The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Embryo Production
2.2. RNA Extraction and Reverse Transcription
2.3. Gene Expression Analysis
2.4. Statistical Analyses
3. Results
3.1. In Vitro Embryo Development
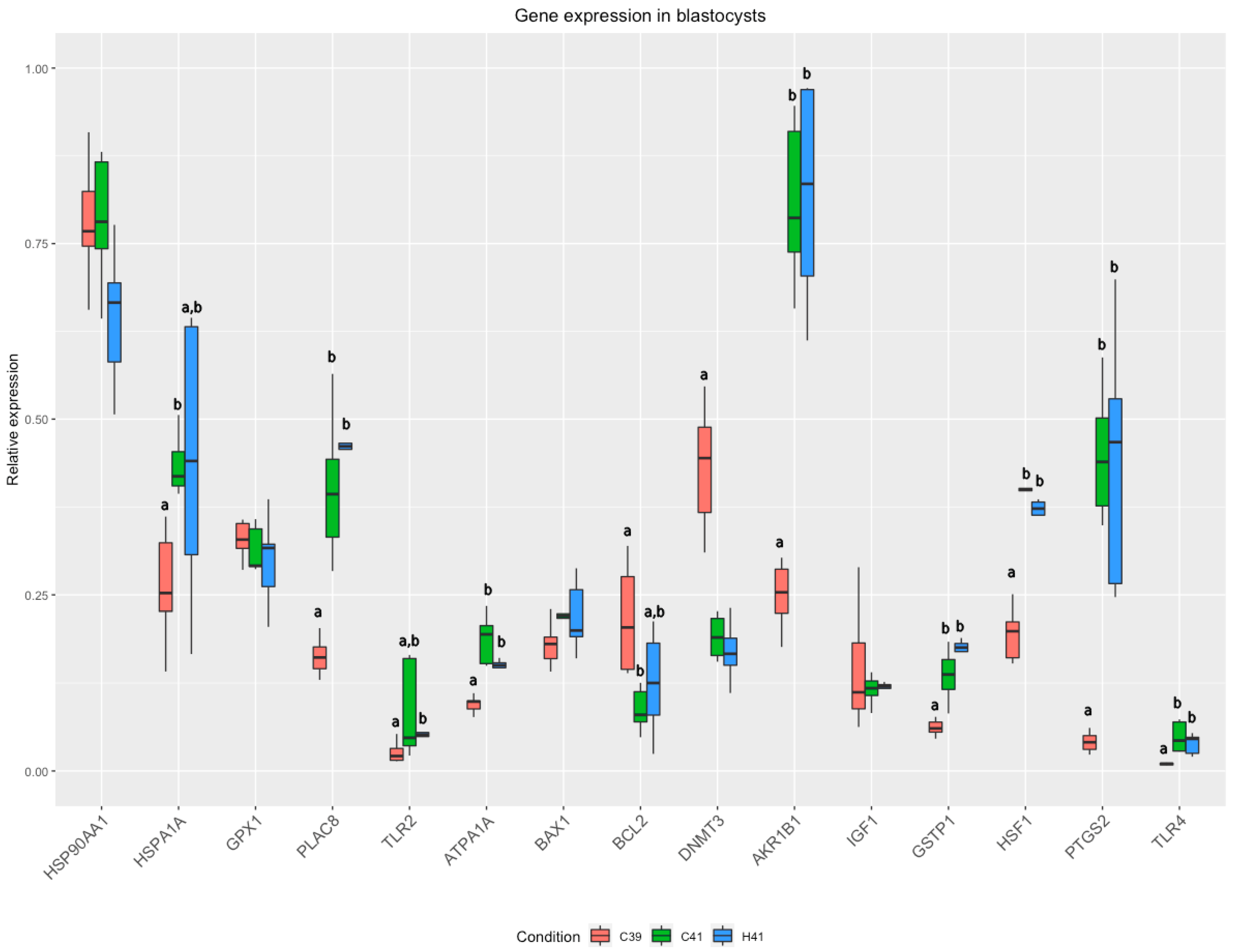
3.2. Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St-Pierre, N.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, 52–77. [Google Scholar] [CrossRef]
- Flamenbaum, I.; Galon, N. Management of heat stress to improve fertility in dairy cows in Israel. J. Reprod. Dev. 2010, 56, 36–41. [Google Scholar] [CrossRef]
- Hansen, P.J. Reproductive physiology of the heat-stressed dairy cow: Implications for fertility and assisted reproduction. Anim. Reprod. 2019, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Nanas, I.; Chouzouris, T.; Dovolou, E.; Dadouli, K.; Stamperna, K.; Kateri, I.; Barbagianni, M.; Amiridis, G.S. Early embryo losses, progesterone and pregnancy associated glycoproteins levels during summer heat stress in dairy cows. J. Therm. Biol. 2021, 98, 102951. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.C.; Parks, J.E.; Yang, X. Thermotolerance of IVM-derived bovine oocytes and embryos after short-term heat shock. Mol. Reprod. Dev. 1999, 53, 336–340. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Nanas, I.; Kalemkeridou, M.; Dadouli, K.; Moutou, K.; Amiridis, G.S.; Dovolou, E. Short term temperature elevation during IVM affects embryo yield and alters gene expression pattern in oocytes, cumulus cells and blastocysts in cattle. Theriogenology 2020, 156, 36–45. [Google Scholar] [CrossRef]
- Edwards, J.L.; Hansen, P.J. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol. Reprod. Dev. 1997, 46, 138–145. [Google Scholar] [CrossRef]
- Ealy, A.D.; Drost, M.; Hansen, P.J. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J. Dairy Sci. 1993, 76, 2899–2905. [Google Scholar] [CrossRef]
- Ealy, A.D.; Hansen, P.J. Induced thermotolerance during early development of murine and bovine embryos. J. Cell. Physiol. 1994, 160, 463–468. [Google Scholar] [CrossRef]
- Dutt, R.H. Critical period for early embryo mortality in ewes exposed to high ambient temperature. J. Anim. Sci. 1963, 22, 713–719. [Google Scholar] [CrossRef]
- Alliston, C.W.; Howarth, B.; Ulberg, L.C. Embryonic mortality following culture in vitro of one- and two-cell rabbit eggs at elevated temperatures. Reproduction 1965, 9, 337–341. [Google Scholar] [CrossRef][Green Version]
- Omtvedt, I.T.; Nelson, R.E.; Edwards, R.L.; Stephens, D.F.; Turman, E.J. Influence of heat stress during early, mid and late pregnancy of gilts. J. Anim. Sci. 1971, 32, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M.; Alvarez, N.V.; Takahashi, M.; Hansen, P.J. Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J. Dairy Sci. 2012, 95, 3080–3091. [Google Scholar] [CrossRef]
