An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section I
Abstract
:Simple Summary
Abstract
1. Introduction
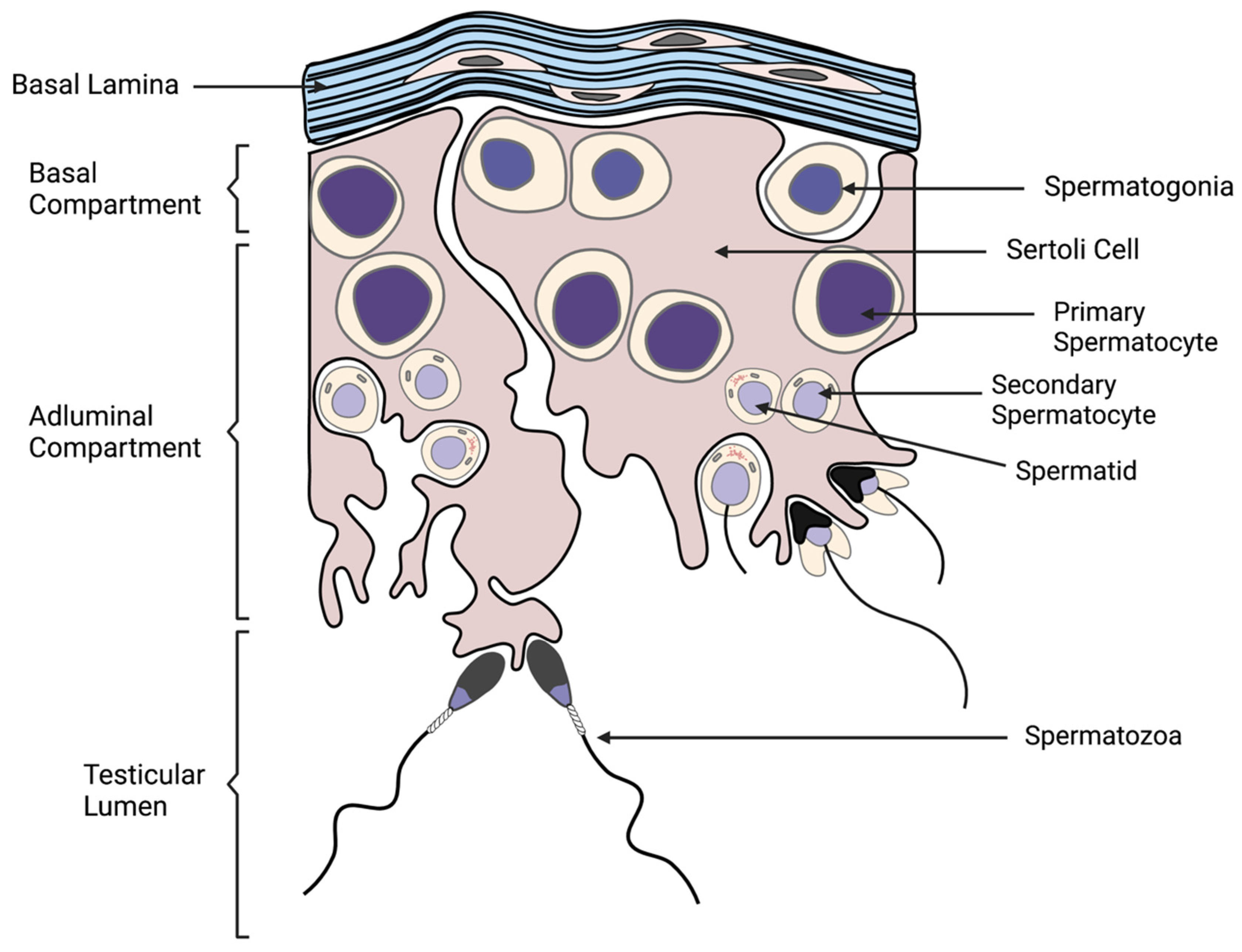
2. Spermatogenesis
3. Sperm Morphology
4. Bioenergetics and Generation of Motility
5. Common Abnormalities and Issues with Fertility
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katila, T. Sperm-uterine interactions. In Equine Reproduction, 2nd ed.; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 1092–1098. [Google Scholar]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R. Spermatogenesis in the stallion: A review. J. Equine Vet. Sci. 1981, 1, 131–139. [Google Scholar] [CrossRef]
- Johnson, L.; Blanchard, T.; Varner, D.; Scrutchfield, W. Factors affecting spermatogenesis in the stallion. Theriogenology 1997, 48, 1199–1216. [Google Scholar] [CrossRef]
- Vasconcelos, A.B.; Santana, M.A.; Santos, A.M.C.; Santoro, M.M.; Lagares, M.A. Metabolic evaluation of cooled equine spermatozoa. Andrologia 2010, 42, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.; Bulkeley, E.; Foutouhi, A. Sperm mitochondrial regulation in motility and fertility in horses. Reprod. Domest. Anim. 2019, 54, 22–28. [Google Scholar] [CrossRef]
- Brito, L.F. Evaluation of Stallion Sperm Morphology. Clin. Tech. Equine Pract. 2007, 6, 249–264. [Google Scholar] [CrossRef]
- Veeramachaneni, D.R.; Moeller, C.L.; Sawyer, H.R. Sperm Morphology in Stallions: Ultrastructure as a Functional and Diagnostic Tool. Vet. Clin. N. Am. Equine Pract. 2006, 22, 683–692. [Google Scholar] [CrossRef]
- Meyers, S. Equine sperm-oocyte interaction: The role of sperm surface hyaluronidase. Anim. Reprod. Sci. 2001, 68, 291–303. [Google Scholar] [CrossRef]
- Thomas, A.; Meyers, S.; Ball, B. Capacitation-like changes in equine spermatozoa following cryopreservation. Theriogenology 2006, 65, 1531–1550. [Google Scholar] [CrossRef]
- Graham, J.K. Methods for Induction of Capacitation and the Acrosome Reaction of Stallion Spermatozoa. Vet. Clin. N. Am. Equine Pract. 1996, 12, 111–117. [Google Scholar] [CrossRef]
- Ball, B.A.; Vo, A.T.; Baumber, J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001, 62, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Squires, E.; Graham, J. Effect of seminal plasma on the cryopreservation of equine spermatozoa. Theriogenology 2005, 63, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Guasti, P.; Souza, F.; Scott, C.; Papa, P.; Camargo, L.; Schmith, R.; Monteiro, G.; Hartwig, F.; Papa, F. Equine seminal plasma and sperm membrane: Functional proteomic assessment. Theriogenology 2020, 156, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Essawe, E.M.; Wallgren, M.; Wulf, M.; Aurich, C.; Macías-García, B.; Sjunnesson, Y.; Morrell, J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 2018, 115, 99–107. [Google Scholar] [CrossRef]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef]
- Swierstra, E.E.; Pickett, B.W.; Gebauer, M.R. Spermatogenesis and duration of transit of spermatozoa through the excurrent ducts of stallions. J. Reprod. Fertil. Suppl. 1975, 23, 53–57. [Google Scholar]
- Johnson, L. Chapter 5—Spermatogenesis. In Reproduction in Domestic Animals, 4th ed.; Cupps, P.T., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 173–219. [Google Scholar]
