Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Growth Performance and Organ Indices
2.3. Analyses of Plasma Biochemical Indicators
2.4. Microbial Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Tributyrin on Growth Performance
3.2. Effects of Tributyrin on Organ Indices
3.3. Effects of Tributyrin on Biochemical Indices
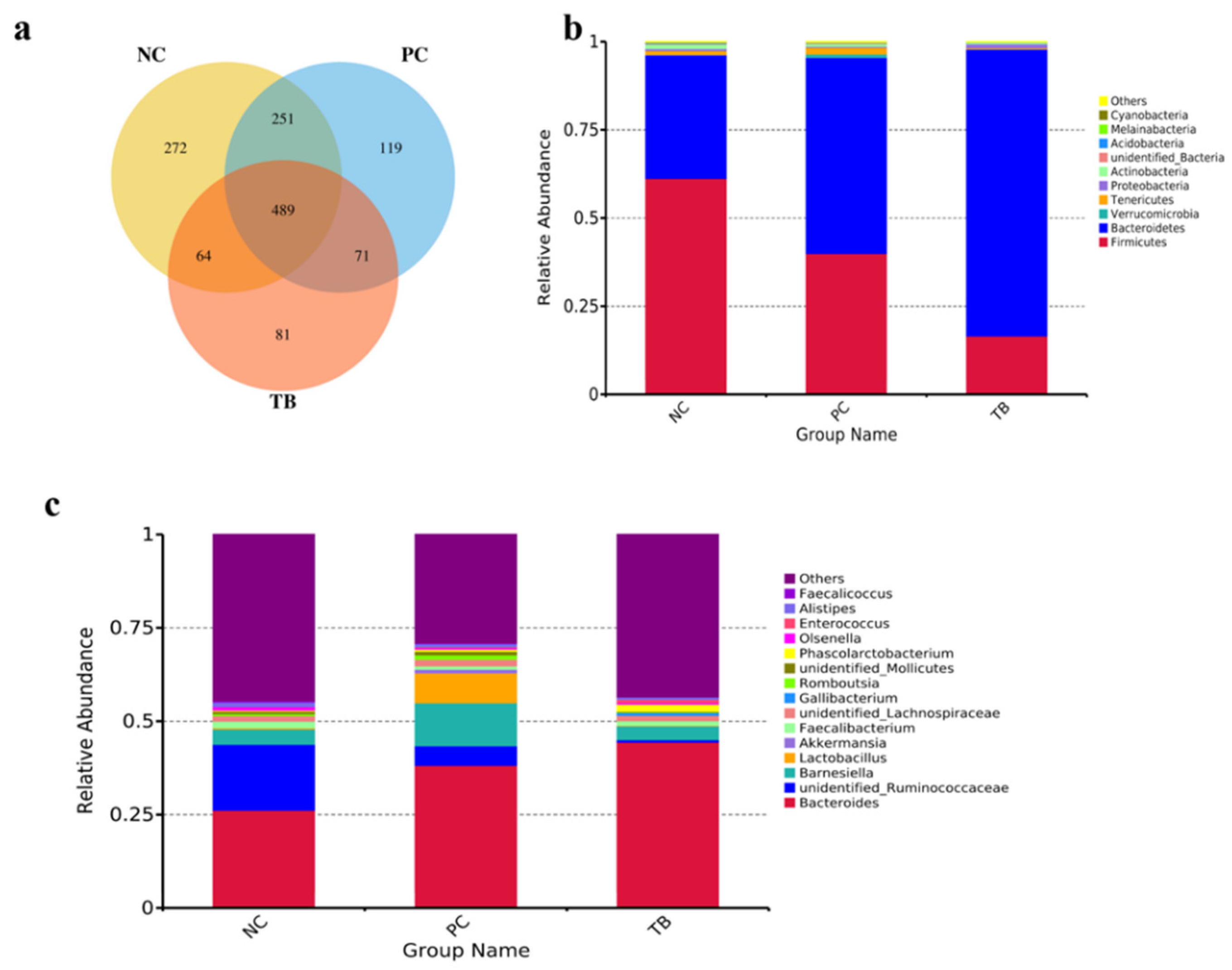
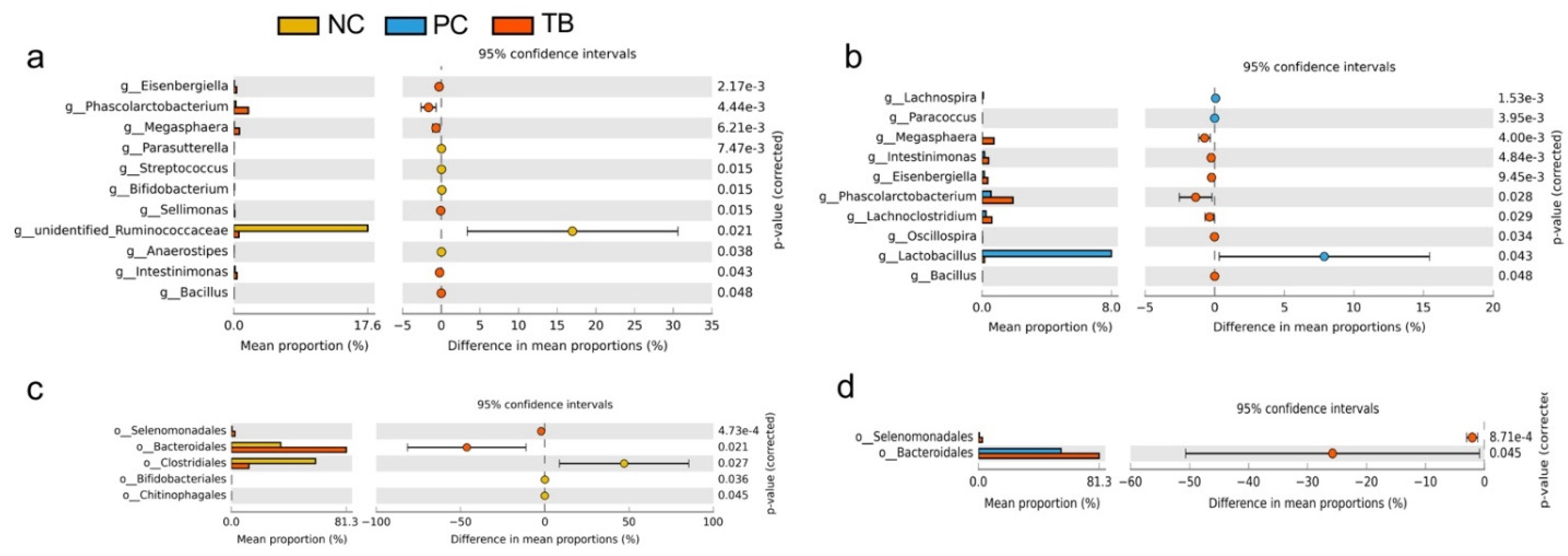
3.4. Effects of Tributyrin on Intestinal Microbiota
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Union, E. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. 2006. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687 (accessed on 16 October 2021).
- Echemi. The Feed Prohibition Order Came into Effect in July. 2020. Available online: https://www.echemi.com/cms/110309.html (accessed on 16 October 2021).
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Onrust, L.; Baeyen, S.; Haesebrouck, F.; Ducatelle, R.; Immerseel, F.V. Effect of in feed administration of different butyrate formulations on Salmonella Enteritidis colonization and cecal microbiota in broilers. Vet. Res. 2020, 51, 56. [Google Scholar] [CrossRef] [PubMed]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S.J.P. Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Ropy, A.; Abdel-Razik, A.H. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Animal 2019, 13, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.B.; Navegantes-Lima, K.C.; Oliveira, A.L.B.; Rodrigues, D.V.S.; Gaspar, S.L.F.; Monteiro, V.V.S.; Moura, D.P.; Monteiro, M.C. Protective Mechanisms of Butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2018, 24, 4154–4166. [Google Scholar] [CrossRef] [PubMed]
- Biagi, G.; Piva, A.; Moschini, M.; Vezzali, E.; Roth, F.X. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 2007, 85, 1184–1191. [Google Scholar] [CrossRef]
- Szentirmai, E.; Millican, N.S.; Massie, A.R.; Kapas, L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Hansen, V.L.; Kahl, S.; Proszkowiec-Weglarz, M.; Jiménez, S.C.; Miska, K.B. The effects of tributyrin supplementation on weight gain and intestinal gene expression in broiler chickens during Eimeria maxima-induced coccidiosis. Poult. Sci. 2021, 100, 100984. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, K.; Ding, X.; Celi, P.; Wang, J. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult. Sci. 2019, 98, 6091–6099. [Google Scholar] [CrossRef]
- Sakdee, J.; Poeikhamph, T.; Rakangthon, C.; Poungpong, K.; Bunchasak, C. Effect of tributyrin supplementation in diet on production performance and gastrointestinal tract of healthy nursery pigs. Pak. J. Nutr. 2016, 15, 954–962. [Google Scholar] [CrossRef]