- Ortega, M.S.; Rocha-Frigoni, N.A.S.; Mingoti, G.Z.; Roth, Z.; Hansen, P.J. Modification of embryonic resistance to heat shock in cattle by melatonin and genetic variation in HSPA1L. J. Dairy Sci. 2016, 99, 9152–9164. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, B.G.; Satrapa, R.A.; Capinzaiki, C.R.L.; Trinca, L.A.; Barros, C.M. Influence of the breed of bull (Bos taurus indicus vs. Bos taurus taurus) and the breed of cow (Bos taurus indicus, Bos taurus taurus and crossbred) on the resistance of bovine embryos to heat. Anim. Reprod. Sci. 2009, 114, 54–61. [Google Scholar] [CrossRef]
- De Sousa, P.A.; Caveney, A.; Westhusin, M.E.; Watson, A.J. Temporal patterns of embryonic gene expression and their dependence on oogenetic factors. Theriogenology 1998, 49, 115–128. [Google Scholar] [CrossRef]
- Memili, E.; First, N.L. Zygotic and embryonic gene expression in cow: A review of timing and mechanisms of early gene expression as compared with other species. Zygote 2000, 8, 87–96. [Google Scholar] [CrossRef]
- Pavlok, A.; Kopečný, V.; Lucas-Hahn, A.; Niemann, H. Transcriptional activity and nuclear ultrastructure of 8-cell bovine embryos developed by in vitro maturation and fertilization of oocytes from different growth categories of antral follicles. Mol. Reprod. Dev. 1993, 35, 233–243. [Google Scholar] [CrossRef]
- Frei, R.E.; Schultz, G.A.; Church, R.B. Qualitative and quantitative changes in protein synthesis occur at the 8-16-cell stage of embryogenesis in the cow. J. Reprod. Fertil. 1989, 86, 637–641. [Google Scholar] [CrossRef]
- Laurincik, J.; Thomsen, P.D.; Hay-Schmidt, A.; Avery, B.; Greve, T.; Ochs, R.L.; Hyttel, P. Nucleolar proteins and nuclear ultrastructure in preimplantation bovine embryos produced in vitro. Biol. Reprod. 2000, 62, 1024–1032. [Google Scholar] [CrossRef][Green Version]
- Lepock, J.R. How do cells respond to their thermal environment? Int. J. Hyperth. 2005, 21, 681–687. [Google Scholar] [CrossRef]
- Sakatani, M.; Bonilla, L.; Dobbs, K.B.; Block, J.; Ozawa, M.; Shanker, S.; Yao, J.Q.; Hansen, P.J. Changes in the transcriptome of morula-stage bovine embryos caused by heat shock: Relationship to developmental acquisition of thermotolerance. Reprod. Biol. Endocrinol. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Yoshinaga, T.; Jalufka, F.L.; Ehsan, H.; Welch, D.B.M.; Kaneko, G. The complex evolution of the metazoan HSP70 gene family. Sci. Rep. 2021, 11, 17794. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila—V. Responses to environmental treatments. Chromosoma 1970, 31, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Ealy, A.D.; Monterroso, V.H.; Hansen, P.J. Ontogeny of temperature-regulated heat shock protein 70 synthesis in preimplantation bovine embryos. Mol. Reprod. Dev. 1997, 48, 25–33. [Google Scholar] [CrossRef]
