Molecular Detection and Characterization of Intestinal and Blood Parasites in Wild Chimpanzees (Pan troglodytes verus) in Senegal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Data Collection
2.3. DNA Extraction and Purification
2.4. Molecular Detection of Cryptosporidium spp.
2.5. Molecular Differential Detection of Entamoeba histolytica and Entamoeba dispar
2.6. Molecular Detection and Characterization of Giardia Duodenalis
2.7. Molecular Detection of Sarcocystis spp.
2.8. Molecular Detection and Characterization of Blastocystis sp.
2.9. Molecular Detection and Characterization of Enterocytozoon bieneusi
2.10. Molecular Detection of Balantioides coli
2.11. Molecular Detection of Troglodytella spp.
2.12. Molecular Detection of Plasmodium spp.
2.13. Molecular Detection of Trypanosomatid Species
2.14. Molecular Detection of Filarial Species
2.15. Sequencing
2.16. Phylogenetic Analysis
2.17. Statistical Analyses
2.18. Parasite Interactions
3. Results
3.1. Prevalence of Intestinal and Blood Parasites
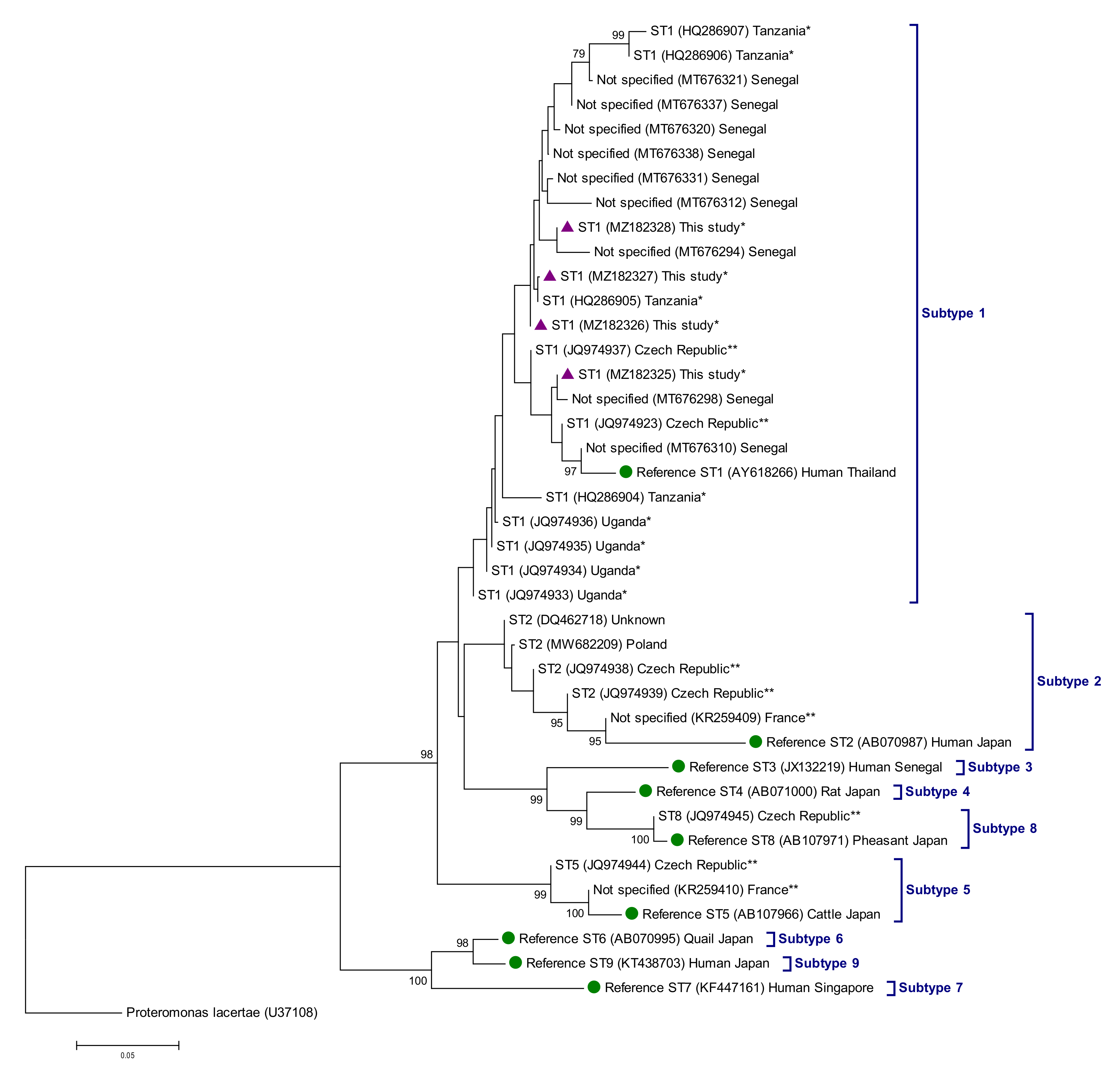
3.2. Molecular Characterization of Intestinal Protist Species
3.3. Molecular Characterization of Blood Protist Parasites
3.4. Molecular Characterization of Filarial Parasites
3.5. Seasonality Effects on Parasites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kühl, H.S.; Sop, T.; Williamson, E.A.; Mundry, R.; Brugière, D.; Campbell, G.; Cohen, H.; Danquah, E.; Ginn, L.; Herbinger, I.; et al. The Critically Endangered western chimpanzee declines by 80%. Am. J. Primatol. 2017, 79, e22681. [Google Scholar] [CrossRef] [PubMed]
- IUCN SSC Primate Specialist Group. Regional Action Plan for the Conservation of Western Chimpanzees (Pan troglodytes verus) 2020–2030; IUCN: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dunay, E.; Apakupakul, K.; Leard, S.; Palmer, J.L.; Deem, S.L. Pathogen transmission from humans to great apes is a growing threat to primate conservation. Ecohealth 2018, 15, 148–162. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef]
- Stuart, P.; Yalcindag, E.; Ali, I.K.M.; Pecková, R.; Nurcahyo, W.; Morrogh-Bernard, H.; Foitová, I. Entamoeba histolytica infections in wild and semi-wild orangutans in Sumatra and Kalimantan. Am. J. Primatol. 2020, 82, e23124. [Google Scholar] [CrossRef]
- Rosenthal, B.M. Zoonotic Sarcocystis. Res. Vet. Sci. 2021, 136, 51–157. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Santin, M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef]
- Ponce-Gordo, F.; García-Rodríguez, J.J. Balantioides coli . Res. Vet. Sci. 2021, 135, 424–431. [Google Scholar] [CrossRef]