- Sotira, S.; Dell’Anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Effects of Tributyrin Supplementation on Growth Performance, Insulin, Blood Metabolites and Gut Microbiota in Weaned Piglets. Animals 2020, 10, 726. [Google Scholar] [CrossRef]
- Whang, K.Y.; Easter, R.A. Blood urea nitrogen as an index of feed efficiency and lean growth potential in growing-finishing swine. Asian Australas. J. Anim. 2000, 13, 811–816. [Google Scholar] [CrossRef]
- He, J.; Dong, L.; Xu, W.; Bai, K.; Lu, C.; Wu, Y.; Huang, Q.; Zhang, L.; Wang, T. Dietary Tributyrin Supplementation Attenuates Insulin Resistance and Abnormal Lipid Metabolism in Suckling Piglets with Intrauterine Growth Retardation. PLoS ONE 2015, 10, e0136848. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, K.; Krauze, M.; Ognik, K. Use of Bacillus subtilis PB6 enriched with choline to improve growth performance, immune status, histological parameters and intestinal microbiota of broiler chickens. Anim. Prod. Sci. 2020, 60, 625–634. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R.J. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Patrone, V.; Miragoli, F.; Prandini, A.; Sigolo, S.; Callegari, M.L. Implications of Tributyrin on Gut Microbiota Shifts Related to Performances of Weaning Piglets. Microorganisms 2021, 9, 854. [Google Scholar]
- Nguyen, T.D.; Prykhodko, O.; Hallenius, F.F.; Nyman, M. Effects of monobutyrin and tributyrin on liver lipid profile, caecal microbiota composition and SCFA in high-fat diet-fed rats. J. Nutr. Sci. 2017, 6, e51. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.G.; Lillehoj, E.P.; Lillehoj, H.S. Dietary Supplementation with Bacillus subtilis Direct-Fed Microbials Alters Chicken Intestinal Metabolite Levels. Front. Vet. Sci. 2020, 7, 123. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Chen, G.; Zhuo, R.; Ding, H.; Yang, K.; Xue, J.; Zhang, S.; Chen, L.; Yin, Y.; Fang, R. Effects of dietary tributyrin and physterol ester supplementation on growth performance, intestinal morphology, microbiota and metabolites in weaned piglets. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sotira, S.; Dell’Anno, M.; Caprarulo, V.; Hejna, M.; Pirrone, F.; Callegari, M.L.; Tucci, T.V.; Rossi, L. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 2021, 100, 101429. [Google Scholar] [CrossRef]
- Liang, H.; Ji, K.; Ge, X.; Xi, B.; Chen, X. Tributyrin Plays an Important Role in Regulating the Growth and Health Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala), as Evidenced by Pathological Examination. Front. Immunol. 2021, 12, 1160. [Google Scholar] [CrossRef]