- Wong, C.; Cheng, C.Y. The Blood-Testis Barrier: Its Biology, Regulation, and Physiological Role in Spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 263–296. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation†. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Teerds, K. Leydig cells. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 30–38. [Google Scholar]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Thompson, D.L. Age-Related and Seasonal Variation in the Sertoli Cell Population, Daily Sperm Production and Serum Concentrations of Follicle-Stimulating Hormone, Luteinizing Hormone and Testosterone in Stallions. Biol. Reprod. 1983, 29, 777–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.-C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.; Tatum, M.E. Temporal Appearance of Seasonal Changes in Numbers of Sertoli Cells, Leydig Cells, and Germ Cells in Stallions. Biol. Reprod. 1989, 40, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, B.; Voss, J. Management of shuttle stallions for maximum reproductive efficiency—Part 1. J. Equine Vet. Sci. 1998, 18, 212–227. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y.-I. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.; Hogarth, C.; Mitchell, D.; Griswold, M. Riding the spermatogenic wave: Profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 2014, 90, 108. [Google Scholar] [CrossRef]
- De Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar]
- De Rooij, D.G.; Griswold, M.D. Questions About Spermatogonia Posed and Answered Since 2000. J. Androl. 2012, 33, 1085–1095. [Google Scholar] [CrossRef]
- Johnson, L. Seasonal Differences in Equine Spermatocytogenesis. Biol. Reprod. 1991, 44, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Steger, K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat. Embryol. 1999, 199, 471–487. [Google Scholar] [CrossRef]
- Druart, X.; de Graaf, S. Seminal plasma proteomes and sperm fertility. Anim. Reprod. Sci. 2018, 194, 33–40. [Google Scholar] [CrossRef]
- Pesch, S.; Bergmann, M. Structure of mammalian spermatozoa in respect to viability, fertility and cryopreservation. Micron 2006, 37, 597–612. [Google Scholar] [CrossRef]
- Gravance, C.; Champion, Z.; Liu, I.; Casey, P. Sperm head morphometry analysis of ejaculate and dismount stallion semen samples. Anim. Reprod. Sci. 1997, 47, 149–155. [Google Scholar] [CrossRef]
- Casey, P.; Gravance, C.; Davis, R.; Chabot, D.; Liu, I. Morphometric differences in sperm head dimensions of fertile and subfertile stallions. Theriogenology 1997, 47, 575–582. [Google Scholar] [CrossRef]
- Bedford, J. Mammalian Fertilization Misread? Sperm Penetration of the Eutherian Zona Pellucida Is Unlikely to be a Lytic Event. Biol. Reprod. 1998, 59, 1275–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.A.; Gerton, G.L. The Acrosomal Matrix. Adv. Anat. Embryol. Cell. Biol. 2016, 220, 15–33. [Google Scholar] [CrossRef] [Green Version]
- López, M.L.; De Souza, W. Distribution of filipin-sterol complexes in the plasma membrane of stallion spermatozoa during the epididymal maturation process. Mol. Reprod. Dev. 1991, 28, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. Plasma membrane composition and organisation during maturation of spermatozoa in the epididymis. In The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens; Robaire, B., Hinton, B.T., Eds.; Springer: Boston, MA, USA, 2002; pp. 405–416. [Google Scholar]
- Flesch, F.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Schroter, S.; Osterhoff, C.; McArdle, W.; Ivell, R. The glycocalyx of the sperm surface. Hum. Reprod. Updat. 1999, 5, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Pan, Q.; Feng, Y.; Choudhury, B.P.; Ma, Q.; Gagneux, P.; Ma, F. Sialylation Facilitates the Maturation of Mammalian Sperm and Affects Its Survival in Female Uterus. Biol. Reprod. 2016, 94, 123. [Google Scholar] [CrossRef]