- Wegele, H.; Müller, L.; Buchner, J. Hsp70 and Hsp90-a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004, 151, 1–44. [Google Scholar] [CrossRef]
- Ju, J.C.; Jiang, S.; Tseng, J.K.; Parks, J.E.; Yang, X. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology 2005, 64, 1677–1689. [Google Scholar] [CrossRef]
- Zhang, B.; Peñagaricano, F.; Driver, A.; Chen, H.; Khatib, H. Differential expression of heat shock protein genes and their splice variants in bovine preimplantation embryos. J. Dairy Sci. 2011, 94, 4174–4182. [Google Scholar] [CrossRef]
- Neuer, A.; Mele, C.; Liu, H.C.; Rosenwaks, Z.; Witkin, S.S. Monoclonal antibodies to mammalian heat shock proteins impair mouse embryo development in vitro. Hum. Reprod. 1998, 13, 987–990. [Google Scholar] [CrossRef]
- Matwee, C.; Kamaruddin, M.; Betts, D.H.; Basrur, P.K.; King, W.A. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol. Hum. Reprod. 2001, 7, 829–837. [Google Scholar] [CrossRef]
- Ju, J. Cellular responses of oocytes and embryos under thermal stress: Hints to molecular signaling. Anim. Reprod 2005, 2, 79–90. [Google Scholar]
- Hansen, P.J. Possible roles for heat shock protein 70 and glutathione in protection of the mammalian preimplantation embryo from heat shock. Ann. Rev. Biomed. Sci. 1999, 1, 5–29. [Google Scholar] [CrossRef]
- Mambula, S.S.; Stevenson, M.A.; Ogawa, K.; Calderwood, S.K. Mechanisms for Hsp70 secretion: Crossing membranes without a leader. Methods 2007, 43, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Andrei, C.; Dazzi, C.; Lotti, L.; Torrisi, M.R.; Chimini, G.; Rubartelli, A. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 1999, 10, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The complicated roles of extracellular HSPs in immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Dadouli, K.; Moutou, K.; Mamuris, Z.; Amiridis, G.S. Heat Shock Protein 70 improves in vitro embryo yield and quality from heat stressed bovine oocytes. Animals 2021, 11, 1794. [Google Scholar] [CrossRef]
- Dovolou, E.; Clemente, M.; Amiridis, G.S.; Messinis, I.; Kallitsaris, A.; Gutierrez-Adan, A.; Rizos, D. Effects of guaiazulene on in vitro bovine embryo production and on mRNA transcripts related to embryo quality. Reprod. Domest. Anim. 2011, 46, 862–869. [Google Scholar] [CrossRef] [PubMed]
- De Loos, F.; van Vliet, C.; van Maurik, P.; Kruip, T.A.M. Morphology of immature bovine oocytes. Gamete Res. 1989, 24, 197–204. [Google Scholar] [CrossRef]
