. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLoS ONE 2020, 15, e0231533. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Giannenas, I.; Papazahariadou, M.; Botsoglou, N.A.; Spais, A.B. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 2004, 53, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, N.; Koinarski, V.; Gadjeva, V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006, 172, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Aljumaah, M.R.; Alkhulaifi, M.M.; Abudabos, A.M.; Alabdullatifb, A.; El-Mubarak, A.H.; Al Suliman, A.R.; Stanley, D. Organic acid blend supplementation increases butyrate and acetate production in Salmonella enterica serovar Typhimurium challenged broilers. PLoS ONE 2020, 15, e0232831. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Nolan, M.J.; Harman, K.; Boulton, K.; Hume, D.A.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS ONE 2017, 12, e0184890. [Google Scholar] [CrossRef]
- Kley, M.-V.; Oviedo-Rondón, E.; Dowd, S.; Hume, M.; Nalian, A. Effect of Eimeria infection on cecal microbiome of broilers fed essential oils. ARS USDA Submiss. 2012, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell. Fact. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gasaway, W.C. Volatile fatty acids and metabolizable energy derived from cecal fermentation in the willow ptarmigan. Comp. Biochem. Physiol. Part A Physiol. 1976, 53, 115–121. [Google Scholar] [CrossRef]
- Santos, T.S.D.; Teng, P.-Y.; Yadav, S.; Castro, F.L.D.S.; Gould, R.L.; Craig, S.W.; Chen, C.; Fuller, A.L.; Pazdro, R.; Sartori, J.R. Effects of Inorganic Zn and Cu Supplementation on Gut Health in Broiler Chickens Challenged with Eimeria spp. Front. Vet. Sci. 2020, 7, 230. [Google Scholar] [CrossRef]
- Renaudeau, D.; Gilbert, H.; Noblet, J. Effect of climatic environment on feed efficiency in swine. In Feed Efficiency in Swine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–210. [Google Scholar]
- Canfora, E.E.; Blaak, E.E. Acetate: A diet-derived key metabolite in energy metabolism: Good or bad in context of obesity and glucose homeostasis? Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Adji, A.V.; Plumeriastuti, H.; Ma’ruf, A.; Legowo, D. Histopathological Alterations of Ceca in Broiler Chickens (Gallus gallus) Exposed to Chronic Heat Stress. World 2019, 9, 211–217. [Google Scholar] [CrossRef]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Clench, M.H.; Mathias, J.R. The avian cecum: A review. Wilson Bull. 1995, 107, 93–121. [Google Scholar]
- Teng, P.-Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: Its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001, 31, 1056–1069. [Google Scholar] [CrossRef]
