Mycobacterium Tuberculosis and Avium Complex Investigation among Malaysian Free-Ranging Wild Boar and Wild Macaques at Wildlife-Livestock-Human Interface
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Animals
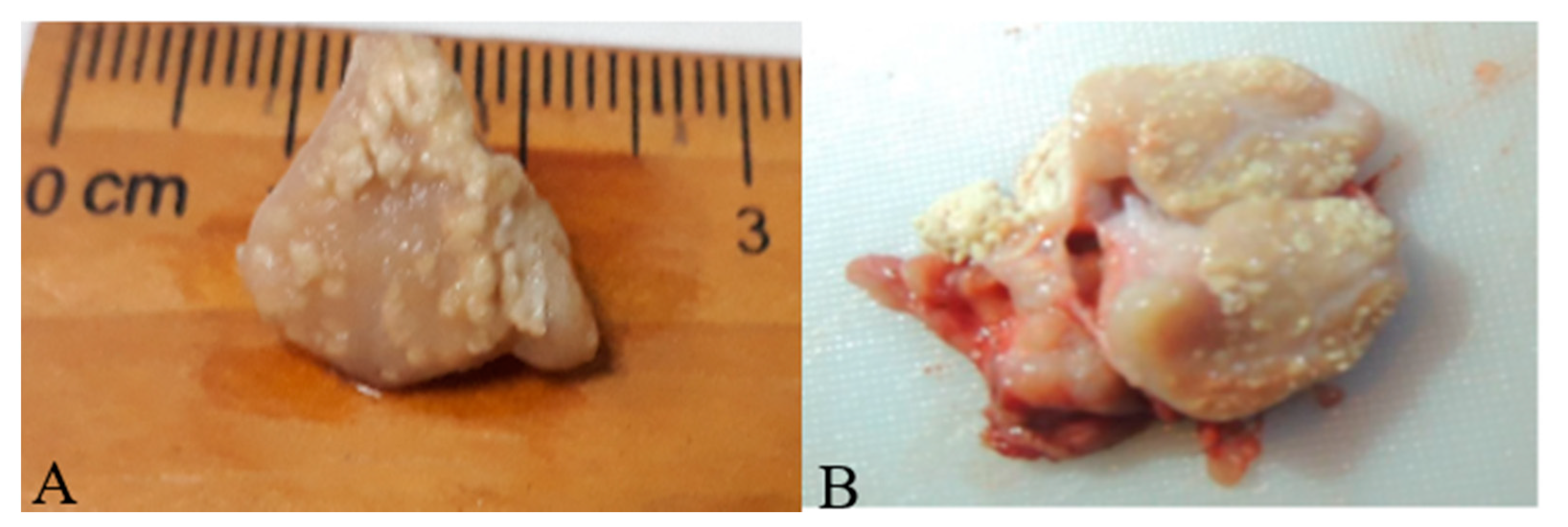
2.2. Sample Collection and Post-Mortem Examination for TBLL
2.3. Stained Smears for Acid-Fast Bacilli (AFB)
2.4. Mycobacterial Culture Isolation
2.5. DNA Extraction from Tissues and Anticoagulated Blood
2.6. PCR for the Genus Mycobacterium, MTBC and MAC
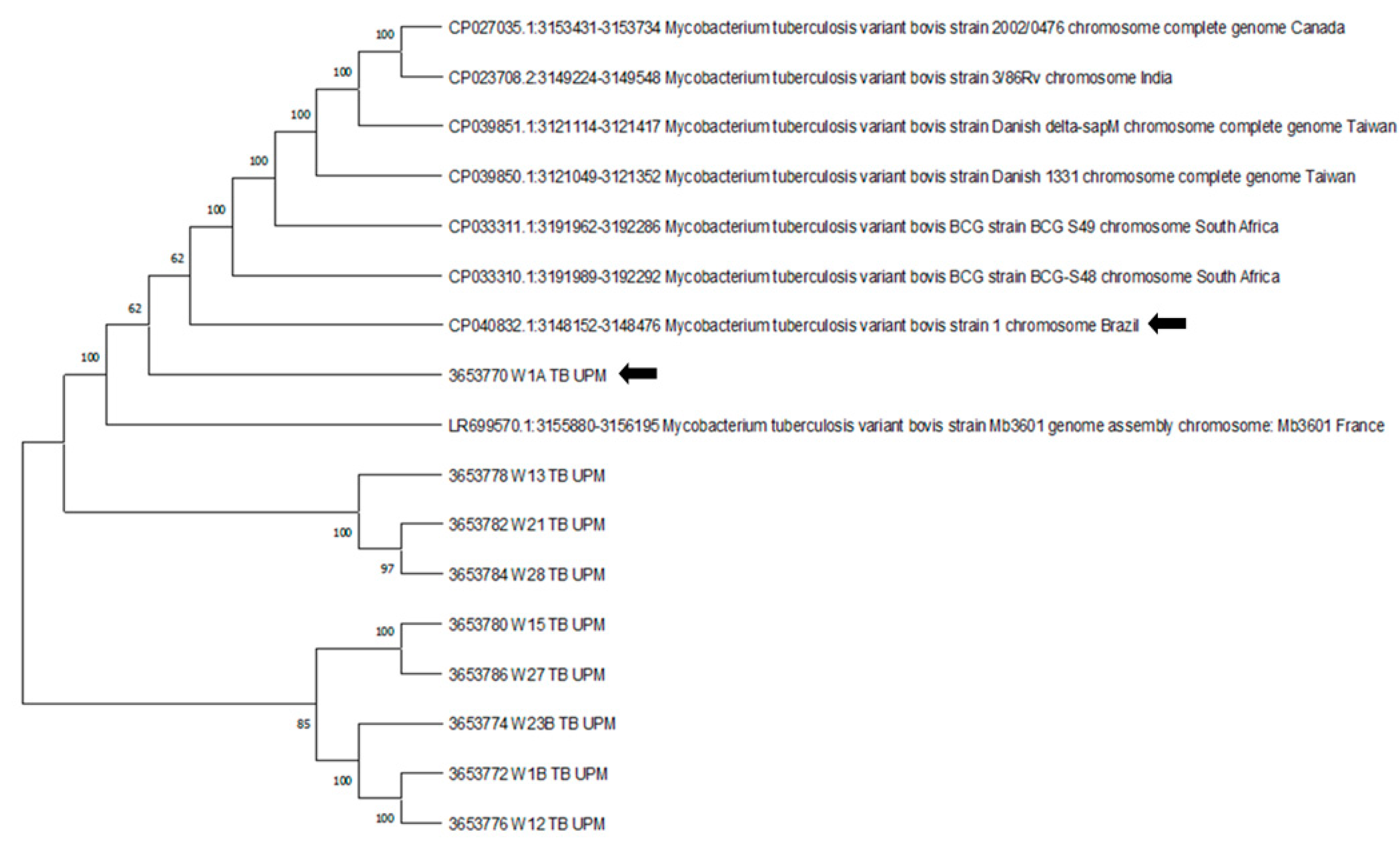
2.7. DNA Sequencing and Phylogenetic Analysis of M. bovis 16S rRNA Gene
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gortázar, C.; Fernández-Calle, L.M.; Collazos-Martínez, J.A.; Mínguez-González, O.; Acevedo, P. Animal tuberculosis maintenance at low abundance of suitable wildlife reservoir hosts: A case study in northern Spain. Prev. Vet. Med. 2017, 146, 150–157. [Google Scholar] [CrossRef]
- Barasona, J.A.; Vicente, J.; Díez-Delgado, I.; Aznar, J.; Gortázar, C.; Torres, M.J. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound. Emerg. Dis. 2016, 64, 1148–1158. [Google Scholar] [CrossRef]
- Sevilla, I.A.; Molina, E.; Elguezabal, N.; Pérez, V.; Garrido, J.M.; Juste, R.A. Detection of mycobacteria, Mycobacterium avium subspecies, and Mycobacterium tuberculosis complex by a novel tetraplex real-time PCR assay. J. Clin. Microbiol. 2015, 53, 930–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, A.C.; Figueira, L.; Martins, M.H.; Matos, M.; Andrade, S.; Álvares, S.; Mendes, A.; Sousa, N.; Coelho, A.; Pinto, M.L. Granulomatous lesions and Mycobacterium avium subsp. paratuberculosis in Portuguese wild boars (Sus scrofa). J. Comp. Pathol. 2013, 148, 85. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Ku, B.K.; Lee, H.N.; Hwang, I.Y.; Jang, Y.B.; Kim, J.; Hyun, B.H.; Jung, S.C. Mycobacterium avium paratuberculosis in wild boars in Korea. J. Wildl. Dis. 2013, 49, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Cowie, C.E.; Hutchings, M.R.; Barasona, J.A.; Gortázar, C.; Vicente, J.; White, P.C.L. Interactions between four species in a complex wildlife: Livestock disease community: Implications for Mycobacterium bovis maintenance and transmission. Eur. J. Wildl. Res. 2016, 62, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Brosch, R.; Gordon, S.V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 2002, 99, 3684–3689. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Flowers, N.; Anderson, J. The National Rheumatic Disease Data Bank: Case mix and severity characteristics of patients in rheumatological practice. Arthritis Rheum. 1998, 41, 132. [Google Scholar]
- Fechner, K.; Mätz-Rensing, K.; Lampe, K.; Kaup, F.J.; Czerny, C.P.; Schäfer, J. Detection of Mycobacterium avium subsp. paratuberculosis in non-human primates. J. Med. Primatol. 2017, 46, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Roller, M.; Hansen, S.; Böhlken-Fascher, S.; Knauf-Witzens, T.; Czerny, C.P.; Goethe, R.; El Wahed, A.A. Molecular and serological footprints of mycobacterium avium subspecies infections in zoo animals. Vet. Sci. 2020, 7, 117. [Google Scholar] [CrossRef]
- Burgess, T.L.; Witte, C.L.; Rideout, B.A. Early-life exposures and Johne’s disease risk in zoo ruminants. J. Vet. Diagnostic Investig. 2018, 30, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biet, F.; Boschiroli, M.L.; Thorel, M.F.; Guilloteau, L.A. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet. Res. 2005, 36, 411–436. [Google Scholar] [CrossRef] [Green Version]
- Chege, G.K.; Warren, R.M.; Van Pittius, N.C.G.; Burgers, W.A.; Wilkinson, R.J.; Shephard, E.G.; Williamson, A.L. Detection of natural infection with Mycobacterium intracellulare in healthy wild-caught Chacma baboons (Papio ursinus) by ESAT-6 and CFP-10 IFN-γ ELISPOT tests following a tuberculosis outbreak. BMC Microbiol. 