- Scott, T.W.; Voglmayr, J.K.; Setchell, B.P. Lipid composition and metabolism in testicular and ejaculated ram spermatozoa. Biochem. J. 1967, 102, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Poulos, A.; Darin-Bennett, A.; White, I. The phospholipid-bound fatty acids and aldehydes of mammalian spermatozoa. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 46, 541–549. [Google Scholar] [CrossRef]
- Aveldaño, M.I.; Rotstein, N.P.; Vermouth, N.T. Lipid remodelling during epididymal maturation of rat spermatozoa. Enrichment in plasmenylcholines containing long-chain polyenoic fatty acids of the n-9 series. Biochem. J. 1992, 283, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.P.; Majumder, G.C.; Misra, S.; Ghosh, A. Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1061, 185–196. [Google Scholar] [CrossRef]
- Retamal, C.; Urzúa, J.; Lorca, C.; López, M.L.; Alves, E.W. Changes in the plasma membrane proteins of stallion spermatozoa during maturation in the epididymis. J. Submicrosc. Cytol. Pathol. 2000, 32, 229–239. [Google Scholar]
- López, M.; Olea, N.; Retamal, C. Post-testicular changes in the density and distribution of intramembrane particles of stallion sperm surface domains. Anim. Reprod. Sci. 2007, 100, 204–210. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Cuasnicú, P.S.; Cohen, D.J.; Ellerman, D.A.; Busso, D.; Da Ros, V.G.; Morgenfeld, M.M. Changes in specific sperm proteins during epididymal maturation. In The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens; Robaire, B., Hinton, B.T., Eds.; Springer: Boston, MA, USA, 2002; pp. 389–403. [Google Scholar]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Belleannée, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015, 17, 730–736. [Google Scholar] [CrossRef]
- Păunescu, T.G.; Shum, W.W.; Huynh, C.; Lechner, L.; Goetze, B.; Brown, D.; Breton, S.; Unescu, T.G.P. High-resolution helium ion microscopy of epididymal epithelial cells and their interaction with spermatozoa. Mol. Hum. Reprod. 2014, 20, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and Prostasomes: Their Roles in Posttesticular Maturation of the Sperm Cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, R.; Baldauf, C.; Koyro, H.-W.; Wennemuth, G.; Suga, Y.; Seitz, J.; Henkel, R.; Meinhardt, A. Influence of macrophage migration inhibitory factor (MIF) on the zinc content and redox state of protein-bound sulphydryl groups in rat sperm: Indications for a new role of MIF in sperm maturation. Mol. Hum. Reprod. 2004, 10, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenette, G.; Lessard, C.; Sullivan, R. Polyol pathway along the bovine epididymis. Mol. Reprod. Dev. 2004, 69, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Murta, D.D.M.; Batista, M.; Silva, E.; Trindade, A.; Henrique, D.; Duarte, A.; Lopes-Da-Costa, L. Notch signaling in the epididymal epithelium regulates sperm motility and is transferred at a distance within epididymosomes. Andrology 2016, 4, 314–327. [Google Scholar] [CrossRef] [Green Version]
- Krapf, D.; Ruan, Y.C.; Wertheimer, E.V.; Battistone, M.A.; Pawlak, J.; Sanjay, A.; Pilder, S.H.; Cuasnicu, P.; Breton, S.; Visconti, P.E. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev. Biol. 2012, 369, 43–53. [Google Scholar] [CrossRef]
- Joshi, C.S.; Suryawanshi, A.R.; Khan, S.A.; Balasinor, N.H.; Khole, V.V. Liprin α3: A putative estrogen regulated acrosomal protein. Histochem. Cell Biol. 2012, 139, 535–548. [Google Scholar] [CrossRef]