- Modrý, D.; Petrzelková, K.J.; Pomajbíková, K.; Tokiwa, T.; Krízek, J.; Imai, S.; Vallo, P.; Profousová, I.; Slapeta, J. The occurrence and ape-to-ape transmission of the entodiniomorphid ciliate Troglodytella abrassarti in captive gorillas. J. Eukaryot. Microbiol. 2009, 56, 83–87. [Google Scholar] [CrossRef]
- Boundenga, L.; Ollomo, B.; Rougeron, V.; Mouele, L.Y.; Mve-Ondo, B.; Delicat-Loembet, L.M.; Moukodoum, N.D.; Okouga, A.P.; Arnathau, C.; Elguero, E.; et al. Diversity of malaria parasites in great apes in Gabon. Malar. J. 2015, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoubangoye, B.; Boundenga, L.; Arnathau, C.; Mombo, I.M.; Durand, P.; Tsoumbou, T.A.; Otoro, B.V.; Sana, R.; Okouga, A.P.; Moukodoum, N.; et al. The host specificity of ape malaria parasites can be broken in confined environments. Int. J. Parasitol. 2016, 46, 737–744. [Google Scholar] [CrossRef]
- Jirků, M.; Votýpka, J.; Petrželková, K.J.; Jirků-Pomajbíková, K.; Kriegová, E.; Vodička, R.; Lankester, F.; Leendertz, S.A.; Wittig, R.M.; Boesch, C.; et al. Wild chimpanzees are infected by Trypanosoma brucei. Int. J. Parasitol. Parasites Wildl. 2015, 4, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, C.M.; Pion, S.D.; Hamou, H.; Sirima, C.; Bizet, C.; Lemarcis, T.; Rodrigues, J.; Esteban, A.; Peeters, M.; Mpoudi Ngole, E.; et al. Detection of DNA of filariae closely related to Mansonella perstans in faecal samples from wild non-human primates from Cameroon and Gabon. Parasites Vectors 2020, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Bolland, S.J.; Oskam, C.L.; Ryan, U. Cryptosporidium abrahamseni n. sp. (Apicomplexa: Cryptosporidiiae) from red-eye tetra (Moenkhausia sanctaefilomenae). Exp. Parasitol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.G.; Santin, M. Mind the gap: New full-length sequences of Blastocystis subtypes generated via Oxford Nanopore Minion sequencing allow for comparisons between full-length and partial sequences of the small subunit of the ribosomal RNA Gene. Microorganisms 2021, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.; Fraixedas, S.; Fernández-Llamazares, Á.; Estela, N.; Mominee, R.; Guallar, F. Perspectives on sustainable resource conservation in community nature reserves: A case study from Senegal. Sustainability 2012, 4, 3158–3179. [Google Scholar] [CrossRef] [Green Version]
- Enthoven, D.; Pacheco, L.; Llana, M.; Reitan, T.; Hernandez-Aguilar, A. Nesting patterns of chimpanzees (Pan troglodytes verus) in a savannah habitat, Dindefelo, Senegal. Folia Primatol. 2017, 88, 114. [Google Scholar]
- JGIS; APES Wiki Team. Dindefelo. 2021. Available online: https://apeswiki.eva.mpg.de/index.php/Dindefelo (accessed on 11 September 2021).