2008, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roller, M.; Roller, M.; Hansen, S.; Knauf-witzens, T.; Oelemann, W.M.R.; Gamble, K.C.; Zoo, L.P.; States, U.; Collins, M.T. Mycobacterium avium subspecies paratuberculosis infection in zoo animals: A review of susceptibility and disease process. Front. Vet. Sci. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Sáez-Royuela, C.; Gomariz, R.P.; Tellería, J.L. Age determination of european wild boar. Wildl. Soc. Bull. 1989, 17, 326–329. [Google Scholar]
- Gortazar, C.; Diez-delgado, I.; Barasona, J.A.; Vicente, J.; Gortazar, C.; Diez-Delgado, I.; Barasona, J.A.; Vicente, J.; De La Fuente, J.; Boadella, M. The wild side of disease control at the wildlife-livestock-human interface: A review. Front. Vet. Sci. 2015, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.S.L.; de la Torre, J.A.; Wong, E.P.; Thuppil, V.; Campos-Arceiz, A. Factors affecting urban and rural tolerance towards conflict-prone endangered megafauna in Peninsular Malaysia. Glob. Ecol. Conserv. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Che-Amat, A.; Ong, B.L. Wildlife tuberculosis in southeast Asia: A less known potential hot-spots and issues in disease surveillance and management. J. Dairy Vet. Sci. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Pesciaroli, M.; Russo, M.; Mazzone, P.; Aronica, V.; Fiasconaro, M.; Boniotti, M.B.; Corneli, S.; Cagiola, M.; Pacciarini, M.; Pasquali, P.; et al. Evaluation of the interferon-gamma (IFN-γ) assay to diagnose Mycobacterium bovis infection in pigs. Vet. Immunol. Immunopathol. 2012, 148, 369–372. [Google Scholar] [CrossRef]
- Maas, M.; Michel, A.L.; Rutten, V.P.M.G. Facts and dilemmas in diagnosis of tuberculosis in wildlife. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Caulfield, A.J.; Wengenack, N.L. Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 4, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Lu, J.J.; Chang, C.Y.; Chou, W.P.; Hsieh, J.C.H.; Lin, C.R.; Wu, M.H. Development of a high sensitivity TaqMan-based PCR assay for the specific detection of Mycobacterium tuberculosis complex in both pulmonary and extrapulmonary specimens. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Costa, P.; Botelho, A.; Couto, I.; Viveiros, M.; Inácio, J. Standing of nucleic acid testing strategies in veterinary diagnosis laboratories to uncover Mycobacterium tuberculosis complex members. Front. Mol. Biosci. 2014, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenas-Montes, A.; García-Bocanegra, I.; Paniagua, J.; Franco, J.J.; Miró, F.; Fernández-Morente, M.; Carbonero, A.; Arenas, A. Blood sampling by puncture in the cavernous sinus from hunted wild boar. Eur. J. Wildl. Res. 2013, 59, 299–303. [Google Scholar] [CrossRef]
- Crawshaw, T.; De La Rua-Domenech, R.; Brown, E. Recognising the gross pathology of tuberculosis in South American camelids, deer, goats, pigs and sheep. Practice 2013, 35, 490–502. [Google Scholar] [CrossRef]
- Santos, N.; Geraldes, M.; Afonso, A.; Almeida, V.; Correia-Neves, M. Diagnosis of tuberculosis in the wild boar (Sus scrofa): A comparison of methods applicable to hunter-harvested animals. PLoS ONE 2010, 5, e12663. [Google Scholar] [CrossRef] [Green Version]
- Corner, L.A.L.; Gormley, E.; Pfeiffer, D.U. Primary isolation of Mycobacterium bovis from bovine tissues: Conditions for maximising the number of positive cultures. Vet. Microbiol. 2012, 156, 162–171. [Google Scholar] [CrossRef]
- Wilton, S.; Cousins, D. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. Genome Res. 1992, 1, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Huard, R.C.; de Oliveira Lazzarini, L.C.; Butler, W.R.; van Soolingen, D.; Ho, J.L. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 2003, 41, 1637–1650. [Google Scholar] [CrossRef] [Green Version]
- Sergeant, E.S.G. Epitools Epidemiological Calculators; Ausvet: Bruce, Australia, 2018; Available online: http://epitools.ausvet.com.au (accessed on 26 September 2021).