- Oh, J.; Woo, J.-M.; Choi, E.; Kim, T.; Cho, B.-N.; Park, Z.Y.; Kim, Y.C.; Kim, D.H.; Cho, C. Molecular, biochemical, and cellular characterization of epididymal ADAMs, ADAM7 and ADAM28. Biochem. Biophys. Res. Commun. 2005, 331, 1374–1383. [Google Scholar] [CrossRef]
- Caballero, J.; Frenette, G.; D’Amours, O.; Belleannée, C.; Lacroix-Pepin, N.; Robert, C.; Sullivan, R. Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida. J. Cell. Physiol. 2012, 227, 3876–3886. [Google Scholar] [CrossRef]
- Gibbs, G.M.; Lo, J.C.Y.; Nixon, B.; Jamsai, D.; O’Connor, A.E.; Rijal, S.; Sanchez-Partida, L.G.; Hearn, M.T.W.; Bianco, D.M.; O’Bryan, M.K. Glioma Pathogenesis-Related 1-Like 1 Is Testis Enriched, Dynamically Modified, and Redistributed during Male Germ Cell Maturation and Has a Potential Role in Sperm-Oocyte Binding. Endocrinology 2010, 151, 2331–2342. [Google Scholar] [CrossRef] [Green Version]
- Frenette, G.; Sullivan, R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 2001, 59, 115–121. [Google Scholar] [CrossRef]
- Dias, A.J.; Maia, M.S.; A Retamal, C.; López, M.L. Identification and partial characterization of α-1,4-glucosidase activity in equine epididymal fluid. Theriogenology 2004, 61, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Tecle, E.; Gagneux, P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015, 82, 635–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishijima, S.A.; Okuno, M.; Mohri, H. Zeta potential of human X- and Y-bearing sperm. Int. J. Androl. 1991, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.H.N.; Deemeh, M.R.; Tavalaee, M.; Sekhavati, M.H.; Gourabi, H. Zeta Sperm Selection Improves Pregnancy Rate and Alters Sex Ratio in Male Factor Infertility Patients: A Double-Blind, Randomized Clinical Trial. Int. J. Fertil. Steril. 2016, 10, 253–260. [Google Scholar] [CrossRef]
- Chan, P.J.; Jacobson, J.D.; Corselli, J.U.; Patton, W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006, 85, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Kondo, T. Electrophoresis of large colloidal particles with surface charge layers. Position of the slipping plane and surface layer thickness. Colloid Polym. Sci. 1986, 264, 1080–1084. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Veres, I. Negative electrical charge of the surface of bull sperm. Mikroskopie 1968, 23, 166–169. [Google Scholar]
- Yanagimachi, R.; Noda, Y.D.; Fujimoto, M.; Nicolson, G.L. The distribution of negative surface charges on mammalian spermatozoa. Am. J. Anat. 1972, 135, 497–519. [Google Scholar] [CrossRef]
- Schröter, S.; Derr, P.; Conradt, H.S.; Nimtz, M.; Hale, G.; Kirchhoff, C. Male-specific Modification of Human CD52. J. Biol. Chem. 1999, 274, 29862–29873. [Google Scholar] [CrossRef] [Green Version]
- Calzada, L.; Salazar, E.L.; Pedrón, N. Presence and Chemical Composition of Glycoproteic Layer on Human Spermatozoa. Arch. Androl. 1994, 33, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Ge, S.-Q.; Carrell, D.T. Sperm Selection Based on Electrostatic Charge. Methods Mol. Biol. 2012, 927, 269–278. [Google Scholar] [CrossRef]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm Membrane Potential: Hyperpolarization during Capacitation Regulates Zona Pellucida-Dependent Acrosomal Secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, C.C.; Varner, D.D.; Thompson, J.A. Intra- and inter-stallion variation in sperm morphology and their relationship with fertility. J. Reprod. Fertil. Suppl. 2000, 56, 93–100. [Google Scholar]
- Varner, D.D.; Johnson, L. From a sperm’s eye view—Revisiting our perception of this intriguing cell. In Proceedings of the 53rd Annual Convention of the American Association of Equine Practitioners, Orlando, FL, USA, 1–5 December 2007; pp. 104–177. [Google Scholar]