- Ramon, M.; Llana, M.; Estela, N.; Pacheco, L.; Hockings, K.; Hill, C. The fruit of discord? Saba senegalensis use by chimpanzees (Pan troglodytes verus) and local people in the Dindefelo Community Nature Reserve (RNCD), Southeastern Senegal. Folia Primatol. 2017, 88, 167–168. [Google Scholar]
- O’Donnell, L.J.; Virjee, J.; Heaton, K.W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990, 300, 439–440. [Google Scholar] [CrossRef] [Green Version]
- Huffman, G.J.; Stocker, E.F.; Bolvin, D.T.; Nelkin, E.J.; Tan, J. GPM IMERG Final Precipitation L3 1 Day 0.1 Degree × 0.1 Degree V06; Savtchenko, A., Ed.; Goddard Earth Sciences Data and Information Services Center (GES DISC): Greenbelt, MD, USA, 2019. [Google Scholar] [CrossRef]
- AIRS Science Team/Joao Teixeira. AIRS/Aqua L3 Daily Standard Physical Retrieval (AIRS-only) 1 Degree × 1 Degree V006; Goddard Earth Sciences Data and Information Services Center (GES DISC): Greenbelt, MD, USA, 2013. [Google Scholar] [CrossRef]
- Tiangtip, R.; Jongwutiwes, S. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop. Med. Int. Health 2002, 7, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Feltus, D.C.; Giddings, C.W.; Schneck, B.L.; Monson, T.; Warshauer, D.; McEvoy, J.M. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 2006, 44, 4303–4308. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Cisneros, M.J.; Cogollos, R.; López-Vélez, R.; Martín-Rabadán, P.; Martínez-Ruiz, R.; Subirats, M.; Merino, F.J.; Fuentes, I. Application of real-time PCR for the differentiation of Entamoeba histolytica and E. dispar in cyst-positive faecal samples from 130 immigrants living in Spain. Ann. Trop. Med. Parasitol. 2010, 104, 145–149. [Google Scholar] [CrossRef]
- Verweij, J.J.; Oostvogel, F.; Brienen, E.A.; Nang-Beifubah, A.; Ziem, J.; Polderman, A.M. Prevalence of Entamoeba histolytica and Entamoeba dispar in northern Ghana. Trop. Med. Int. Health 2003, 8, 1153–1156. [Google Scholar] [CrossRef]
- Verweij, J.J.; Schinkel, J.; Laeijendecker, D.; van Rooyen, M.A.; van Lieshout, L.; Polderman, A.M. Real-time PCR for the detection of Giardia lamblia. Mol. Cell. Probes 2003, 17, 223–225. [Google Scholar] [CrossRef]
- Read, C.M.; Monis, P.T.; Thompson, R.C. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 2004, 4, 125–130. [Google Scholar] [CrossRef]
- Lalle, M.; Pozio, E.; Capelli, G.; Bruschi, F.; Crotti, D.; Cacciò, S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005, 35, 207–213. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Fayer, R.; Bern, C.; Gilman, R.H.; Trout, J.M.; Schantz, P.M.; Das, P.; Lal, A.A.; Xiao, L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003, 9, 1444–1452. [Google Scholar] [CrossRef]
- Scicluna, S.M.; Tawari, B.; Clark, C.G. DNA barcoding of Blastocystis. Protist 2006, 157, 77–85. [Google Scholar] [CrossRef]
- Buckholt, M.A.; Lee, J.H.; Tzipori, S. Prevalence of Enterocytozoon bieneusi in swine: An 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 2002, 68, 2595–2599. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Gordo, F.; Fonseca-Salamanca, F.; Martínez-Díaz, R.A. Genetic heterogeneity in internal transcribed spacer genes of Balantidium coli (Litostomatea, Ciliophora). Protist 2011, 162, 774–794. [Google Scholar] [CrossRef]
- Vallo, P.; Petrželková, K.J.; Profousová, I.; Petrášová, J.; Pomajbíková, K.; Leendertz, F.; Hashimoto, C.; Simmons, N.; Babweteera, F.; Machanda, Z.; et al. Molecular diversity of entodiniomorphid ciliate Troglodytella abrassarti and its coevolution with chimpanzees. Am. J. Phys. Anthropol. 2012, 148, 525–533. [Google Scholar] [CrossRef]
- Ta, T.-H.; Moya, L.; Nguema, J.; Aparicio, P.; Miguel-Oteo, M.; Cenzual, G.; Canorea, I.; Lanza, M.; Benito, A.; Crainey, J.L.; et al. Geographical distribution and species identification of human filariasis and onchocerciasis in Bioko Island, Equatorial Guinea. Acta Trop. 2018, 180, 12–17. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 11 September 2021).
