Regulation of FSH Synthesis by Differentially Expressed miR-488 in Anterior Adenohypophyseal Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Tissue Collection
2.3. Cell Culture
2.4. GnRH Analogue Treatment for Cows
2.5. GnRH Analogue Treatment for Rats
2.6. GnRH Treatment for Cells
2.7. Transfection
2.8. RNA Extraction, Reverse Transcription, and Real-Time q-PCR
2.9. Dual Luciferase Reporter Assay
2.10. ELISA
2.11. Apoptosis Analysis
2.12. MiRNA Target Prediction
2.13. Statistical Analysis
3. Results
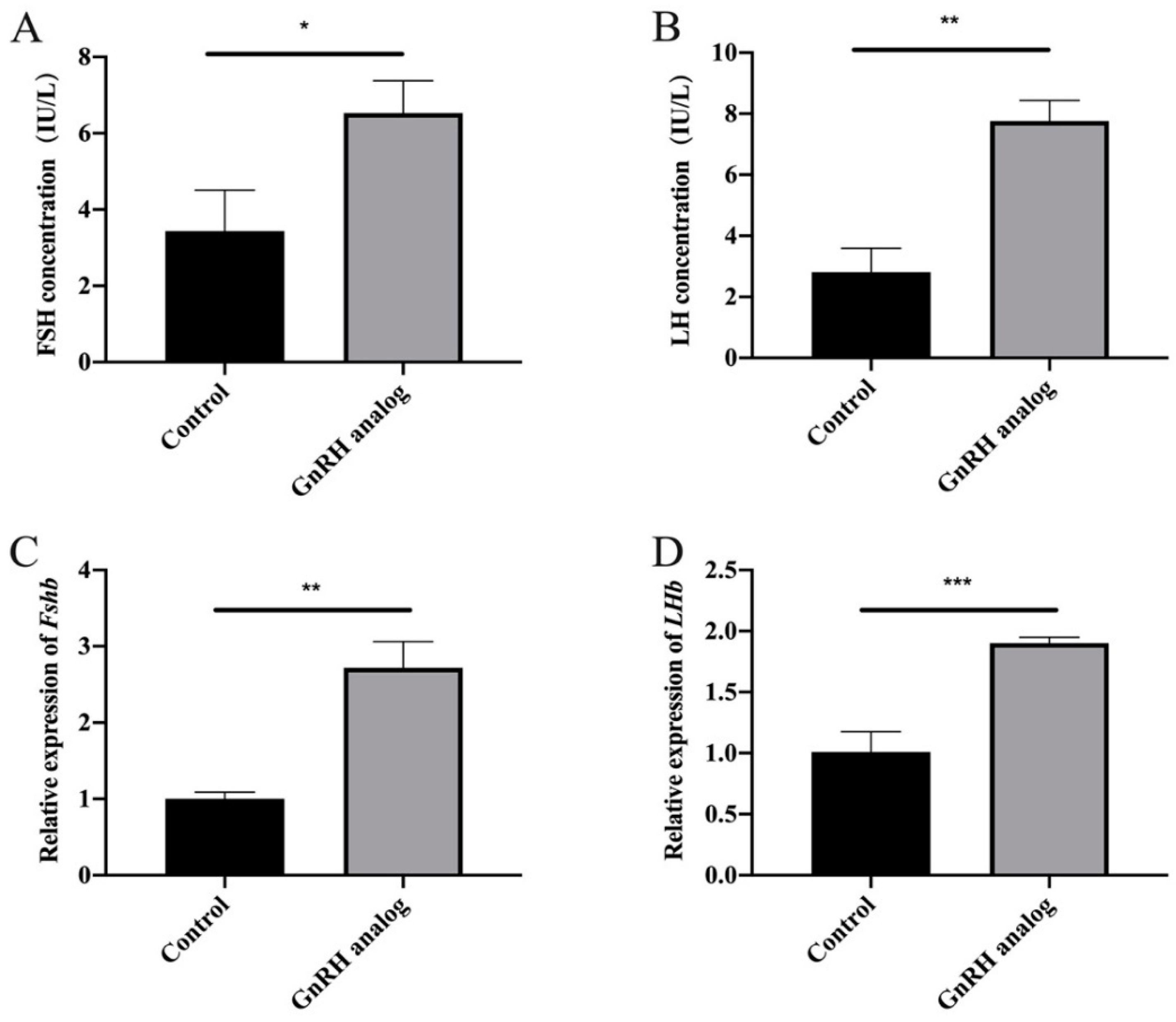
3.1. GnRH Analogue Treatment Promoted the Synthesis and Secretion of FSH and LH in Cows
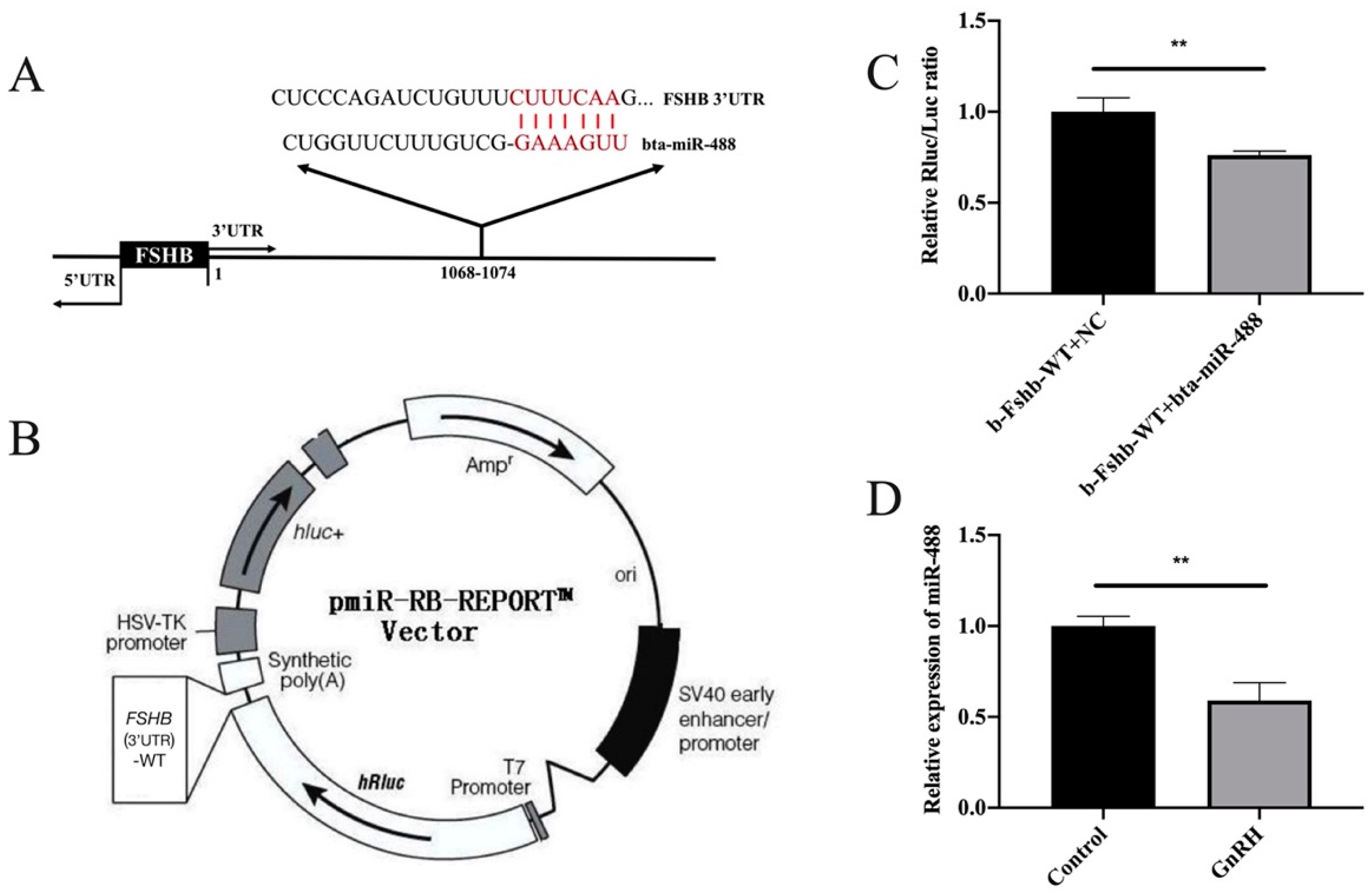
3.2. MiR-488 Targeted the FSHB 3’UTR
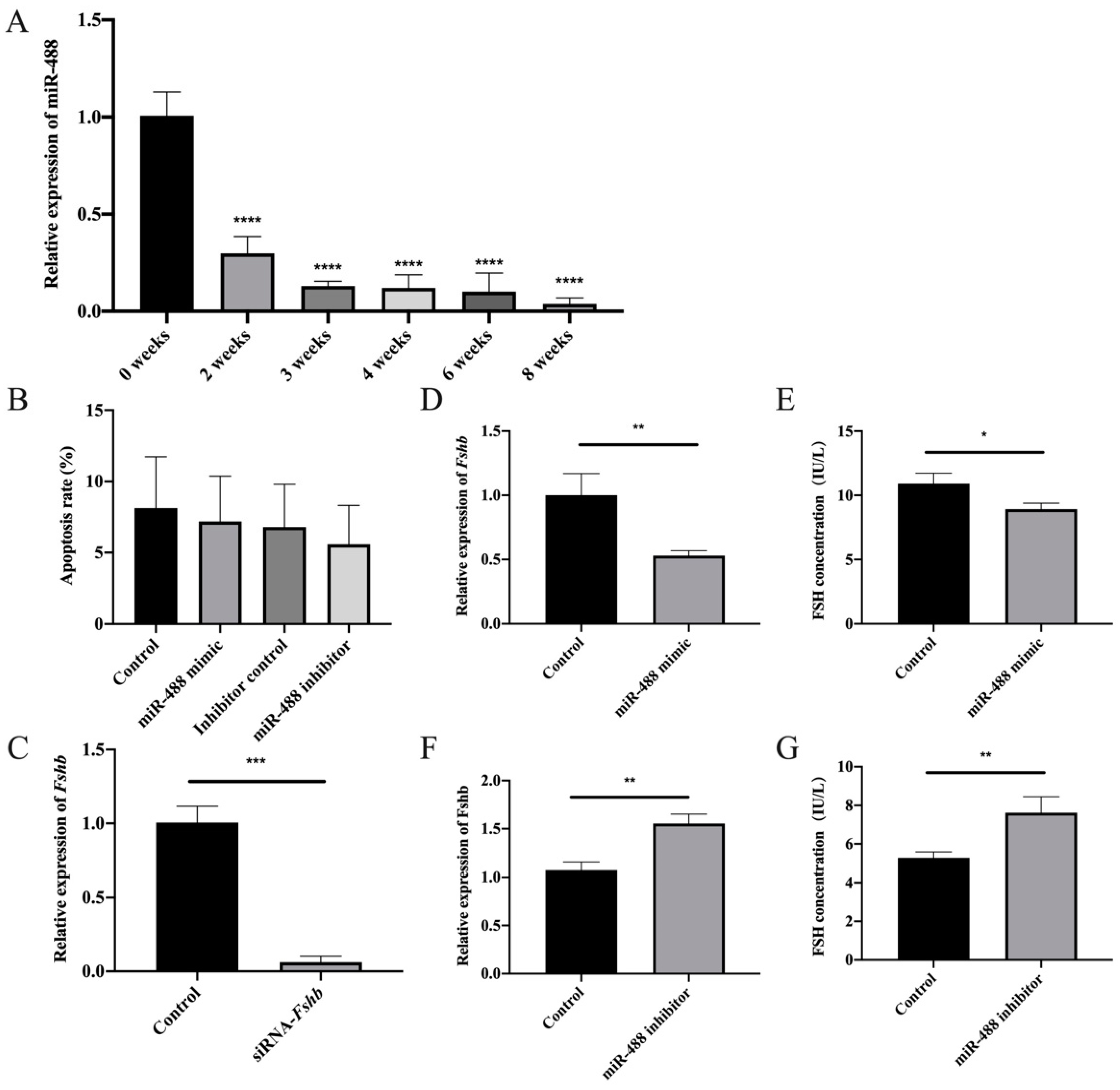
3.3. Low-Frequency GnRH Analogue Treatment Promoted the Synthesis and Secretion of FSH and LH in Rats
3.4. Regulation of FSH Synthesis by Differentially Expressed miR-488 in Rat Anterior Adenohypophyseal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaprara, A.; Huhtaniemi, I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 2018, 86, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Stamatiades, G.A.; Carroll, R.S.; Kaiser, U.B. GnRH-A Key Regulator of FSH. Endocrinology 2019, 160, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Ge, J.; Wei, J.; Liu, J.; Liu, C.; Ma, C.; Zhao, X.; Wei, Q.; Ma, B. Effect of FSH on E2/GPR30-mediated mouse oocyte maturation in vitro. Cell Signal. 2020, 66, 109464. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.A.; Desrosier, S.; Assidi, M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology 2007, 68 (Suppl. 1), S71–S76. [Google Scholar] [CrossRef]