- Ramalho-Santos, J.; Amaral, S. Mitochondria and mammalian reproduction. Mol. Cell. Endocrinol. 2013, 379, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.P.; Amaral, A.; Baptista, M.; Tavares, R.; Campo, P.C.; Peregrín, P.C.; Freitas, A.; Paiva, A.; Almeida-Santos, T.; Ramalho-Santos, J. Not All Sperm Are Equal: Functional Mitochondria Characterize a Subpopulation of Human Sperm with Better Fertilization Potential. PLoS ONE 2011, 6, e18112. [Google Scholar] [CrossRef] [Green Version]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia 2011, 44, 136–141. [Google Scholar] [CrossRef]
- Mannella, C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta BBA Mol. Cell Res. 2006, 1763, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The Paradoxical Relationship Between Stallion Fertility and Oxidative Stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Saraste, M. Oxidative Phosphorylation at the fin de siècle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wolken, G.G.; Arriaga, E.A. Simultaneous Measurement of Individual Mitochondrial Membrane Potential and Electrophoretic Mobility by Capillary Electrophoresis. Anal. Chem. 2014, 86, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.P.; Muñoz, P.M.; Tapia, J.A.; Ortega-Ferrusola, C.; Balao da Silva, C.C.; Peña, F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [Green Version]
- Darr, C.R.; Cortopassi, G.A.; Datta, S.; Varner, D.D.; Meyers, S.A. Mitochondrial oxygen consumption is a unique indicator of stallion spermatozoal health and varies with cryopreservation media. Theriogenology 2016, 86, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Nijs, M.; Vanderzwalmen, P.; Vandamme, B.; Segal-Bertin, G.; Lejeune, B.; Segal, L.; van Roosendaal, E.; Schoysman, R. Andrology: Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum. Reprod. 1996, 11, 2180–2185. [Google Scholar] [CrossRef] [Green Version]
- Gaddum-Rosse, P. Some observations on sperm transport through the uterotubal junction of the rat. Am. J. Anat. 1981, 160, 333–341. [Google Scholar] [CrossRef]
- Fraser, L.R.; Quinn, P.J. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. Reproduction 1981, 61, 25–35. [Google Scholar] [CrossRef]
- Turner, R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003, 24, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar] [CrossRef]
- Jasko, D.J.; Little, T.V.; Lein, D.H.; Foote, R.H. Comparison of spermatozoal movement and semen characteristics with fertility in stallions: 64 cases (1987–1988). J. Am. Vet. Med. Assoc. 1992, 200, 979–985. [Google Scholar] [PubMed]
- Hurtgen, J.P. Evaluation of the Stallion for Breeding Soundness. Vet. Clin. N. Am. Equine Pract. 1992, 8, 149–165. [Google Scholar] [CrossRef]
- Voss, J.L.; Pickett, B.W.; Squires, E.L. Stallion spermatozoal morphology and motility and their relationships to fertility. J. Am. Vet. Med. Assoc. 1981, 178, 287–289. [Google Scholar]
- Johnson, S.; Nguyen, V.; Coder, D. Assessment of Cell Viability. Curr. Protoc. Cytom. 2013, 64, 9.2.1–9.2.26. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pintado, B.; de la Fuente, J.; Roldan, E.R. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: Accuracy in the assessment of cell viability. J. Reprod. Fertil. 2000, 118, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Glazar, A.I. Assessment of Sperm Plasma Membrane Integrity and Viability: Propidium Iodide/SYBR-14. Equine Reprod. Proced. 2014, 476–477. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C.G. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar]