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The upper tail probabilities of Spearman’s rho. Appl. Stat. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Mehta, C.R.; Patel, N.R. A network algorithm for performing Fisher’s exact test in r × c contingency tables. J. Am. Stat. Assoc. 1983, 78, 427–434. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Claes, F.; Büscher, P.; Touratier, L.; Goddeeris, B.M. Trypanosoma equiperdum: Master of disguise or historical mistake? Trends Parasitol. 2005, 21, 316–321. [Google Scholar] [CrossRef]
- Mapua, M.I.; Petrželková, K.J.; Burgunder, J.; Dadáková, E.; Brožová, K.; Hrazdilová, K.; Stewart, F.A.; Piel, A.K.; Vallo, P.; Fuehrer, H.P.; et al. A comparative molecular survey of malaria prevalence among Eastern chimpanzee populations in Issa Valley (Tanzania) and Kalinzu (Uganda). Malar. J. 2016, 15, 423. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.F.; Löhrich, T.; Sachse, A.; Mundry, R.; Wittig, R.M.; Calvignac-Spencer, S.; Deschner, T.; Leendertz, F.H. Seasonal and inter-annual variation of malaria parasite detection in wild chimpanzees. Malar. J. 2018, 17, 38. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, T.R.; Morgan, D.; Deutsch, J.C.; Kuhlenschmidt, M.S.; Salzer, J.S.; Cameron, K.; Reed, T.; Sanz, C. A legacy of low-impact logging does not elevate prevalence of potentially pathogenic protozoa in free-ranging gorillas and chimpanzees in the Republic of Congo: Logging and parasitism in African apes. Ecohealth 2009, 6, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Moreno, O.; Hernandez-Aguilar, R.A.; Piel, A.K.; Stewart, F.A.; Gracenea, M.; Moore, J. Prevalence and climatic associated factors of Cryptosporidium sp. infections in savanna chimpanzees from Ugalla, Western Tanzania. Parasitol. Res. 2013, 112, 393–399. [Google Scholar] [CrossRef]
- Renelies-Hamilton, J.; Noguera-Julian, M.; Parera, M.; Paredes, R.; Pacheco, L.; Dacal, E.; Saugar, J.M.; Rubio, J.M.; Poulsen, M.; Köster, P.C.; et al. Exploring interactions between Blastocystis sp., Strongyloides spp. and the gut microbiomes of wild chimpanzees in Senegal. Infect. Genet. Evol. 2019, 74, 104010. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.B.; Travis, D.; Lonsdorf, E.V.; Lipende, I.; Roellig, D.M.; Collins, A.; Kamenya, S.; Zhang, H.; Xiao, L.; Gillespie, T.R. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0003529. [Google Scholar] [CrossRef] [Green Version]
- Squire, S.A.; Ryan, U. Cryptosporidium and Giardia in Africa: Current and future challenges. Parasites Vectors 2017, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Widmer, G.; Köster, P.C.; Carmena, D. Cryptosporidium hominis infections in non-human animal species: Revisiting the concept of host specificity. Int. J. Parasitol. 2020, 50, 253–262. [Google Scholar] [CrossRef]
- Ragazzo, L.J.; Zohdy, S.; Velonabison, M.; Herrera, J.; Wright, P.C.; Gillespie, T.R. Entamoeba histolytica infection in wild lemurs associated with proximity to humans. Vet. Parasitol. 2018, 249, 98–101. [Google Scholar] [CrossRef]
- Abdallah, M.D.; Wolf, R.F.; White, G.L.; Kosanke, S.D.; Carey, D.W.; Verweij, J.J.; El-Dessouky, Y.M.; Zhang, M.J.; Ravdin, J.I. Natural infection of baboons by Entamoeba histolytica elicits anti-gal-lectin heavy subunit IgA and IgG antibodies with shared epitope specificity to that of humans. J. Egypt Soc. Parasitol. 2013, 43, 723–735. [Google Scholar]
- Howells, M.E.; Pruetz, J.; Gillespie, T.R. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: The case of sympatric western chimpanzees (Pan troglodytes verus) and guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am. J. Primatol. 2011, 73, 173–179. [Google Scholar] [CrossRef]
- Jirků-Pomajbíková, K.; Čepička, I.; Kalousová, B.; Jirků, M.; Stewart, F.; Levecke, B.; Modrý, D.; Piel, A.K.; Petrželková, K.J. Molecular identification of Entamoeba species in savanna woodland chimpanzees (Pan troglodytes schweinfurthii). Parasitology 2016, 143, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Deere, J.R.; Parsons, M.B.; Lonsdorf, E.V.; Lipende, I.; Kamenya, S.; Collins, D.A.; Travis, D.A.; Gillespie, T.R. Entamoeba Histolytica–1122; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Vlčková, K.; Kreisinger, J.; Pafčo, B.; Čížková, D.; Tagg, N.; Hehl, A.B.; Modrý, D. Diversity of Entamoeba spp. in African great apes and humans: An insight from Illumina MiSeq high-throughput sequencing. Int. J. Parasitol. 2018, 48, 519–530. [Google Scholar] [CrossRef]