- Martín-Hernando, M.P.; Höfle, U.; Vicente, J.; Ruiz-Fons, F.; Vidal, D.; Barral, M.; Garrido, J.M.; de la Fuente, J.; Gortazar, C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 2007, 87, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.; Höfle, U.; Garrido, J.M.; Fernández-De-Mera, I.G.; Juste, R.; Barral, M.; Gortazar, C. Wild boar and red deer display high prevalences of tuberculosis-like lesions in Spain. Vet. Res. 2006, 37, 107–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de la Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Andrade, S.; Figueira, L.; Matos, M.; Pires, M.A.; Coelho, A.C.; Pinto, M.L. Mesenteric lymph node granulomatous lesions in naturally infected wild boar (Sus scrofa) in Portugal—Histological, immunohistochemical and molecular aspects. Vet. Immunol. Immunopathol. 2016, 173, 21–26. [Google Scholar] [CrossRef]
- Mentaberre, G.; Romero, B.; De Juan, L.; Navarro-González, N.; Velarde, R.; Mateos, A.; Marco, I.; Olivé-Boix, X.; Domínguez, L.; Lavín, S.; et al. Long-term assessment of wild boar harvesting and cattle removal for bovine tuberculosis control in free ranging populations. PLoS ONE 2014, 9, e88824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Delgado, I.; Boadella, M.; Martín-Hernando, M.; Barasona, J.A.; Beltrán-Beck, B.; González-Barrio, D.; Sibila, M.; Vicente, J.; Garrido, J.M.; Segalés, J.; et al. Complex links between natural tuberculosis and porcine circovirus type 2 infection in wild boar. BioMed Res. Int. 2014, 2014, 765715 . [Google Scholar] [CrossRef]
- Che’ Amat, A.; González-Barrio, D.; Ortiz, J.A.; Díez-Delgado, I.; Boadella, M.; Barasona, J.A.; Bezos, J.; Romero, B.; Armenteros, J.A.; Lyashchenko, K.P.; et al. Testing Eurasian wild boar piglets for serum antibodies against Mycobacterium bovis. Prev. Vet. Med. 2015, 121, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barasona, J.A.; Acevedo, P.; Diez-Delgado, I.; Queiros, J.; Carrasco-García, R.; Gortazar, C.; Vicente, J. Tuberculosis-associated death among adult wild boars, Spain, 2009–2014. Emerg. Infect. Dis. 2016, 22, 2178–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.L.; Sarmiento, M.E.; Norazmi, M.N.; Acosta, A. DNA markers for tuberculosis diagnosis. Tuberculosis 2018, 113, 139–152. [Google Scholar] [CrossRef]
- De Lisle, G.W.; Bengis, R.G.; Schmitt, S.M.; Brien, D.J.O. Tuberculosis in free-ranging wildlife: Detection, in wildlife ante-mortem diagnosis. Sci. Tech. Rev. Off. Int. Des. Epizoot. 2002, 21, 317–334. [Google Scholar] [CrossRef]
- García-Jiménez, W.L.; Salguero, F.J.; Fernández-Llario, P.; Martínez, R.; Risco, D.; Gough, J.; Ortiz-Peláez, A.; Hermoso-de-Mendoza, J.; Gómez, L. Immunopathology of granulomas produced by Mycobacterium bovis in naturally infected wild boar. Vet. Immunol. Immunopathol. 2013, 156, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Asselineau, C.C.; Asselineau, J.; Laneélle, G.; Laneélle, M.-A. The biosynthesis of mycolic acids by Mycobacteria: Current and alternative hypotheses. Prog. Lipid Res. 2002, 41, 501–523. [Google Scholar] [CrossRef]
- Mahapatra, S.; Brennan, P.J.; Crick, D.C.; Basu, J. Structure, biosynthesis, and genetics of the mycolic acid-arabinogalactan-peptidoglycan complex. In Tuberculosis and the Tubercle Bacillus; American Society of Microbiology: Washington, DC, USA, 2014; pp. 275–286. [Google Scholar]