- Russell, L.D.; Kershaw, M.; Borg, K.E.; El Shennawy, A.; Rulli, S.S.; Gates, R.J.; Calandra, R.S. Hormonal regulation of spermatogenesis in the hypophysectomized rat: FSH maintenance of cellular viability during pubertal spermatogenesis. J. Androl. 1998, 19, 308–319, discussion 341–302. [Google Scholar]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- Gobello, C. New GnRH analogs in canine reproduction. Anim. Reprod. Sci. 2007, 100, 1–13. [Google Scholar] [CrossRef]
- Roser, J.F.; Meyers-Brown, G. Enhancing Fertility in Mares: Recombinant Equine Gonadotropins. J. Equine Vet. Sci. 2019, 76, 6–13. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Liu, C.; Chen, M.; Wang, M.; Pi, W.; Li, N.; Meng, Q. MiR-18a regulates myoblasts proliferation by targeting Fgf1. PLoS ONE 2018, 13, e0201551. [Google Scholar] [CrossRef]
- Liao, H.Q.; Liu, H.; Sun, H.L.; Xiang, J.B.; Wang, X.X.; Jiang, C.X.; Ma, L.; Cao, Z.G. MiR-361-3p/Nfat5 Signaling Axis Controls Cementoblast Differentiation. J. Dent. Res. 2019, 98, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int. J. Mol. Med. 2019, 43, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Cotrim-Sousa, L.; Freire-Assis, A.; Pezzi, N.; Tanaka, P.P.; Oliveira, E.H.; Passos, G.A. Adhesion between medullary thymic epithelial cells and thymocytes is regulated by miR-181b-5p and miR-30b. Mol. Immunol. 2019, 114, 600–611. [Google Scholar] [CrossRef]
- Byun, Y.; Choi, Y.C.; Jeong, Y.; Lee, G.; Yoon, S.; Jeong, Y.; Yoon, J.; Baek, K. MiR-200c downregulates HIF-1alpha and inhibits migration of lung cancer cells. Cell Mol. Biol. Lett. 2019, 24, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazare, S.S.; Wojtowicz, E.E.; Bystrykh, L.V.; de Haan, G. microRNAs in hematopoiesis. Exp. Cell Res. 2014, 329, 234–238. [Google Scholar] [CrossRef]