- Sá, R.M.; Petrášová, J.; Pomajbíková, K.; Profousová, I.; Petrželková, K.J.; Sousa, C.; Cable, J.; Bruford, M.W.; Modrý, D. Gastrointestinal symbionts of chimpanzees in Cantanhez National Park, Guinea-Bissau with respect to habitat fragmentation. Am. J. Primatol. 2013, 75, 1032–1041. [Google Scholar] [CrossRef]
- Sak, B.; Petrzelkova, K.J.; Kvetonova, D.; Mynarova, A.; Shutt, K.A.; Pomajbikova, K.; Kalousova, B.; Modry, D.; Benavides, J.; Todd, A.; et al. Long-term monitoring of microsporidia, Cryptosporidium and Giardia infections in western Lowland Gorillas (Gorilla gorilla gorilla) at different stages of habituation in Dzanga Sangha Protected Areas, Central African Republic. PLoS ONE 2013, 8, e71840. [Google Scholar] [CrossRef]
- Hogan, J.N.; Miller, W.A.; Cranfield, M.R.; Ramer, J.; Hassell, J.; Noheri, J.B.; Conrad, P.A.; Gilardi, K.V. Giardia in mountain gorillas (Gorilla beringei beringei), forest buffalo (Syncerus caffer), and domestic cattle in Volcanoes National Park, Rwanda. J. Wildl. Dis. 2014, 50, 21–30. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Bosco-Nizeyi, J.; Ssebide, B.; Thompson, R.C.; Read, C.; Cranfield, M.R. Anthropozoonotic Giardia duodenalis genotype (assemblage) a infections in habitats of free-ranging human-habituated gorillas, Uganda. J. Parasitol. 2002, 88, 905–909. [Google Scholar] [CrossRef] [Green Version]
- Teichroeb, J.A.; Kutz, S.J.; Parkar, U.; Thompson, R.C.; Sicotte, P. Ecology of the gastrointestinal parasites of Colobus vellerosus at Boabeng-Fiema, Ghana: Possible anthropozoonotic transmission. Am. J. Phys. Anthropol. 2009, 140, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.R.; Gillespie, T.R.; Rwego, I.B.; McLachlan, T.L.; Kent, A.D.; Goldberg, T.L. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl. Trop. Dis. 2010, 4, e683. [Google Scholar] [CrossRef] [Green Version]
- Heydorn, A.O.; Gestrich, R.; Janitschke, K. Beiträge zum lebenszyklus der Sarkosporidien. VIII. Sporozysten von Sarcocystis bovihominis in den fäzes von rhesusaffen (Macaca rhesus) und pavianen (Papio cynocephalus). Berl. Munch. Tierarztl. Wochenschr. 1976, 89, 116–120. [Google Scholar]
- Fayer, R.; Heydorn, A.O.; Johnson, A.J.; Leek, R.G. Transmission of Sarcocystis suihominis from humans to swine to nonhuman primates (Pan troglodytes, Macaca mulatta, Macaca irus). Z. Parasitenkd. 1979, 59, 15–20. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gjerde, B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from Cattle (Bos taurus) and Sarcocystis sinensis from Water Buffaloes (Bubalus bubalis). Parasitol. Res. 2016, 115, 1473–1492. [Google Scholar] [CrossRef]
- Abe, N.; Matsuo, K.; Moribe, J.; Takashima, Y.; Irie, T.; Baba, T.; Gjerde, B. Morphological and molecular characteristics of seven Sarcocystis species from sika deer (Cervus nippon centralis) in Japan, including three new species. Int. J. Parasitol. Parasites Wildl. 2019, 10, 252–262. [Google Scholar] [CrossRef]
- Drakulovski, P.; Bertout, S.; Locatelli, S.; Butel, C.; Pion, S.; Mpoudi-Ngole, E.; Delaporte, E.; Peeters, M.; Mallié, M. Assessment of gastrointestinal parasites in wild chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon. Parasitol. Res. 2014, 113, 2541–2550. [Google Scholar] [CrossRef] [Green Version]
- Petrášová, J.; Uzlíková, M.; Kostka, M.; Petrželková, K.J.; Huffman, M.A.; Modrý, D. Diversity and host specificity of Blastocystis in syntopic primates on Rubondo Island, Tanzania. Int. J. Parasitol. 2011, 41, 1113–1120. [Google Scholar] [CrossRef]