- Inabu, Y.; Murayama, K.; Inouchi, K.; Sugino, T. The effect of tributyrin supplementation to milk replacer on plasma glucagon-like peptide 2 concentrations in pre-weaning calves. Anim. Sci. J. 2019, 90, 1185–1192. [Google Scholar] [CrossRef]
- Siavoshian, S.; Segain, J.P.; Kornprobst, M.; Bonnet, C.; Cherbut, C.; Galmiche, J.-P.; Blottière, H.M. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: Induction of cyclin D3 and p21 expression. Gut 2000, 46, 507. [Google Scholar]
- Liu, J.D.; Bayir, H.O.; Cosby, D.E.; Cox, N.A.; Williams, S.M.; Fowler, J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017, 96, 3638–3644. [Google Scholar] [CrossRef]
- Hu, Q.; Yin, F.; Li, B.; Guo, Y.; Yin, Y. Dietary Tributyrin Administration Improves Intestinal Morphology and Selected Bacterial and Short-Chain Fatty Acid Profiles in Broilers Under an Isocaloric Feeding Regime. Front. Microbiol. 2021, 12, 715712. [Google Scholar] [CrossRef] [PubMed]
- Antongiovanni, M.; Buccioni, A.; Petacchi, F.; Leeson, S.; Minieri, S.; Martini, A.; Cecchi, R.J.I. Butyric acid glycerides in the diet of broiler chickens: Effects on gut histology and carcass composition. Ital. J. Anim. Sci. 2010, 6, 19–25. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Hernandez, M.; Squires, E.J.; Leeson, S.; Hou, Y.; Gong, J. Response of Ross 308 and 708 broiler strains in growth performance and lipid metabolism to diets containing tributyrate glycerides. Can. J. Anim. Sci. 2017, 98, 98–108. [Google Scholar] [CrossRef]
- Elazab, S.T.; Elshater, N.S.; Kishaway, A.T.Y.; Ei-Emam, H.A. Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens. Animals 2021, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, G.; Wang, G.; Liu, Y.; Zhang, L.; Wang, W.; Chen, N. Association of serum uric acid levels with the incident of kidney disease and rapid eGFR decline in Chinese individuals with eGFR > 60 mL/min/1.73 m2 and negative proteinuria. Clin. Exp. Nephrol. 2019, 23, 871–879. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Yang, Y.; Ma, H.; Gong, Q.; Kan, X.; Ran, X.; Cao, Y.; Wang, J.; Fu, S.J. Rumen-bypassed tributyrin alleviates heat stress by reducing the inflammatory responses of immune cells. Poult. Sci. 2021, 100, 348–356. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Liao, X.D.; Wu, Y.; Liang, J.B.; Laudadio, V.; Tufarelli, V. Sodium butyrate mitigates in vitro ammonia generation in cecal content of laying hens. Environ. Sci. Pollut Res. Int. 2016, 23, 16272–16279. [Google Scholar] [CrossRef]
- Becker, B.F. Towards the physiological function of uric acid. Free Radic. Biol. Med. 1993, 14, 615–631. [Google Scholar] [CrossRef]