- Gravance, C.; Garner, D.; Baumber, J.; Ball, B. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology 2000, 53, 1691–1703. [Google Scholar] [CrossRef]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef]
- Glazar, A.I.; McCue, P.M. Assessment of Sperm Mitochondrial Function. Equine Reprod. Proced. 2021, 637–638. [Google Scholar] [CrossRef]
- Baumber, J. Changes in Membrane Lipid Order with Capacitation in Rhesus Macaque (Macaca mulatta) Spermatozoa. J. Androl. 2006, 27, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, A.; Sabeur, K.; Ball, B. Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology 2008, 69, 1041–1055. [Google Scholar] [CrossRef]
- Cheng, F.-P.; Gadella, B.M.; Voorhout, W.F.; Fazeli, A.; Bevers, M.M.; Colenbrander, B. Progesterone-Induced Acrosome Reaction in Stallion Spermatozoa Is Mediated by a Plasma Membrane Progesterone Receptor. Biol. Reprod. 1998, 59, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.P.; Fazeli, A.; Voorhout, W.F.; Marks, A.; Bevers, M.M.; Colenbrander, B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996, 17, 674–682. [Google Scholar]
- Franken, D.R. How accurate is sperm morphology as an indicator of sperm function? Andrologia 2014, 47, 720–723. [Google Scholar] [CrossRef]
- Dariš, B.; Goropevšek, A.; Hojnik, N.; Vlaisavljević, V. Sperm morphological abnormalities as indicators of DNA fragmentation and fertilization in ICSI. Arch. Gynecol. Obstet. 2009, 281, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Oumaima, A.; Tesnim, A.; Zohra, H.; Amira, S.; Ines, Z.; Sana, C.; Intissar, G.; Lobna, E.; Ali, J.; Meriem, M. Investigation on the origin of sperm morphological defects: Oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ. Sci. Pollut. Res. 2018, 25, 13775–13786. [Google Scholar] [CrossRef]
- Kruger, T.F.; Acosta, A.A.; Simmons, K.F.; Swanson, R.J.; Matta, J.F.; Oehninger, S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil. Steril. 1988, 49, 112–117. [Google Scholar] [CrossRef]
- Oehninger, S.; Mahony, M.; Özgür, K.; Kolm, P.; Kruger, T.; Franken, D. Clinical significance of human sperm-zona pellucida binding. Fertil. Steril. 1997, 67, 1121–1127. [Google Scholar] [CrossRef]
- Love, C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology 2011, 76, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization†. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Yanagimachi, R.; Hamana, K.; Hafez, E.S.E. The movement of golden hamster spermatozoa before and after capacitation. Reproduction 1970, 23, 193–196. [Google Scholar] [CrossRef]
- Leemans, B.; Stout, T.A.E.; De Schauwer, C.; Heras, S.; Nelis, H.; Hoogewijs, M.; Van Soom, A.; Gadella, B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction 2019, 157, R181–R197. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.P.; Fazeli, A.; Voorhout, W.F.; Tremoleda, J.L.; Bevers, M.M.; Colenbrander, B. Progesterone in mare follicular fluid induces the acrosome reaction in stallion spermatozoa and enhances In Vitro binding to the zona pellucida. Int. J. Androl. 2002, 21, 57–66. [Google Scholar] [CrossRef]
- McPartlin, L.; Littell, J.; Mark, E.; Nelson, J.; Travis, A.; Bedford-Guaus, S. A defined medium supports changes consistent with capacitation in stallion sperm, as evidenced by increases in protein tyrosine phosphorylation and high rates of acrosomal exocytosis. Theriogenology 2008, 69, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.A.; Overstreet, J.W.; Liu, I.K.; Drobnis, E.Z. Capacitation in vitro of stallion spermatozoa: Comparison of progesterone-induced acrosome reactions in fertile and subfertile males. J. Androl. 1995, 16, 47–54. [Google Scholar] [PubMed]