- Muadica, A.S.; Köster, P.C.; Dashti, A.; Bailo, B.; Hernández-de-Mingo, M.; Reh, L.; Balasegaram, S.; Verlander, N.Q.; Ruiz Chércoles, E.; Carmena, D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp. and Blastocystis sp. in asymptomatic school children in Leganés, Madrid (Spain). Microorganisms 2020, 8, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muadica, A.S.; Köster, P.C.; Dashti, A.; Bailo, B.; Hernández-de-Mingo, M.; Balasegaram, S.; Carmena, D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp., and Blastocystis sp. in symptomatic and asymptomatic schoolchildren in Zambézia Province (Mozambique). Pathogens 2021, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Kvác, M.; Petrzelková, K.; Kvetonová, D.; Pomajbíková, K.; Mulama, M.; Kiyang, J.; Modrý, D. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: Evidence for zoonotic transmission? Folia Parasitol. 2011, 58, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Boudenga, L.; Ngoubangoye, B.; Moukodoum, N.; Dibakou, S.-E.; Moussadji, C.; Hugot, J.P. Diversity of parasites in two captive chimpanzee populations in souther Gabon. Infect. Genet. Evol. 2021, 91, 104807. [Google Scholar] [CrossRef]
- Bakuza, J.S.; Nkwengulila, G. Variation over time in parasite prevalence among free-ranging chimpanzees at Gombe National Park, Tanzania. Int. J. Primatol. 2009, 30, 43–53. [Google Scholar] [CrossRef]
- Pomajbíková, K.; Petrželková, K.J.; Profousová, I.; Petrášová, J.; Modrý, D. Discrepancies in the occurrence of Balantidium coli between wild and captive African great apes. J. Parasitol. 2010, 96, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Pomajbíková, K.; Petrželková, K.J.; Profousová, I.; Petrášova, J.; Kišidayová, S.; Varádyová, Z.; Modrý, D. A survey of entodiniomorphid ciliates in chimpanzees and bonobos. Am. J. Phys. Anthropol. 2010, 142, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lilly, A.A.; Mehlman, P.T.; Doran, D. Intestinal parasites in gorillas, chimpanzees, and humans at Mondika Research Site, Dzanga-Ndoki National Park, Central African Republic. Int. J. Primatol. 2002, 23, 555–573. [Google Scholar] [CrossRef]
- Zommers, Z.; Macdonald, D.W.; Johnson, P.J.; Gillespie, T.R. Impact of human activities on chimpanzee ground use and parasitism (Pan troglodytes). Conserv. Lett. 2013, 6, 264–273. [Google Scholar] [CrossRef]
- Profousová, I.; Petrželková, K.J.; Pomajbíková, K.; Modrý, D. Survival and morphological changes of entodiniomorphid ciliate Troglodytella abrassarti in chimpanzee feces. Zoo Wild Med. 2011, 42, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Jirků, M.; Pomajbíková, K.; Petrželková, K.J.; Hůzová, Z.; Modrý, D.; Lukeš, J. Detection of Plasmodium spp. in human feces. Emerg. Infect. Dis. 2012, 18, 634–636. [Google Scholar] [CrossRef]
- Assis, G.M.; Alvarenga, D.A.; Costa, D.C.; Souza, J.C., Jr.; Hirano, Z.M.; Kano, F.S.; Sousa, T.N.; Brito, C.F. Detection of Plasmodium in faeces of the New World primate Alouatta clamitans. Mem. Inst. Oswaldo Cruz 2016, 111, 570–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shehri, H.; Power, B.J.; Archer, J.; Cousins, A.; Atuhaire, A.; Adriko, M.; Arinaitwe, M.; Alanazi, A.D.; LaCourse, E.J.; Kabatereine, N.B.; et al. Non-invasive surveillance of Plasmodium infection by real-time PCR analysis of ethanol preserved faeces from Ugandan school children with intestinal schistosomiasis. Malar. J. 2019, 18, 109. [Google Scholar] [CrossRef]
- Ta, T.H.; Hisam, S.; Lanza, M.; Jiram, A.I.; Ismail, N.; Rubio, J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Ta, T.T.; Salas, A.; Ali-Tammam, M.; Martínez, M.D.C.; Lanza, M.; Arroyo, E.; Rubio, J.M. First case of detection of Plasmodium knowlesi in Spain by Real Time PCR in a traveller from Southeast Asia. Malar. J. 2010, 27, 219. [Google Scholar]
- Coatney, G.R.; Collins, W.E.; Warren, M.; Contacos, P.G. CD-ROM. The Primate Malarias; Original Book Published, 1971; Division of Parasitic Disease, Producers; Version 1.0; CDC: Atlanta, GA, USA, 2003. [Google Scholar]
- Hayakawa, T.; Arisue, N.; Udono, T.; Hirai, H.; Sattabongkot, J.; Toyama, T.; Tsuboi, T.; Horii, T.; Tanabe, K. Identification of Plasmodium malariae, a human malaria parasite, in imported chimpanzees. PLoS ONE 2009, 4, e7412. [Google Scholar] [CrossRef]