- Nanas, I.; Chouzouris, T.M.; Dadouli, K.; Dovolou, E.; Stamperna, K.; Barbagianni, M.; Valasi, I.; Tsiaras, A.; Amiridis, G.S. A study on stress response and fertility parameters in phenotypically thermotolerant and thermosensitive dairy cows during summer heat stress. Reprod. Domest. Anim. 2020, 55, 1774–1783. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Lekanne Deprez, R.H.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Sartorelli, E.S.; Castilho, A.C.S.; Satrapa, R.A.; Puelker, R.Z.; Razza, E.M.; Ticianelli, J.S.; Eduardo, H.P.; Loureiro, B.; Barros, C.M. Effects of heat stress on development, quality and survival of Bos indicus and Bos taurus embryos produced in vitro. Theriogenology 2013, 79, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Kelley, K.L.; Erdos, G.W.; Hansen, P.J. Alterations in ultrastructural morphology of two-cell bovine embryos produced in vitro and in vivo following a physiologically relevant heat shock. Biol. Reprod. 2003, 69, 2068–2077. [Google Scholar] [CrossRef]
- Ealy, A.D.; Howell, J.L.; Monterroso, V.H.; Aréchiga, C.F.; Hansen, P.J. Developmental changes in sensitivity of bovine embryos to heat shock and use of antioxidants as thermoprotectants. J. Anim. Sci. 1995, 73, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. To be or not to be-Determinants of embryonic survival following heat shock. Theriogenology 2007, 68, 40–48. [Google Scholar] [CrossRef]
- Eyestone, W.H.; First, N.L. A Study of the 8 to16 Cell developmental block in bovine embryos cultured in vitro. Theriogenology 1986, 25, 152. [Google Scholar] [CrossRef]
- Krininger, C.E.; Stephens, S.H.; Hansen, P.J. Developmental changes in inhibitory effects of arsenic and heat shock on growth of pre-implantation bovine embryos. Mol. Reprod. Dev. 2002, 63, 335–340. [Google Scholar] [CrossRef]
- Barnes, F.L.; First, N.L. Embryonic Transcription in in vitro cultured bovine embryos. Mol. Reprod. Dev. 1991, 29, 117–123. [Google Scholar] [CrossRef]
- Edwards, J.L.; Hansen, P.J. Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biol. Reprod. 1996, 55, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, J.-M.; Peynot, N.; Ruffini, S.; Laffont, L.; Le Bourhis, D.; Girard, P.-M.; Duranthon, V. Regulation of heat-inducible HSPA1A gene expression during maternal-to-embryo transition and in response to heat in in vitro-produced bovine embryos. Reprod. Fertil. Dev. 2017, 29, 1868–1881. [Google Scholar] [CrossRef]
- Al-Katanani, Y.M.; Hansen, P.J. Induced thermotolerance in bovine two-cell embryos and the role of heat shock protein 70 in embryonic development. Mol. Reprod. Dev. 2002, 62, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.M.L.; Alfaro, N.S.; Dupuy, C.R.F.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Heat shock protein patterns in the bovine ovary and relation with cystic ovarian disease. Anim. Reprod. Sci. 2010, 118, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Barna, J.; Csermely, P.; Vellai, T. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Coccia, M.; Trotta, E.; Angelini, M.; Santoro, M.G. Regulation of cyclooxygenase-2 expression by heat: A novel aspect of heat shock factor 1 function in human cells. PLoS ONE 2012, 7, e31304. [Google Scholar] [CrossRef]