- Mortimer, D.; Camenzind, A. The role of follicular fluid in inducing the acrosome reaction of human spermatozoa incubated in vitro. Hum. Reprod. 1989, 4, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T.; Chung, C.M.; Chan, H.C. Acrosome reaction in the cumulus oophorus revisited: Involvement of a novel sperm-released factor NYD-SP8. Protein Cell 2011, 2, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siiteri, J.E.; Dandekar, P.; Meizel, S. Human sperm acrosome reaction-initiating activity associated with the human cumulus oophorus and mural granulosa cells. J. Exp. Zool. 1988, 246, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in horses. Reproduction 2016, 152, R233–R245. [Google Scholar] [CrossRef] [Green Version]
- Brucker, C. The human sperm acrosome reaction: Physiology and regulatory mechanisms. An update. Hum. Reprod. Updat. 1995, 1, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C. Relationship of in Vitro Acrosome Reaction to Sperm Function: An Update. Open Reprod. Sci. J. 2011, 3, 72–84. [Google Scholar] [CrossRef]
- Avella, M.A.; Dean, J. Fertilization with acrosome-reacted mouse sperm: Implications for the site of exocytosis. Proc. Natl. Acad. Sci. USA 2011, 108, 19843–19844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahlay, G.K.; Rajput, N. The enigmatic sperm proteins in mammalian fertilization: An overview†. Biol. Reprod. 2020, 103, 1171–1185. [Google Scholar] [CrossRef]
- Katz, D.F.; Yanagimachi, R.; Dresdner, R.D. Movement characteristics and power output of guinea-pig and hamster spermatozoa in relation to activation. Reproduction 1978, 52, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Fraser, L.R. Sperm capacitation and the acrosome reaction. Hum. Reprod. 1998, 13, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Cummins, J.M.; Yanagimachi, R. Development of ability to penetrate the cumulus oophorus by hamster spermatozoa capacitated in vitro, in relation to the timing of the acrosome reaction. Gamete Res. 1986, 15, 187–212. [Google Scholar] [CrossRef]
- Florman, H.M.; Jungnickel, M.K.; Sutton, K.A. Regulating the acrosome reaction. Int. J. Dev. Biol. 2008, 52, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesarik, J.; Mendoza, C. Alleviation of acrosome reaction prematurity by sperm treatment with egg yolk. Fertil. Steril. 1995, 63, 153–157. [Google Scholar] [CrossRef]
- Meyers, S.A.; Liu, I.K.; Overstreet, J.W.; Vadas, S.; Drobnis, E.Z. Zona pellucida binding and zona-induced acrosome reactions in horse spermatozoa: Comparisons between fertile and subfertile stallions. Theriogenology 1996, 46, 1277–1288. [Google Scholar] [CrossRef]
- Peña, F.; Martínez, H.R.; Tapia, J.; Ferrusola, C.O.; Fernández, L.G.; García, B.M. Mitochondria in Mammalian Sperm Physiology and Pathology: A Review. Reprod. Domest. Anim. 2009, 44, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 2008, 107, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabeur, K.; Ball, B.A. Detection of superoxide anion generation by equine spermatozoa. Am. J. Vet. Res. 2006, 67, 701–706. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Imam, S.N.; Dada, R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J. Assist. Reprod. Genet. 2011, 28, 1073–1085. [Google Scholar] [CrossRef] [Green Version]