- Domingo, M.; Vidal, E.; Marco, A. Pathology of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.A.; Trajstman, A.C.; Lund, K. Determination of the optimum concentration of decontaminants for the primary isolation of Mycobacterium bovis. N. Z. Vet. J. 1995, 43, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gavier-Widén, D.; Cooke, M.; Gallagher, J.; Chambers, M.; Gortázar, C. A review of infection of wildlife hosts with Mycobacterium bovis and the diagnostic difficulties of the ‘no visible lesion’ presentation. N. Z. Vet. J. 2009, 57, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Gormley, E.; Corner, L.A.L.; Costello, E.; Rodriguez-Campos, S. Bacteriological diagnosis and molecular strain typing of Mycobacterium bovis and Mycobacterium caprae. Res. Vet. Sci. 2014, 97, S30–S43. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.A. Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet. Microbiol. 1994, 40, 53–63. [Google Scholar] [CrossRef]
- Bollo, E.; Ferroglio, E.; Dini, V.; Mignone, W.; Biollatti, B.; Rossi, L. Detection of Mycobacterium tuberculosis complex in lymph nodes of wild boar (Sus scrofa) by a target-amplified test system. J. Vet. Med. Ser. B 2000, 47, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Fell, S.; Bröckl, S.; Büttner, M.; Rettinger, A.; Zimmermann, P.; Straubinger, R.K. Two alternative DNA extraction methods to improve the detection of Mycobacterium-tuberculosis-complex members in cattle and red deer tissue samples. BMC Microbiol. 2016, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Radomski, N.; Kreitmann, L.; McIntosh, F.; Behr, M.A. The Critical Role of DNA Extraction for detection of Mycobacteria in tissues. PLoS ONE 2013, 8, e78749. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.C.T.; Vasconcellos, S.E.G.; Issa, M.D.A.; Filho, P.M.S.; Mota, P.M.P.C.; De Araújo, F.R.; Carvalho, A.C.D.S.; Magdiniergomes, H.; Suffys, P.N.; Figueiredo, E.E.D.S.; et al. Molecular typing of Mycobacterium bovis from cattle reared in midwest Brazil. PLoS ONE 2016, 11, e0162459. [Google Scholar] [CrossRef]
- Fink, M.; Schleicher, C.; Gonano, M.; Prodinger, W.M.; Pacciarini, M.; Glawischnig, W.; Ryser-Degiorgis, M.P.; Walzer, C.; Stalder, G.L.; Lombardo, D.; et al. Red deer as maintenance host for bovine tuberculosis, Alpine region. Emerg. Infect. Dis. 2015, 21, 464–467. [Google Scholar] [CrossRef] [Green Version]
- Andrievskaia, O.; Turcotte, C.; Berlie-Surujballi, G.; Battaion, H.; Lloyd, D. Genotypes of Mycobacterium bovis strains isolated from domestic animals and wildlife in Canada in 1985–2015. Vet. Microbiol. 2018, 214, 44–50. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, W.L.; Benítez-Medina, J.M.; Carranza, J.; Cerrato, R.; García-Sánchez, A.; Risco, D.; Moreno, J.C.; Sequeda, M.; Gómez, L.; Martínez, R.; et al. Non-tuberculous Mycobacteria in wild boar (Sus scrofa) from southern Spain: Epidemiological, clinical and diagnostic concerns. Transbound. Emerg. Dis. 2013, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tirkkonen, T.; Pakarinen, J.; Moisander, A.M.; Mäkinen, J.; Soini, H.; Ali-Vehmas, T. High genetic relatedness among Mycobacterium avium strains isolated from pigs and humans revealed by comparative IS1245 RFLP analysis. Vet. Microbiol. 2007, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kopecna, M.; Trcka, I.; Lamka, J.; Moravkova, M.; Koubek, P. The wildlife hosts of Mycobacterium avium subsp. paratuberculosis in the Czech Republic during the years 2002–2007. Vet. Med. 2008, 53, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.V.; Singh, A.V.; Singh, P.K.; Kumar, A.; Singh, B. Molecular identification and characterization of Mycobacterium avium subspecies paratuberculosis in free living non-human primate (Rhesus macaques) from North India. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Münster, P.; Fechner, K.; Völkel, I.; von Buchholz, A.; Czerny, C.P. Distribution of Mycobacterium avium ssp. paratuberculosis in a German zoological garden determined by IS900 semi-nested and quantitative real-time PCR. Vet. Microbiol. 2013, 163, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hibiya, K.; Utsunomiya, K.; Yoshida, T.; Toma, S.; Higa, F. Pathogenesis of systemic Mycobacterium avium infection in pigs through histological analysis of hepatic lesions. Can. J. Vet. Res. 2010, 74, 252–257. [Google Scholar] [PubMed]
- Shin, J.I.; Shin, S.J.; Shin, M.K. Differential genotyping of Mycobacterium avium complex and its implications in clinical and environmental epidemiology. Microorganisms 2020, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulinova Stromerova, N.; Faldyna, M. Mycobacterium avium complex infection in pigs: A review. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 62–68. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Name | Nucleotide Sequence | bp | Target |
|---|---|---|---|---|
| 16S rRNA | MYCGEN-F | AGAGTTTGATCCTGGCTCAG | 1030 | MTBC |
| MYCGEN-R | TGCACACAGGCCACAAGGGA | |||
| MYCGEN-F | AGAGTTTGATCCTGGCTCAG | 180 | M. avium | |
| MYCAV-R | ACCAGAAGACATGCGTCTTG | |||
| MYCINT-F | CCTTTAGGCGCATGTCTTTA | 850 | M. intracellulare | |
| MYCGEN-R | TGCACACAGGCCACAAGGGA | |||
| TBI-F | GAACAATCCGGAGTTGACAA | 372 | M. bovis | |
| TB1R | AGCACGCTGTCAATCATGTA | |||
| oxyR285 | oxyRF | 5′CTATGCGATCAGGCGTACTTG 3′ | 556 | M. bovis |
| oxyRR | 5′GGT GAT ATA TCA CAC CAT A 3′ | |||
| L1F | 5′CCCGCTGATGCAAGTGCC 3′ | 460 | M. bovis | |
| L2R | CCCGCACATCCCAACACC 3′ | |||
| Rv2073c | Rv2073cF | 5′TCGCCGCTGCCAGATGAGTC 3′ | 600 | M. tb |
| (RD9) | Rv2073cR | 5′TTTGGGAGCCGCCGGTGGTGATGA3′ | ||
| hsp65631 | hsp65F | 5′ACC AAC GAT GGT GTG TCC AT 3′ | 441 | MTBC |
| hsp65R | 5′CTT GTC GAA CCG CAT ACC CT 3′ |
| Diagnostic Test | Samples (N) | Positive | Percentage (95% CI) |
|---|---|---|---|
| Tuberculosis-like lesion wild boar wild macaques | 30 42 | 9 0 | 30% (16.7–47.9) 0 (0–8.4) |
| Acid fast staining wild boar wild macaques | 30 42 | 0 0 | 0 (0–11.4) 0 (0–8.4) |
| Mycobacterial culture Total samples | 93 | 0 | |
| PCR MTBC—wild boar PCR MAC—wild boar PCR MTBC— macaques | 12 12 30 | 9 11 0 | 75% (46.8–91.1) 91% (64.6–98.5) 0 (0–11.4) |
| PCR MAC—macaques | 30 | 10 | 33% (19.2–51.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekko, Y.M.; Che-Amat, A.; Ooi, P.T.; Omar, S.; Ramanoon, S.Z.; Mazlan, M.; Jesse, F.F.A.; Jasni, S.; Ariff Abdul-Razak, M.F. Mycobacterium Tuberculosis and Avium Complex Investigation among Malaysian Free-Ranging Wild Boar and Wild Macaques at Wildlife-Livestock-Human Interface. Animals 2021, 11, 3252. https://doi.org/10.3390/ani11113252
Lekko YM, Che-Amat A, Ooi PT, Omar S, Ramanoon SZ, Mazlan M, Jesse FFA, Jasni S, Ariff Abdul-Razak MF. Mycobacterium Tuberculosis and Avium Complex Investigation among Malaysian Free-Ranging Wild Boar and Wild Macaques at Wildlife-Livestock-Human Interface. Animals. 2021; 11(11):3252. https://doi.org/10.3390/ani11113252
Chicago/Turabian StyleLekko, Yusuf Madaki, Azlan Che-Amat, Peck Toung Ooi, Sharina Omar, Siti Zubaidah Ramanoon, Mazlina Mazlan, Faez Firdaus Abdullah Jesse, Sabri Jasni, and Mohd Firdaus Ariff Abdul-Razak. 2021. "Mycobacterium Tuberculosis and Avium Complex Investigation among Malaysian Free-Ranging Wild Boar and Wild Macaques at Wildlife-Livestock-Human Interface" Animals 11, no. 11: 3252. https://doi.org/10.3390/ani11113252