- Chadwick, E.; Rahimi, S.; Grimes, J.; Pitts, J.; Beckstead, R. Sodium bisulfate feed additive aids broilers in growth and intestinal health during a coccidiosis challenge. Poult. Sci. 2020, 99, 5324–5330. [Google Scholar] [CrossRef]
- Nakahiro, Y.; Isshiki, Y. Effect of cecal ligation on digestibility of crude fiber, cellulose and pentosan in chickens. Jpn. Poult. Sci. 1975, 12, 138–140. [Google Scholar] [CrossRef] [Green Version]
- Van der Hoeven-Hangoor, E.; Van de Linde, I.; Paton, N.; Verstegen, M.; Hendriks, W. Effect of different magnesium sources on digesta and excreta moisture content and production performance in broiler chickens. Poult. Sci. 2013, 92, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Faradilla, Z.S.; Yunus, M.; Hermadi, H.A. The Effect of Dietary Administration of Virgin Coconut oil on Differential Leukocytes in Infected Chicken with Eimeria tenella. J. World’s Poult. Res. 2020, 10, 615–622. [Google Scholar] [CrossRef]



| Ingredients | D 14 to 23 |
|---|---|
| Corn | 700.8 |
| Soybean meal (480 g crude protein/kg) | 241.73 |
| Soybean oil | 15.84 |
| Deflour phosphate | 13.99 |
| Sand | 7.00 |
| Limestone | 6.11 |
| Titanium dioxide | 3.00 |
| DL-Methionine 99% | 2.86 |
| L-Lysine HCl 78% | 2.80 |
| Vitamin Premix 1 | 2.50 |
| Common Salt | 1.79 |
| L-threonine | 0.77 |
| Mineral Premix 2 | 0.80 |
| Total | 1,000 |
| Calculated energy and nutrient value, % | |
| Metabolizable energy, Mcal/kg | 3,100 |
| Crude protein | 18.375 |
| SID 3 Methionine | 0.552 |
| SID Total sulfur amino acids | 0.8 |
| SID Lysine | 1.02 |
| SID Threonine | 0.66 |
| Total calcium | 0.76 |
| Available phosphate | 0.38 |
| Items | Eimeria tenella-Challenged | Polynomial Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | SEM | p Value | Lin. | Quad. | |
| Initial BW | 359.4 | 360.3 | 356 | 359.5 | 358 | 3.72 | 0.38 | ||
| 0 to 6 dpi | |||||||||
| BW | 703.4 | 705.5 | 697 | 699.5 | 680.77 | 27.28 | 0.55 | 0.16 | 0.43 |
| ADG | 57.33 | 57.53 | 57.01 | 56.54 | 53.80 | 4.32 | 0.56 | 0.16 | 0.37 |
| ADFI | 84.92 | 87.24 | 88.63 | 86.05 | 84.31 | 4.61 | 0.55 | 0.69 | 0.11 |
| FCR | 1.48 | 1.52 | 1.55 | 1.52 | 1.57 | 0.06 | 0.19 | 0.04 | 0.64 |
| 6 to 9 dpi | |||||||||
| BW | 881.8 | 859 | 876.9 | 885.73 | 889.19 | 79.63 | 0.97 | 0.69 | 0.72 |
| ADG | 65.81 | 57.40 | 61.68 | 63.83 | 69.70 | 13.74 | 0.62 | 0.43 | 0.22 |
| ADFI | 115.4 | 120.9 | 123.77 | 123.1 | 123.1 | 14.86 | 0.87 | 0.4 | 0.55 |
| FCR | 1.9 | 2.17 | 2.03 | 1.97 | 1.77 | 0.39 | 0.50 | 0.38 | 0.16 |
| 0 to 9 dpi | |||||||||
| ADG | 60.16 | 57.49 | 58.83 | 58.97 | 59.10 | 6.47 | 0.97 | 0.94 | 0.66 |
| ADFI | 95.09 | 98.47 | 100.6 | 98.16 | 97.24 | 6.81 | 0.72 | 0.65 | 0.22 |
| FCR | 1.62 | 1.74 | 1.71 | 1.67 | 1.64 | 0.14 | 0.54 | 0.85 | 0.15 |