- Madelaine, R.; Sloan, S.A.; Huber, N.; Notwell, J.H.; Leung, L.C.; Skariah, G.; Halluin, C.; Pasca, S.P.; Bejerano, G.; Krasnow, M.A.; et al. MicroRNA-9 Couples Brain Neurogenesis and Angiogenesis. Cell Rep. 2017, 20, 1533–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.X.; Sun, X.L.; Xu, M.Q.; Chen, C.Z.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Roles of differential expression of microRNA-21-3p and microRNA-433 in FSH regulation in rat anterior pituitary cells. Oncotarget 2017, 8, 36553–36565. [Google Scholar] [CrossRef]
- Pace, J.N.; Miller, J.L.; Rose, L.I. GnRH agonists: Gonadorelin, leuprolide and nafarelin. Am. Fam. Physician 1991, 44, 1777–1782. [Google Scholar]
- Hashem, N.M.; Sallam, S.M. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest. Anim. Endocrinol. 2020, 71, 106390. [Google Scholar] [CrossRef]
- Newton, C.L.; Riekert, C.; Millar, R.P. Gonadotropin-releasing hormone analog therapeutics. Minerva Ginecol. 2018, 70, 497–515. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, L.; Li, J.; Cui, L.; Huang, W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst. Rev. 2019, 3, CD008018. [Google Scholar] [CrossRef] [PubMed]
- Freick, M.; Weber, O.; Passarge, O.; Neubert, T. [Use of gonadorelin[6-D-Phe] at day 0 or 12 after insemination to increase the conception rate in a large dairy herd in Saxony/Germany]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2014, 42, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.N.; Habermacher, R.; Martine, U.; Closs, E.I.; Filipowicz, W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 2006, 125, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Simoes da Silva, C.J.; Sospedra, I.; Aparicio, R.; Busturia, A. The microRNA-306/abrupt regulatory axis controls wing and haltere growth in Drosophila. Mech. Dev. 2019, 158, 103555. [Google Scholar] [CrossRef] [PubMed]
- Gummalla, M.; Galetti, S.; Maeda, R.K.; Karch, F. Hox gene regulation in the central nervous system of Drosophila. Front. Cell Neurosci. 2014, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2017, 219, 346–361. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Wernicke, D.; Croce, C.M. MicroRNA and cancer—A brief overview. Adv. Biol. Regul. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef]