- Scenna, F.N.; Edwards, J.L.; Rohrbach, N.R.; Hockett, M.E.; Saxton, A.M.; Schrick, F.N. Detrimental effects of prostaglandin F2α on preimplantation bovine embryos. Prostaglandins Other Lipid Mediat. 2004, 73, 215–226. [Google Scholar] [CrossRef]
- Putney, D.J.; Malayer, J.R.; Gross, T.S.; Thatcher, W.W.; Hansen, P.J.; Drost, M. Heat stress-induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol. Reprod. 1988, 39, 717–728. [Google Scholar] [CrossRef]
- Malayer, J.R.; Hansen, P.J.; Gross, T.S.; Thatcher, W.W. Regulation of heat shock-induced alterations in the release of prostaglandins by the uterine endometrium of cows. Theriogenology 1990, 34, 219–230. [Google Scholar] [CrossRef]
- Sagirkaya, H.; Misirlioglu, M.; Kaya, A.; First, N.L.; Parrish, J.J.; Memili, E. Developmental and molecular correlates of bovine preimplantation embryos. Reproduction 2006, 131, 895–904. [Google Scholar] [CrossRef]
- Gómez, E.; Gutiérrez-Adán, A.; Díez, C.; Bermejo-Alvarez, P.; Muñoz, M.; Rodriguez, A.; Otero, J.; Alvarez-Viejo, M.; Martín, D.; Carrocera, S.; et al. Biological differences between in vitro produced bovine embryos and parthenotes. Reproduction 2009, 137, 285–295. [Google Scholar] [CrossRef]
- Ghanem, N.; Salilew-Wondim, D.; Gad, A.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K.; Hoelker, M. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction 2011, 142, 551–564. [Google Scholar] [CrossRef]
- O’Neill, L.A.J. How Toll-like receptors signal: What we know and what we don’t know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.D.; McMunn, K.A.; Hammon, H.M.; Donkin, S.S. Toll-like receptors 2 and 4, and acute phase cytokine gene expression in dexamethasone and growth hormone treated dairy calves. Vet. Immunol. Immunopathol. 2004, 98, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; An, H.; Xu, H.; Liu, S.; Cao, X. Heat shock up-regulates expression of Toll-like receptor-2 and Toll-like receptor-4 in human monocytes via p38 kinase signal pathway. Immunology 2005, 114, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Kwon, A.; Lee, E.; Chung, H. Characterization of genes and pathways that respond to heat stress in Holstein calves through transcriptome analysis. Cell Stress Chaperones 2017, 22, 29–42. [Google Scholar] [CrossRef]
- Das, R.; Gupta, I.; Verma, A.; Singh, A.; Chaudhari, M.; Sailo, L.; Upadhyay, R.; Goswami, J. Genetic polymorphisms in ATP1A1 gene and their association with heat tolerance in Jersey crossbred cows. Indian J. Dairy Sci. 2015, 68, 50–54. [Google Scholar]
- Rolfe, D.F.S.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Geering, K.; Kraehenbuhl, J.P.; Rossier, B.C. Maturation of the catalytic α-subunit of Na,K-ATPase during intracellular transport. J. Cell Biol. 1987, 105, 2613–2619. [Google Scholar] [CrossRef]