- Makanga, B.; Yangari, P.; Rahola, N.; Rougeron, V.; Elguero, E.; Boundenga, L.; Moukodoum, N.D.; Okouga, A.P.; Arnathau, C.; Durand, P.; et al. Ape malaria transmission and potential for ape-to-human transfers in Africa. Proc. Natl. Acad. Sci. USA 2016, 113, 5329–5334. [Google Scholar] [CrossRef] [Green Version]
- Simarro, P.P.; Diarra, A.; Postigo, J.A.R.; Franco, J.R.; Jannin, J.G. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: The way forward. PLoS Negl. Trop. Dis. 2011, 5, e1007. [Google Scholar] [CrossRef] [Green Version]
- Cordon-Obras, C.; Berzosa, P.; Ndong-Mabale, N.; Bobuakasi, L.; Buatiche, J.N.; Ndongo-Asumu, P.; Benito, A.; Cano, J. Trypanosoma brucei gambiense in domestic livestock of Kogo and Mbini foci (Equatorial Guinea). Trop. Med. Int. Health 2009, 14, 535–541. [Google Scholar] [CrossRef]
- Godfrey, D.G.; Killick-Kendrick, R. Cyclically transmitted infections of Trypanosoma brucei, T. rhodesiense and T. gambiense in chimpanzees. Trans. R. Soc. Trop. Med. Hyg. 1967, 61, 781–791. [Google Scholar] [CrossRef]
- Votýpka, J.; Pafčo, B.; Modrý, D.; Mbohli, D.; Tagg, N.; Petrželková, K.J. An unexpected diversity of trypanosomatids in fecal samples of great apes. Int. J. Parasitol. Parasites Wildl. 2018, 7, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Ta-Tang, T.H.; Luz, S.L.; Crainey, J.L.; Rubio, J.M. An overview of the management of Mansonellosis. Res. Rep. Trop. Med. 2021, 12, 93–105. [Google Scholar] [CrossRef]
- McLennan, M.R.; Hasegawa, H.; Bardi, M.; Huffman, M.A. Gastrointestinal parasite infections and self-medication in wild chimpanzees surviving in degraded forest fragments within an agricultural landscape mosaic in Uganda. PLoS ONE 2017, 12, e0180431. [Google Scholar] [CrossRef] [Green Version]
- Petrášová, J.; Modrý, D.; Huffman, M.A.; Mapua, M.I.; Bobáková, L.; Mazoch, V.; Singh, J.; Kaur, T.; Petrželková, K.J. Gastrointestinal parasites of indigenous and introduced primate species of Rubondo Island National Park, Tanzania. Int. J. Primatol. 2010, 31, 920–936. [Google Scholar] [CrossRef]
- Kalousová, B.; Piel, A.K.; Pomajbíková, K.; Modrý, D.; Stewart, F.A.; Petrželková, K.J. Gastrointestinal parasites of savanna chimpanzees (Pan troglodytes schweinfurthii) in Ugalla, Tanzania. Int. J. Primatol. 2014, 35, 463–475. [Google Scholar] [CrossRef]
- Ashford, R.W.; Reid, G.D.; Wrangham, R.W. Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Ann. Trop. Med. Parasitol. 2000, 94, 173–179. [Google Scholar] [CrossRef]
- Masi, S.; Chauffour, S.; Bain, O.; Todd, A.; Guillot, J.; Krief, S. Seasonal effects on great ape health: A case study of wild chimpanzees and Western gorillas. PLoS ONE 2012, 7, e49805. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; De Wachter, R. TREECON for Windows: A software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. BioSci. 1994, 10, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Species | Positive (n) | Prevalence (%) | 95% Confidence Interval |
|---|---|---|---|
| Intestinal protists | |||
| Entamoeba dispar | 43 | 18.4 | 13.6–23.9 |
| Sarcocystis spp. | 27 | 11.5 | 7.7–16.3 |
| Blastocystis sp. | 13 | 5.6 | 3.0–9.3 |
| Troglodytella abrassarti | 13 | 5.6 | 3.0–9.3 |
| Giardia duodenalis | 5 | 2.1 | 0.7–4.9 |
| Cryptosporidium hominis | 2 | 0.9 | 0.1–3.1 |
| Balantioides coli | 0 | 0.0 | – |
| Entamoeba histolytica | 0 | 0.0 | ‒ |
| Intestinal microsporidia | |||
| Enterocytozoon bieneusi | 0 | 0.0 | ‒ |
| Blood protists | |||
| Trypanosoma brucei | 3 | 1.3 | 0.27–3.7 |
| Plasmodium malariae | 1 | 0.4 | 0.01–2.4 |
| Plasmodium spp. | 1 | 0.4 | 0.01–2.4 |
| Filarial nematodes | |||
| Mansonella perstans | 23 | 9.8 | 6.3–14.4 |
| Species | Genotype | Sub- Genotype | No. Isolates | Locus | Reference Sequence | Stretch | Single Nucleotide Polymorphisms | GenBank ID |
|---|---|---|---|---|---|---|---|---|
| Cryptosporidium hominis | ‒ | ‒ | 1 | ssu rRNA | AF108865 | 574–997 | None | MZ182324 |
| ‒ | ‒ | 1 | ssu rRNA | AF108865 | 579–983 | C805Y | MZ182323 | |
| Blastocystis sp. | ST1 | Allele 1 | 1 | ssu rRNA | AB107968 | 79–568 | A132G | MZ182325 |
| ST1 | Allele 7 | 1 | ssu rRNA | HQ286905 | 14–506 | A476G | MZ182326 | |