- Li, Y.; Ren, W.; Wang, X.; Yu, X.; Cui, L.; Li, X.; Zhang, X.; Shi, B. MicroRNA-150 relieves vascular remodeling and fibrosis in hypoxia-induced pulmonary hypertension. Biomed. Pharmacother. 2019, 109, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, L.; Jia, K.; Wang, H.; Wang, X. miR-9-5p modulates the progression of Parkinson’s disease by targeting SIRT1. Neurosci. Lett. 2019, 701, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matarese, A.; Gambardella, J.; Lombardi, A.; Wang, X.; Santulli, G. miR-7 Regulates GLP-1-Mediated Insulin Release by Targeting beta-Arrestin 1. Cells 2020, 9, 1621. [Google Scholar] [CrossRef]
- Elias, A.E.; Kreiling, J.A. MicroRNA dysregulation influences growth hormone signaling. Aging 2019, 11, 5294–5295. [Google Scholar] [CrossRef]
- Han, D.X.; Xiao, Y.; Wang, C.J.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Regulation of FSH expression by differentially expressed miR-186-5p in rat anterior adenohypophyseal cells. PLoS ONE 2018, 13, e0194300. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.J.; Guo, H.X.; Han, D.X.; Yu, Z.W.; Zheng, Y.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Pituitary tissue-specific miR-7a-5p regulates FSH expression in rat anterior adenohypophyseal cells. PeerJ 2019, 7, e6458. [Google Scholar] [CrossRef]
- Wei, X.; Yu, L.; Kong, X. miR-488 inhibits cell growth and metastasis in renal cell carcinoma by targeting HMGN5. OncoTargets Ther. 2018, 11, 2205–2216. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.Y.; Wang, X.Q.; Sun, L.F. MicroRNA-488 inhibits ovarian cancer cell metastasis through regulating CCNG1 and p53 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2902–2910. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.R.; Ciccone, N.A.; Xu, S.; Zaytseva, S.; Carroll, R.S.; Kaiser, U.B. GnRH pulse frequency-dependent stimulation of FSHbeta transcription is mediated via activation of PKA and CREB. Mol. Endocrinol. 2013, 27, 606–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Species | Primer Name | Sequence (5’–3’) | |
|---|---|---|---|
| Rat | Gapdh | Forward primer | GGAAACCCATCACCATCTTC |
| Reverse primer | GTGGTTCACACCCATCACAA | ||
| Fshβ | Forward primer | ATACCACTTGGTGTGAGGGC | |
| Reverse primer | TAGAGGGAGTCTGAGTGGCG | ||
| Lhβ | Forward primer | CAAAAGCCAGGTCAGGGATA | |
| Reverse primer | GTACTCGAACCATGCTAGGACA | ||
| U6 | Forward primer | GCTTCGGCAGCACATATACTAAAAT | |
| Reverse primer | CGCTTCACGAATTTGCGTGTCAT | ||
| U6 | Reverse transcription | CGCTTCACGAATTTGCGTGTCAT | |
| miR-488 | Reverse transcription | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTGAG | |
| miR-488 | Forward primer | ACACTCCAGCTGGGGCCTTCCGGT | |
| universal reverse | CTCAAGTGTCGTGGAGTCGGCAA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Q.; Wang, W.-H.; Chen, C.-Z.; Guo, H.-X.; Jiang, H.; Yuan, B.; Zhang, J.-B. Regulation of FSH Synthesis by Differentially Expressed miR-488 in Anterior Adenohypophyseal Cells. Animals 2021, 11, 3262. https://doi.org/10.3390/ani11113262
Wang H-Q, Wang W-H, Chen C-Z, Guo H-X, Jiang H, Yuan B, Zhang J-B. Regulation of FSH Synthesis by Differentially Expressed miR-488 in Anterior Adenohypophyseal Cells. Animals. 2021; 11(11):3262. https://doi.org/10.3390/ani11113262
Chicago/Turabian StyleWang, Hao-Qi, Wen-Hua Wang, Cheng-Zhen Chen, Hai-Xiang Guo, Hao Jiang, Bao Yuan, and Jia-Bao Zhang. 2021. "Regulation of FSH Synthesis by Differentially Expressed miR-488 in Anterior Adenohypophyseal Cells" Animals 11, no. 11: 3262. https://doi.org/10.3390/ani11113262