- Spitsin, S.V.; Scott, G.S.; Kean, R.B.; Mikheeva, T.; Hooper, D.C. Protection of myelin basic protein immunized mice from free-radical mediated inflammatory cell invasion of the central nervous system by the natural peroxynitrite scavenger uric acid. Neurosci. Lett. 2000, 292, 137–141. [Google Scholar] [CrossRef]
- Ozer, E.K.; Goktas, M.T.; Kilinc, I.; Bariskaner, H.; Iskit, A.B.J.B.; Biomedecine, P. Celecoxib administration reduced mortality, mesenteric hypoperfusion, aortic dysfunction and multiple organ injury in septic rats. Biomed. Pharmacother. 2016, 86, 583. [Google Scholar] [CrossRef] [PubMed]
- Shaufi, M.A.M.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, H.; Song, Y.; Sun, C.; Zhang, Z.; Chen, S. Community composition of cecal microbiota in commercial yellow broilers with high and low feed efficiencies. Poult. Sci. 2021, 100, 100996. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilan, C.G. Shaping the metabolism of intestinal Bacteroides population through diet to improve human health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Cho, K.H.; Cho, D.; Wang, G.R.; Salyers, A.A. New regulatory gene that contributes to control of Bacteroides thetaiotaomicron starch utilization genes. J. Bacteriol. 2001, 183, 7198–7205. [Google Scholar] [CrossRef][Green Version]
- Zheng, M.; Mao, P.; Tian, X.; Guo, Q.; Meng, L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult. Sci. 2019, 98, 2250–2259. [Google Scholar] [CrossRef]
- Dahiya, D.; Renuka, D.A.; Shandilya, U.; Puniya, A.; Shukla, P. New-generation probiotics: Perspectives and applications. In Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications; Elsevier Ltd., Academic Press: Amsterdam, The Netherland, 2019; pp. 417–424. [Google Scholar]
- Klaering, K.; Hanske, L.; Bui, N.; Charrier, C.; Blaut, M.; Haller, D.; Plugge, C.M.; Clavel, T. Intestinimonas butyriciproducens gen. nov., sp. nov., a butyrate-producing bacterium from the mouse intestine. Int. J. Syst. Evol. Microbiol. 2013, 63, 4606–4612. [Google Scholar] [CrossRef]
- Khan, A.; Chattopadhyay, P.; Devi, P.; Pandey, R. Epigenetics of Host–Human Gut Microbiome Interactions; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Velge, P.; Bottreau, E.; Fievez, V.; Haesebrouck, F.; Ducatelle, R. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003, 85, 237–248. [Google Scholar] [CrossRef]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, H.; Wang, Y.; Zheng, X.; He, L.; Qi, W.; Wang, T.; Guo, B.; Guo, G.; Zhang, Z. Echinococcus granulosus infection results in an increase in Eisenbergiella and Parabacteroides genera in the gut of mice. Front. Microbiol. 2018, 9, 2890. [Google Scholar] [CrossRef]
- Ariefdjohan, M.W.; Dilk, A.; Brown-Esters, O.N.; Savaiano, D.A. Intestinal Microbiota and Diet in Health. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 811–834. [Google Scholar]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548. [Google Scholar] [CrossRef]