| ST1 | Allele 8 | 10 | ssu rRNA | HQ286907 | 1–553 | T1A, 10_11DelAG, 71DelC, 513DelC, T551A, A553C | MZ182327 | |
| ST1 | Alleles 7 + 8 | 1 | ssu rRNA | HQ286907 | 73–553 | G141R, 513DelC, T551A, A553C | MZ182328 | |
| Troglodytella abrassarti | ‒ | ‒ | 13 | ITS | EU680311 | 1–418 | T82C, G177A | MZ224016 |
| Variable | Contrast | Estimate | SE | Z Ratio | p Value |
|---|---|---|---|---|---|
| Blastocystis sp. | Seasonality | 0.4770 | 0.3520 | 1.355 | 0.1756 |
| Rainfall | 0.0548 | 0.0235 | 2.330 | 0.0198 | |
| Temperature min. | −0.0263 | 0.2118 | −0.124 | 0.9010 | |
| Stool consistency | 0.1069 | 0.1632 | 0.655 | 0.5120 | |
| Giardia duodenalis | Seasonality | 0.6270 | 1.1300 | 0.556 | 0.5780 |
| Rainfall | −0.4564 | 0.4465 | −1.022 | 0.3067 | |
| Temperature min. | 1.7000 | 0.0754 | 2.255 | 0.0241 | |
| Stool consistency | −0.0967 | 0.0460 | −2.100 | 0.0357 | |
| Mansonella perstans | Seasonality | 0.3569 | 0.1588 | 2.303 | 0.0213 |
| Rainfall | −0.0435 | 0.0237 | −1.838 | 0.0066 | |
| Temperature min. | −0.1081 | 0.1177 | −0.918 | 0.3590 | |
| Stool consistency | −0.1626 | 0.1151 | −1.412 | 0.1579 | |
| Entamoeba dispar | Seasonality | −1.4600 | 0.5770 | −2.525 | 0.0570 |
| Rainfall | 0.2614 | 0.1591 | 0.164 | 0.1000 | |
| Temperature min. | 0.1270 | 0.4470 | 0.285 | 0.7758 | |
| Stool consistency | 0.0228 | 0.0200 | 1.141 | 0.2538 | |
| Sarcocystis spp. | Seasonality | 1.7000 | 0.0754 | 2.255 | 0.0241 |
| Rainfall | −0.2865 | 0.1756 | −1.631 | 0.1028 | |
| Temperature min. | 1.0200 | 0.4400 | 2.323 | 0.0202 | |
| Stool consistency | −0.0435 | 0.0237 | −1.838 | 0.0066 | |
| Bristol Stool Scale | Seasonality | −1.6200 | 0.2230 | −7.285 | <0.0001 |
| Rainfall * | t = 5.961 | df = 210 | – | <0.0001 | |
| Temperature min. * | t = 2.030 | df = 210 | – | 0.04361 |
| Parasite 1 | Parasite 2 | p Value | p Adjusted Value |
|---|---|---|---|
| Blastocystis sp. | Giardia duodenalis | 1.0000 | 1.0000 |
| Blastocystis sp. | Mansonella perstans | 1.0000 | 1.0000 |
| Blastocystis sp. | Entamoeba dispar | 0.2654 | 1.0000 |
| Blastocystis sp. | Sarcocystis spp. | 1.0000 | 1.0000 |
| Giardia duodenalis | Mansonella perstans | 1.0000 | 1.0000 |
| Giardia duodenalis | Entamoeba dispar | 0.5875 | 1.0000 |
| Giardia duodenalis | Sarcocystis spp. | 0.4660 | 1.0000 |
| Mansonella perstans | Entamoeba dispar | 0.0874 | 0.8740 |
| Mansonella perstans | Sarcocystis spp. | 0.7384 | 1.0000 |
| Entamoeba dispar | Sarcocystis spp. | 0.7934 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köster, P.C.; Renelies-Hamilton, J.; Dotras, L.; Llana, M.; Vinagre-Izquierdo, C.; Prakas, P.; Sneideris, D.; Dashti, A.; Bailo, B.; Lanza, M.; et al. Molecular Detection and Characterization of Intestinal and Blood Parasites in Wild Chimpanzees (Pan troglodytes verus) in Senegal. Animals 2021, 11, 3291. https://doi.org/10.3390/ani11113291
Köster PC, Renelies-Hamilton J, Dotras L, Llana M, Vinagre-Izquierdo C, Prakas P, Sneideris D, Dashti A, Bailo B, Lanza M, et al. Molecular Detection and Characterization of Intestinal and Blood Parasites in Wild Chimpanzees (Pan troglodytes verus) in Senegal. Animals. 2021; 11(11):3291. https://doi.org/10.3390/ani11113291
Chicago/Turabian StyleKöster, Pamela C., Justinn Renelies-Hamilton, Laia Dotras, Manuel Llana, Celia Vinagre-Izquierdo, Petras Prakas, Donatas Sneideris, Alejandro Dashti, Begoña Bailo, Marta Lanza, and et al. 2021. "Molecular Detection and Characterization of Intestinal and Blood Parasites in Wild Chimpanzees (Pan troglodytes verus) in Senegal" Animals 11, no. 11: 3291. https://doi.org/10.3390/ani11113291
APA StyleKöster, P. C., Renelies-Hamilton, J., Dotras, L., Llana, M., Vinagre-Izquierdo, C., Prakas, P., Sneideris, D., Dashti, A., Bailo, B., Lanza, M., Jiménez-Mejías, A., Muñoz-García, C., Muadica, A. S., González-Barrio, D., Rubio, J. M., Fuentes, I., Ponce-Gordo, F., Calero-Bernal, R., & Carmena, D. (2021). Molecular Detection and Characterization of Intestinal and Blood Parasites in Wild Chimpanzees (Pan troglodytes verus) in Senegal. Animals, 11(11), 3291. https://doi.org/10.3390/ani11113291