- Wang, L.; Schumann, U.; Liu, Y.; Prokopchuk, O.; Steinacker, J.M. Heat shock protein 70 (Hsp70) inhibits oxidative phosphorylation and compensates ATP balance through enhanced glycolytic activity. J. Appl. Physiol. 2012, 113, 1669–1676. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, P.; Deshmukh, B.; Bhat, S.; Kumar, A.; Chauhan, A.; Bhushan, B.; Singh, G.; Sharma, D. Association of ATP1A1 gene polymorphism with thermotolerance in Tharparkar and Vrindavani cattle. Vet. World 2015, 8, 892–897. [Google Scholar] [CrossRef][Green Version]
- Elayadeth-Meethal, M.; Thazhathu Veettil, A.; Asaf, M.; Pramod, S.; Maloney, S.K.; Martin, G.B.; Rivero, M.J.; Sejian, V.; Naseef, P.P.; Kuruniyan, M.S.; et al. Comparative expression profiling and sequence characterization of ATP1A1 gene associated with heat tolerance in tropically adapted cattle. Animals 2021, 11, 2368. [Google Scholar] [CrossRef]
- Kaushik, R.; Goel, A.; Rout, P.K. Differential expression and characterization of ATP1A1 exon17 gene by high resolution melting analysis and RT-PCR in Indian goats. Mol. Biol. Rep. 2019, 46, 5273–5286. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Yamada, T. Glutathione Transferases. In Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Liu, H.; Lu, Z.; Shi, X.; Liu, L.; Zhang, P.; Golemis, E.A.; Tu, Z. HSP90 inhibition downregulates DNA replication and repair genes via E2F1 repression. J. Biol. Chem. 2021, 297, 100996. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Accession Number | Gene Description | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|---|---|
| EEF1A1 | ENSBTAG00000014534 | Eukaryotic translation elongation factor 1 alpha 1 | CCCCAGGACACAGAGACTTC | ATTCACCAACACCAGCAGCA | 93 |
| YWHAZ | ENSBTAG00000000236 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | CTGTAACTGAGCAAGGAGC | CCAAGATGACCTACGGGC | 95 |
| UBA52 | ENSBTAG00000007737 | Ubiquitin A-52 residue ribosomal protein fusion product 1 | CCGCAAGAAGAAGTGTGGC | GCAAAGGAGAAGCAGGTGGA | 84 |
| HSPA1A | ENSBTAG00000025441 | Heat shock protein family A (Hsp70) member 1A | GGACCTGCTGTTGCTGGAC | TTCGTGGGGATGGTGGAGTT | 102 |
| HSP90AA1 | ENSBTAG00000006270 | Heat shock protein 90 alpha family class A member 1 | CTGGAAGGAGACGACGACAC | ACACACTGGAGGGAATGGAG | 104 |
| GPX1 | ENSBTAG00000054195 | Glutathione peroxidase 1 | GAAAAGTGCGAGGTGAATGG | GAGAGCAGTGGCGTCGTC | 93 |
| PLAC8 | ENSBTAG00000009849 | Placenta specific 8 A | GTTTCACAGCCAGGTTACAGC | AGAGCCCCACAGAGACAGAT | 104 |
| TLR2 | ENSBTAG00000008008 | Toll like receptor 2 | GCTGCCATTCTGATTCTGCT | GCCACTCCAGGTAGGTCTTG | 103 |
| ATP1A1 | ENSBTAG00000001246 | ATPase Na +/K+ transporting subunit alpha 1 | CGCCAGGGTTTATCCAGTT | AGGGGAAGCCAGTTTTTGTT | 80 |
| BAX | ENSBTAG00000013340 | BCL2 associated X, apoptosis regulator | TTTGCTTCAGGGTTTCATCC | CGCTTCAGACACTCGCTCAG | 120 |