| Items | Eimeria tenella-Challenged | Polynomial Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | SEM | p Value | Lin. | Quad. | |
| Feces | |||||||||
| 5 to 6 dpi | 78.23 | 76.76 | 75.91 | 76.56 | 76.99 | 1.66 | 0.22 | 0.22 | 0.04 |
| 6 to 7 dpi | 68.14 | 69.68 | 73.38 | 74.64 | 68.66 | 4.87 | 0.12 | 0.35 | 0.03 |
| 7 to 8 dpi | 70.81 | 71.87 | 73.09 | 75.07 | 74.86 | 2.52 | 0.03 | <0.01 | 0.65 |
| Ileum | |||||||||
| 6 dpi | 81.75 | 81.83 | 80.6 | 80.94 | 80.81 | 0.76 | 0.03 | 0.01 | 0.33 |
| 9 dpi | 80.34 | 80.1 | 81.24 | 80.62 | 79.93 | 1.22 | 0.42 | 0.9 | 0.18 |
| Items | Eimeria tenella-Challenged | Polynomial Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | SEM | p Value | Lin. | Quad. | |
| 6 dpi | |||||||||
| Duodenum | |||||||||
| VH | 2459.97 | 2585.52 | 2442.42 | 2154.12 | 2485.42 | 300.16 | 0.22 | 0.34 | 0.56 |
| CD | 282.46 | 245.17 | 253.82 | 227.51 | 255.55 | 35.36 | 0.17 | 0.13 | 0.08 |
| VH:CD | 9.05 | 10.8 | 9.86 | 10.39 | 9.85 | 1.78 | 0.53 | 0.61 | 0.26 |
| Jejunum | |||||||||
| VH | 1411.46 | 1375.62 | 1332.65 | 1321.93 | 1303.21 | 147 | 0.71 | 0.17 | 0.76 |
| CD | 234.92 | 222.03 | 223.93 | 221.29 | 225.43 | 34.52 | 34.52 | 0.66 | 0.57 |
| VH:CD | 6.38 | 6.56 | 6.08 | 6.28 | 5.94 | 1.22 | 0.91 | 0.47 | 0.85 |
| Ileum | |||||||||
| VH | 899.01 | 936.51 | 934.37 | 894.06 | 879.21 | 113.9 | 0.87 | 0.58 | 0.41 |
| CD | 194.11 | 181.49 | 183.02 | 184.97 | 161.46 | 24.03 | 0.24 | 0.06 | 0.59 |
| VH:CD | 4.92 | 5.28 | 5.18 | 5.09 | 5.72 | 0.59 | 0.22 | 0.08 | 0.54 |
| Cecal CD | 217.21 b | 410.22 a | 451.86 a | 593.18 a | 477.05 a | 111.85 | <0.01 | <0.01 | <0.01 |
| 9 dpi | |||||||||
| Duodenum | |||||||||
| VH | 2475 | 2574.81 | 2452.6 | 2150.15 | 2375.54 | 278.63 | 0.17 | 0.1 | 0.87 |
| CD | 250.69 | 226.78 | 233.48 | 211.26 | 234.56 | 35.58 | 0.49 | 0.31 | 0.23 |
| VH:CD | 10.26 | 11.55 | 11.04 | 10.74 | 10.32 | 1.95 | 0.77 | 0.78 | 0.28 |
| Jejunum | |||||||||
| VH | 1410.12 | 1348.81 | 1320.96 | 1288.44 | 1257.51 | 159.32 | 0.54 | 0.09 | 0.82 |
| CD | 208.29 | 217.18 | 197.49 | 211.8 | 210.85 | 34.22 | 0.87 | 0.99 | 0.78 |
| VH:CD | 7.21 | 6.55 | 7.06 | 6.5 | 6.21 | 1.86 | 0.69 | 0.26 | 0.87 |
| Ileum | |||||||||
| VH | 888.68 | 917.4 | 879.2 | 885.43 | 876.32 | 108.22 | 0.97 | 0.69 | 0.85 |
| CD | 173.99 | 167.73 | 158.63 | 161.9 | 155.46 | 28.8 | 0.8 | 0.26 | 0.78 |
| VH:CD | 5.35 | 5.57 | 5.74 | 5.7 | 5.95 | 0.72 | 0.69 | 0.17 | 0.89 |
| Cecal CD | 87.57 b | 163.55 a,b | 182.47 a | 224.19 a | 196.3 a | 47.71 | <0.01 | <0.01 | 0.02 |
| Items | Eimeria tenella-Challenged 1 | Polynomial Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | SEM | p Value | Lin. | Quad. | |
| 6 dpi | |||||||||