| Day 1–21 | Day 22–42 | Day 43–63 | |
|---|---|---|---|
| Feed ingredients (%) | |||
| Corn | 61.00 | 63.26 | 65.52 |
| Soybean meal | 32.00 | 28.00 | 24.00 |
| Corn gluten meal | 1.50 | 2.00 | 3.00 |
| Soybean oil | 1.40 | 2.50 | 3.50 |
| Limestone | 1.41 | 1.41 | 1.35 |
| Dicalcium phosphate | 1.33 | 1.33 | 1.33 |
| DL-Methionine (99%) | 0.18 | 0.15 | 0.12 |
| L-Lysine-HCl (78%) | 0.18 | 0.18 | 0.18 |
| Wheat middling | 0.11 | 0.17 | 0.00 |
| Vitamin-mineral premix a | 1.00 | 1.00 | 1.00 |
| Calculated nutrient composition b | |||
| ME (MJ/kg) | 12.12 | 12.54 | 12.96 |
| CP (%) | 19.91 | 18.63 | 17.60 |
| Lys (%) | 1.09 | 1.00 | 0.92 |
| Met (%) | 0.51 | 0.46 | 0.42 |
| Ca (%) | 0.87 | 0.88 | 0.84 |
| Available Phosphorus (%) | 0.42 | 0.40 | 0.38 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| NC | PC | TB | |||
| Initial BW, g | 36.57 | 36.95 | 37.41 | 0.50 | 0.519 |
| Final BW, g | 1581.96 b | 1624.97 a | 1609.84 | 13.56 | 0.103 |
| ADG, g/d | |||||
| 1–21 d | 16.98 | 17.73 | 17.55 | 0.28 | 0.185 |
| 22–42 d | 34.03 | 34.40 | 35.94 | 0.59 | 0.524 |
| 43–63 d | 26.23 | 27.10 | 26.40 | 0.57 | 0.286 |
| 1–63 d | 25.74 | 26.41 | 26.24 | 0.35 | 0.064 |
| ADFI, g/d | |||||
| 1–21 d | 27.26 | 26.96 | 27.50 | 0.44 | 0.185 |
| 22–42 d | 72.39 | 72.98 | 70.99 | 0.85 | 0.542 |
| 43–63 d | 79.79 | 82.56 | 80.05 | 0.95 | 0.798 |
| 1–63 d | 59.81 | 60.83 | 59.51 | 0.56 | 0.363 |
| FCR | |||||
| 1–21 d | 1.61 | 1.52 | 1.57 | 0.02 | 0.061 |
| 22–42 d | 2.13 b | 2.12 | 2.04 a | 0.03 | 0.349 |
| 43–63 d | 3.04 | 3.05 | 3.03 | 0.06 | 0.617 |
| 1–63 d | 2.32 | 2.30 | 2.27 | 0.02 | 0.341 |
| Items (% BW) | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| NC | PC | TB | |||
| Bursa index | |||||
| 21 d | 0.26 | 0.25 | 0.24 | 0.007 | 0.5053 |
| 42 d | 0.18 | 0.23 | 0.21 | 0.008 | 0.0748 |
| 63 d | 0.11 | 0.13 | 0.11 | 0.005 | 0.1552 |
| Spleen index | |||||
| 21 d | 0.17 | 0.19 | 0.18 | 0.006 | 0.7373 |
| 42 d | 0.18 | 0.17 | 0.17 | 0.005 | 0.6141 |
| 63 d | 0.16 | 0.17 | 0.14 | 0.007 | 0.1878 |
| Liver index | |||||
| 21 d | 3.53 | 3.48 | 3.87 | 0.083 | 0.1168 |
| 42 d | 2.46 | 2.20 | 2.41 | 0.062 | 0.2286 |
| 63 d | 1.66 | 1.59 | 1.73 | 0.039 | 0.3232 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| NC | PC | TB | |||
| Uric acid (μmol/L) | |||||
| 21 d | 181.50 b | 269.67 a | 219.67 ab | 12.02 | 0.0034 |
| 42 d | 211.33 | 226.16 | 177.16 | 9.86 | 0.1102 |
| 63 d | 257.50 a | 241.66 ab | 212.33 b | 7.39 | 0.0290 |
| Lactate dehydrogenase (U/L) | |||||
| 21 d | 551.00 | 440.33 | 539.83 | 23.91 | 0.1105 |