- Esteves, S.C.; Sharma, R.K.; Gosálvez, J.; Agarwal, A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int. Urol. Nephrol. 2014, 46, 1037–1052. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Kumar, R.; Dada, R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J. Med. Res. 2008, 127, 115–123. [Google Scholar]
- Sakkas, D.; Moffatt, O.; Manicardi, G.C.; Mariethoz, E.; Tarozzi, N.; Bizzaro, D. Nature of DNA Damage in Ejaculated Human Spermatozoa and the Possible Involvement of Apoptosis. Biol. Reprod. 2002, 66, 1061–1067. [Google Scholar] [CrossRef]
- Henkel, R.; Kierspel, E.; Stalf, T.; Mehnert, C.; Menkveld, R.; Tinneberg, H.-R.; Schill, W.-B.; Kruger, T.F. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil. Steril. 2005, 83, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Gardner, D.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004, 82, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Aitken, R.J. Reactive oxygen species in spermatozoa: Methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 2005, 3, 67–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Goriely, A.; McVean, G.A.T.; Röjmyr, M.; Ingemarsson, B.; Wilkie, A.O.M. Evidence for Selective Advantage of Pathogenic FGFR2 Mutations in the Male Germ Line. Science 2003, 301, 643–646. [Google Scholar] [CrossRef]
- Tiemann-Boege, I.; Navidi, W.; Grewal, R.; Cohn, D.; Eskenazi, B.; Wyrobek, A.; Arnheim, N. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc. Natl. Acad. Sci. USA 2002, 99, 14952–14957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, V.; Pandolfi, C.; Santucci, R.; Pelliccione, F.; Macerola, B.; Focarelli, R.; Rosati, F.; Della Giovampaola, C.; Francavilla, F. Expression of gp20, a human sperm antigen of epididymal origin, is reduced in spermatozoa from subfertile men. Mol. Reprod. Dev. 2004, 69, 235–240. [Google Scholar] [CrossRef]
- Ionov, M.; Gontarek, W.; Bryszewska, M. Zeta potential technique for analyzing semen quality. MethodsX 2020, 7, 100895. [Google Scholar] [CrossRef]
- Kaneko, S.; Oshio, S.; Kobayashi, T.; Iizuka, R.; Mohri, H. Human X- and Y-bearing sperm differ in cell surface sialic acid content. Biochem. Biophys. Res. Commun. 1984, 124, 950–955. [Google Scholar] [CrossRef]
- Focarelli, R.; Rosati, F.; Terrana, B. Sialylglycoconjugates Release During In Vitro Capacitation of Human Spermatozoa. J. Androl. 1990, 11, 97–104. [Google Scholar] [CrossRef]
- Levinsky, H.; Singer, R.; Malik, Z.; Sagiv, M.; Cohen, A.M.; Servadio, C.; Allalouf, D. Distribution of Sialic Acid in Human Sperm Membranes. Arch. Androl. 1983, 10, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, J.G.; Canovas, S.; Barajas, P.; Marcos, J.; Jimenez-Movilla, M.; Gallego, R.G.; Ballesta, J.; Avilés, M.; Coy, P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Repeod. Dev. 2006, 74, 617–628. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsolini, M.F.; Meyers, S.A.; Dini, P. An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section I. Animals 2021, 11, 3248. https://doi.org/10.3390/ani11113248
Orsolini MF, Meyers SA, Dini P. An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section I. Animals. 2021; 11(11):3248. https://doi.org/10.3390/ani11113248
Chicago/Turabian StyleOrsolini, Morgan F., Stuart A. Meyers, and Pouya Dini. 2021. "An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section I" Animals 11, no. 11: 3248. https://doi.org/10.3390/ani11113248
APA StyleOrsolini, M. F., Meyers, S. A., & Dini, P. (2021). An Update on Semen Physiology, Technologies, and Selection Techniques for the Advancement of In Vitro Equine Embryo Production: Section I. Animals, 11(11), 3248. https://doi.org/10.3390/ani11113248






