Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products
Abstract
Simple Summary
Abstract
1. Introduction
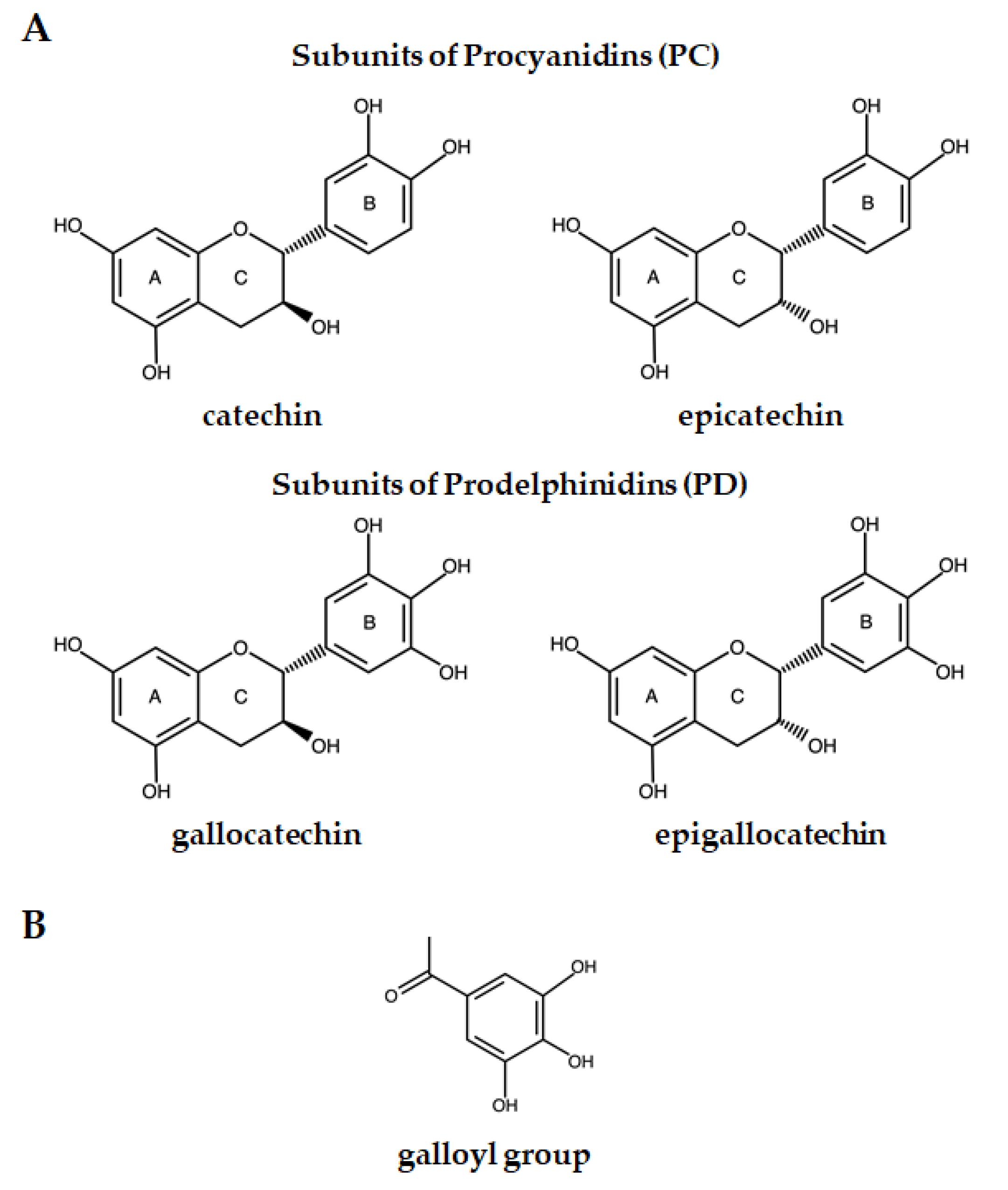
2. Condensed Tannins’ Chemical Structure
3. Condensed Tannins as Antioxidants in Ruminant Diets
3.1. Condensed Tannin-Rich Plants and Agro-Industrial By-Products
3.2. Condensed Tannin Extracts
4. Condensed Tannins’ Antioxidant Action Mechanisms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.A.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid stability in meat and meat products. Meat Sci. 1998, 49, S73–S86. [Google Scholar] [CrossRef]
- Hess, B.W.; Moss, G.E.; Rule, D.C. A decade of developments in the area of fat supplementation research with beef cattle and sheep. J. Anim. Sci. 2008, 86, E188–E204. [Google Scholar] [CrossRef]
- Bessa, R.J.B.; Alves, S.P.; Santos-Silva, J. Constraints and potentials for the nutritional modulation of the fatty acid composition of ruminant meat. Eur. J. Lipid Sci. Technol. 2015, 117, 1325–1344. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of heat stress-induced oxidative stress on the milk protein biosynthesis of dairy cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Deters, E.L.; Hansen, S.L. Invited Review: Linking road transportation with oxidative stress in cattle and other species. Appl. Anim. Sci. 2020, 36, 183–200. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Samples, S. Oxidation and antioxidants in fish and meat from farm to fork. In Food Industry; Muzzalupo, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Jacobsen, C. Some strategies for the stabilization of long chain n-3 PUFA-enriched foods: A Review. Eur. J. Lipid Sci. Technol. 2015, 117, 1853–1866. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous antioxidants: A review of their role in oxidative stress. In The Transcription Factor Nrf2; Navarro, B.C.H., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Salami, S.A.; Guinguina, A.; Agboola, J.O.; Omede, A.A.; Agbonlahor, E.M.; Tayyab, U. Review: In vivo and postmortem effects of feed antioxidants in livestock: A review of the implications on authorization of antioxidant feed additives. Animal 2016, 10, 1375–1390. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the mechanism of the health benefits of proanthocyanidins: Absorption, metabolism, and interaction with gut microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Capela e Silva, F. Tannins in ruminant nutrition-Impact on animal performance and quality of edible products. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 121–168. ISBN 978-1-63484-150-4. [Google Scholar]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed. Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Tao, W.; Li, L.; Wei, C.; Duan, J.; Chen, S.; Ye, X. Antioxidant and antiproliferative activities of proanthocyanidins from Chinese bayberry (Myrica Rubra Sieb. et Zucc.) leaves. J. Funct. Foods 2016, 27, 645–654. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (Buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “Chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, S. Applications of tannins in industry. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Girard, M.; Lehtimäki, A.; Bee, G.; Dohme-Meier, F.; Karonen, M.; Salminen, J.-P. Changes in feed proanthocyanidin profiles during silage production and digestion by lamb. Molecules 2020, 25, 5887. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop. Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Desrues, O.; Mueller-Harvey, I.; Pellikaan, W.F.; Enemark, H.L.; Thamsborg, S.M. Condensed tannins in the gastrointestinal tract of cattle after sainfoin (Onobrychis viciifolia) intake and their possible relationship with anthelmintic effects. J. Agric. Food Chem. 2017, 65, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic Inhibition of haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Tam, N.F.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Lin, Y.-M.; Li, Y.-Y.; Li, M.; Wei, S.-D.; Chai, W.-M.; Tam, N.F. Antioxidant properties of polymeric proanthocyanidins from fruit stones and pericarps of Litchi chinensis sonn. Food Res. Int. 2011, 44, 613–620. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Guyot, S.; Ducrot, P.-H. NMR, ESI/MS, and MALDI-TOF/MS analysis of pear juice polymeric proanthocyanidins with potent free radical scavenging activity. J. Agric. Food Chem. 2006, 54, 6969–6977. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Natalello, A.; Luciano, G.; Fondevila, M.; Priolo, A.; Toral, P.G. Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed. Sci. Technol. 2020, 269, 114623. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop. Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed. Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, Y.; Du, M.; Liu, W.; Ren, Y.; Zhang, C.; Zhang, J. The effect of dietary grape pomace supplementation on epididymal sperm quality and testicular antioxidant ability in ram lambs. Theriogenology 2017, 97, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Guerra-Rivas, C.; Martínez, B.; Rubio, B.; Manso, T. Effects of grape pomace supplementation on the diet of lactating ewes as compared to vitamin E on the meat shelf life of suckling lambs. Meat Sci. 2021, 184, 108666. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Moelich, E.; Gouws, P.; Muchenje, V.; Nolte, J.V.E.; Dugan, M.E.R.; Mapiye, C. Effects of feeding increasing levels of grape (Vitis vinifera Cv. Pinotage) pomace on lamb shelf-life and eating quality. Meat Sci. 2019, 157, 107887. [Google Scholar] [CrossRef]
- Tayengwa, T.; Chikwanha, O.C.; Gouws, P.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Dietary citrus pulp and grape pomace as potential natural preservatives for extending beef shelf life. Meat Sci. 2020, 162, 108029. [Google Scholar] [CrossRef]
- Santos, N.W.; Santos, G.T.D.; Silva-Kazama, D.C.; Grande, P.A.; Pintro, P.M.; de Marchi, F.E.; Jobim, C.C.; Petit, H.V. Production, composition and antioxidants in milk of dairy cows fed diets containing soybean oil and grape residue silage. Livest. Sci. 2014, 159, 37–45. [Google Scholar] [CrossRef]
- Gravador, R.S.; Luciano, G.; Jongberg, S.; Bognanno, M.; Scerra, M.; Andersen, M.L.; Lund, M.N.; Priolo, A. Fatty acids and oxidative stability of meat from lambs fed carob-containing diets. Food Chem. 2015, 182, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Min, B.R.; Lemma, B.B. Quality characteristics of goat meat as influenced by condensed tannins-containing pine bark. Small Rumin. Res. 2017, 146, 28–32. [Google Scholar] [CrossRef]