| 42 d | 733.83 a | 524.16 b | 461.66 b | 34.54 | 0.0003 |
| 63 d | 539.83 | 540.83 | 440.00 | 20.24 | 0.0548 |
| Creatinine (μmol/L) | |||||
| 21 d | 5.50 a | 6.00 a | 4.50 b | 0.19 | 0.0014 |
| 42 d | 5.16 a | 4.83 a | 3.33 b | 0.28 | 0.0087 |
| 63 d | 5.00 a | 4.83 a | 3.50 b | 0.22 | 0.0021 |
| Alkaline phosphatase (U/L) | |||||
| 21 d | 1182.16 | 722.33 | 1312.50 | 118.67 | 0.0967 |
| 42 d | 318.33 | 187.00 | 207.00 | 39.64 | 0.3675 |
| 63 d | 64.83 | 78.33 | 79.66 | 8.80 | 0.7710 |
| Cholesterol (mmol/L) | |||||
| 21 d | 3.53 | 3.48 | 3.70 | 0.12 | 0.7758 |
| 42 d | 3.98 | 3.64 | 3.70 | 0.13 | 0.5541 |
| 63 d | 3.56 | 3.10 | 3.55 | 0.09 | 0.0752 |
| Triacylglycerols (mmol/L) | |||||
| 21 d | 0.44 | 0.50 | 0.62 | 0.03 | 0.0191 |
| 42 d | 0.58 | 0.53 | 0.52 | 0.03 | 0.6933 |
| 63 d | 0.51 | 0.53 | 0.59 | 0.02 | 0.4078 |
| High density lipoprotein (mmol/L) | |||||
| 21 d | 2.46 | 2.28 | 2.44 | 0.07 | 0.5421 |
| 42 d | 2.58 | 2.34 | 2.52 | 0.09 | 0.5494 |
| 63 d | 2.33 | 2.03 | 2.24 | 0.06 | 0.1862 |
| Low density lipoprotein (mmol/L) | |||||
| 21 d | 0.73 | 0.82 | 0.72 | 0.03 | 0.5318 |
| 42 d | 0.66 | 0.72 | 0.63 | 0.03 | 0.4990 |
| 63 d | 0.75 | 0.64 | 0.79 | 0.04 | 0.3524 |
| Alanine aminotransferase (U/L) | |||||
| 21 d | 2.00 b | 3.16 a | 2.66 ab | 0.18 | 0.0216 |
| 42 d | 1.83 | 2.00 | 1.83 | 0.13 | 0.8644 |
| 63 d | 2.00 b | 3.16 a | 2.16 b | 0.1661 | 0.0019 |
| Aspartate aminotransferase (U/L) | |||||
| 21 d | 201.50 | 222.66 | 212.16 | 4.88 | 0.2180 |
| 42 d | 196.16 | 218.16 | 187.83 | 7.11 | 0.2052 |
| 63 d | 214.00 | 210.66 | 221.83 | 6.84 | 0.8111 |
| γ-glutamyl transpeptidase (U/L) | |||||
| 21 d | 27.66 | 26.83 | 23.50 | 1.30 | 0.4096 |
| 42 d | 28.83 | 22.66 | 26.16 | 1.29 | 0.1488 |
| 63 d | 24.66 | 26.66 | 23.33 | 1.23 | 0.5671 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Xiao, G.; Zheng, L.; Yan, X.; Qi, Q.; Zhu, C.; Feng, X.; Huang, W.; Zhang, H. Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers. Animals 2021, 11, 3425. https://doi.org/10.3390/ani11123425
Gong L, Xiao G, Zheng L, Yan X, Qi Q, Zhu C, Feng X, Huang W, Zhang H. Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers. Animals. 2021; 11(12):3425. https://doi.org/10.3390/ani11123425
Chicago/Turabian StyleGong, Li, Gengsheng Xiao, Liwei Zheng, Xia Yan, Qien Qi, Cui Zhu, Xin Feng, Weilong Huang, and Huihua Zhang. 2021. "Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers" Animals 11, no. 12: 3425. https://doi.org/10.3390/ani11123425
APA StyleGong, L., Xiao, G., Zheng, L., Yan, X., Qi, Q., Zhu, C., Feng, X., Huang, W., & Zhang, H. (2021). Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers. Animals, 11(12), 3425. https://doi.org/10.3390/ani11123425