| DM | 69.16 | 70.31 | 68.35 | 71.53 | 70.19 | 1.96 | 0.09 | 0.21 | 0.96 |
| OM | 70.85 | 72.08 | 70.13 | 73.32 | 71.79 | 1.94 | 0.09 | 0.23 | 0.89 |
| Ash | 40.16 | 40 | 37.66 | 40.82 | 42.66 | 5.47 | 0.6 | 0.42 | 0.26 |
| 9 dpi | |||||||||
| DM | 72.71 | 70.31 | 71.86 | 72.44 | 73.5 | 2.8 | 0.42 | 0.36 | 0.2 |
| OM | 75.02 | 72.29 | 74.32 | 74.68 | 75.8 | 2.69 | 0.29 | 0.31 | 0.18 |
| Ash | 33.03 | 36.24 | 29.47 | 33.86 | 33.82 | 9.49 | 0.81 | 0.95 | 0.76 |
| Items | Eimeria tenella-Challenged 1 | Polynomial Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | SEM | p Value | Lin. | Quad. | |
| 6 dpi | |||||||||
| Acetate | 48.21 a | 35.2 a,b | 35.64 a,b | 36.94 a,b | 26.91 b | 10 | 0.02 | <0.01 | 0.65 |
| Propionate | 8.37 | 9.03 | 0.84 | 7.27 | 6.1 | 2.95 | 0.5 | 0.11 | 0.45 |
| Isobutyrate | 0.26 | 0.17 | 0.19 | 0.15 | 0.12 | 0.08 | 0.07 | <0.01 | 00.73 |
| Butyrate | 8.69 | 5.8 | 7.4 | 5.34 | 4.96 | 3.8 | 0.42 | 0.12 | 0.82 |
| Isovalerate | 0.42 | 0.42 | 0.45 | 0.32 | 0.37 | 0.17 | 0.72 | 0.35 | 0.82 |
| Valerate | 1.09 | 0.95 | 0.78 | 0.69 | 0.8 | 0.51 | 0.72 | 0.22 | 0.47 |
| Total VFA | 67.04 a | 51.58 a,b | 52.52 a,b | 50.73 a,b | 39.26 b | 13.95 | 0.04 | <0.01 | 0.8 |
| 9 dpi | |||||||||
| Acetate | 50.77 | 76.52 | 62.01 | 46.47 | 48.11 | 27.04 | 0.31 | 0.32 | 0.25 |
| Propionate | 6.14 b | 11.87 a | 8.17 a,b | 7.82 a,b | 7.07 a,b | 3.34 | 0.06 | 0.62 | 0.07 |
| Isobutyrate | 0.16 | 0.32 | 0.15 | 0.16 | 0.16 | 0.14 | 0.22 | 0.39 | 0.55 |
| Butyrate | 8.64 | 13.04 | 8.58 | 6.24 | 6.3 | 4.94 | 0.16 | 0.09 | 0.4 |
| Isovalerate | 0.26 | 0.53 | 0.39 | 0.37 | 0.6 | 0.39 | 0.59 | 0.31 | 0.96 |
| Valerate | 0.96 a,b | 1.65 a | 0.86 a,b | 0.8 a,b | 0.57 b | 0.55 | 0.03 | 0.03 | 0.21 |
| Total VFA | 66.94 | 103.94 | 68.72 | 61.86 | 62.83 | 35.97 | 0.26 | 0.29 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Ko, H.; Tompkins, Y.H.; Teng, P.-Y.; Lourenco, J.M.; Callaway, T.R.; Kim, W.K. Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals 2021, 11, 3428. https://doi.org/10.3390/ani11123428
Choi J, Ko H, Tompkins YH, Teng P-Y, Lourenco JM, Callaway TR, Kim WK. Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals. 2021; 11(12):3428. https://doi.org/10.3390/ani11123428
Chicago/Turabian StyleChoi, Janghan, Hanseo Ko, Yuguo Hou Tompkins, Po-Yun Teng, Jeferson M. Lourenco, Todd R. Callaway, and Woo Kyun Kim. 2021. "Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens" Animals 11, no. 12: 3428. https://doi.org/10.3390/ani11123428
APA StyleChoi, J., Ko, H., Tompkins, Y. H., Teng, P.-Y., Lourenco, J. M., Callaway, T. R., & Kim, W. K. (2021). Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals, 11(12), 3428. https://doi.org/10.3390/ani11123428