| BCL2 | ENSBTAG00000019302 | BCL2 apoptosis regulator | CCCTGTTTGATTTCTCCTGGC | CTGTGGGCTTCACTTATGGC | 107 |
| DNMT3A | ENSBTAG00000021143 | DNA methyltransferase 3 alpha | GAAGGAGCATTTGGGAACAG | GTTATTGCGTGAGCCTGGAT | 118 |
| AKR1B1 | ENSBTAG00000009902 | Aldo-keto reductase family 1, member B1 (aldose reductase) | GAAAGTGGTGAAGCGTGAGG | TAGAGGTCCAGGTAGTCCAGC | 129 |
| IGF1 | ENSBTAG00000011082 | Insulin like growth factor 1 | TCACATCCTCCTCGCATCTCTT | AGCATCCACCAACTCAGCC | 107 |
| GSTP1 | ENSBTAG00000003548 | Glutathione S-transferase pi 1 | TGGAAGGAGGAGGTGGTGAC | CAGGTGACGCAGGATGGTATTG | 211 |
| HSF1 | ENSBTAG00000020751 | Heat shock transcription factor 1 | ATGAAGCACGAGAACGAGGC | GCACCAGCGAGATGAGGAACT | 112 |
| PTGS2 | ENSBTAG00000014127 | Prostaglandin-endoperoxide synthase 2 | AGTCTTTGGTCTGGTGCCTG | AACAACTGCTCATCGCCCC | 117 |
| TLR4 | ENSBTAG00000006240 | Toll like receptor 4 | AGGTAGCCCAGACAGCATTT | GAGCGAGTGGAGTGGTTCA | 110 |
| Stage of Embryos | Factor | B- Coefficients with 95% CI | Sig. |
|---|---|---|---|
| Day-7 blastocysts | Temperature | −9.1 (−10.7, −7.6) | <0.001 |
| HSP70 | 3.9 (0.8, 6.9) | 0.017 | |
| Day-8 blastocysts | Temperature | −10.0 (−12.5, −7.5) | <0.001 |
| HSP70 | 4.1 (−0.8, 9.0) | 0.096 | |
| Day-9 blastocysts | Temperature | −9.9 (−12.2, −7.5) | <0.001 |
| HSP70 | 4.8 (0.2, 9.5) | 0.043 |
| Group | Presumptive Zygotes | Cleavage (%) | Day-7 Blastocysts (%) | Day-8 Blastocysts (%) | Day-9 Blastocysts (%) |
|---|---|---|---|---|---|
| C | 396 | 327 (82.6 ± 2.3) | 108 (27.3 ± 2.1) a | 137 (34.6 ± 5.4) a | 146 (36.9 ± 5.1) a |
| H41 | 532 | 427 (80.2 ± 2.1) | 68 (12.8 ± 2.0) b | 96 (18.0 ± 3.2) b | 114 (21.4 ± 3.5) b |
| C41 | 514 | 411 (80.3 ± 3.5) | 47 (8.9 ± 3.8) c | 70 (13.6 ± 4.3) b | 82 (15.9 ± 3.6) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamperna, K.; Giannoulis, T.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Dadouli, K.; Moutou, K.; Mamuris, Z.; Amiridis, G.S. The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos. Animals 2021, 11, 3347. https://doi.org/10.3390/ani11123347
Stamperna K, Giannoulis T, Dovolou E, Kalemkeridou M, Nanas I, Dadouli K, Moutou K, Mamuris Z, Amiridis GS. The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos. Animals. 2021; 11(12):3347. https://doi.org/10.3390/ani11123347
Chicago/Turabian StyleStamperna, Konstantina, Themistoklis Giannoulis, Eleni Dovolou, Maria Kalemkeridou, Ioannis Nanas, Katerina Dadouli, Katerina Moutou, Zissis Mamuris, and Georgios S. Amiridis. 2021. "The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos" Animals 11, no. 12: 3347. https://doi.org/10.3390/ani11123347
APA StyleStamperna, K., Giannoulis, T., Dovolou, E., Kalemkeridou, M., Nanas, I., Dadouli, K., Moutou, K., Mamuris, Z., & Amiridis, G. S. (2021). The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos. Animals, 11(12), 3347. https://doi.org/10.3390/ani11123347