- Kafle, D.; Lee, J.H.; Min, B.R.; Kouakou, B. Carcass and meat quality of goats supplemented with tannin-rich peanut skin. J. Agric. Food Res. 2021, 5, 100159. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.M.; Alves, S.P.; Dentinho, M.T.P.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- Francisco, A.; Dentinho, M.T.; Alves, S.P.; Portugal, P.V.; Fernandes, F.; Sengo, S.; Jerónimo, E.; Oliveira, M.A.; Costa, P.; Sequeira, A.; et al. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Sci. 2015, 100, 275–282. [Google Scholar] [CrossRef]
- Jerónimo, E.; Soldado, D.; Sengo, S.; Francisco, A.; Fernandes, F.; Portugal, A.P.V.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J.B. Increasing the α-tocopherol content and lipid oxidative stability of meat through dietary Cistus ladanifer L. in lamb fed increasing levels of polyunsaturated fatty acid rich vegetable oils. Meat Sci. 2020, 164, 108092. [Google Scholar] [CrossRef]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Dentinho, M.T.; Jerónimo, E.; Sengo, S.; Almeida, J.; Bressan, M.C.; Pires, V.M.R.; Alfaia, C.M.; et al. Effects of dietary inclusion of citrus pulp and rockrose soft stems and leaves on lamb meat quality and fatty acid composition. Animal 2018, 12, 872–881. [Google Scholar] [CrossRef]
- García, E.M.; López, A.; Zimerman, M.; Hernández, O.; Arroquy, J.I.; Nazareno, M.A. Enhanced oxidative stability of meat by including tannin-rich leaves of woody plants in goat diet. Asian-Australas J. Anim. Sci. 2019, 32, 1439–1447. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Morales-Romero, A.M.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Goats’ Feeding Supplementation with Acacia Farnesiana Pods and Their Relationship with Milk Composition: Fatty Acids, Polyphenols, and Antioxidant Activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef]
- Dey, A.; De, P.S. Influence of Condensed Tannins from Ficus Bengalensis Leaves on Feed Utilization, Milk Production and Antioxidant Status of Crossbred Cows. Asian-Australas. J. Anim. Sci. 2014, 27, 342–348. [Google Scholar] [CrossRef]
- Dey, A.; Dutta, N.; Pattanaik, A.K.; Sharma, K. Antioxidant status, metabolic profile and immune response of lambs supplemented with tannin tich Ficus infectoria leaf meal. Jpn. J. Vet. Res. 2015, 63, 15–24. [Google Scholar]
- Pathak, A.K.P.; Dutta, N.; Pattanaik, A.K.; Sharma, K.; Banerjee, P.S.; Goswami, T.K. The effect of condensed tannin supplementation through Ficus infectoria and Psidium guajava leaf meal mixture on erythrocytic antioxidant status, immune response and gastrointestinal nematodes in lambs (Ovis aries). Vet. Arh. 2017, 87, 139–156. [Google Scholar]
- Chaurasiya, A.; Tamboli, P.; Chaurasiya, P.; Nehra, A.; Sahoo, B.; Kuriyal, A.; Sankar, M. Effect of feeding tannin rich oak (Quercus leucotrichophora) leaves on immunological parameters, antioxidant status and microbial nitrogen supply of parasitic infected goats in Kumaon hills. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 455–465. [Google Scholar] [CrossRef]
- Luciano, G.; Natalello, A.; Mattioli, S.; Pauselli, M.; Sebastiani, B.; Niderkorn, V.; Copani, G.; Benhissi, H.; Amanpour, A.; Valenti, B. Feeding lambs with silage mixtures of grass, sainfoin and red clover improves meat oxidative stability under high oxidative challenge. Meat Sci. 2019, 156, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Bertolín, J.R.; Joy, M. Meat quality of light lambs is more affected by the dam’s feeding system during lactation than by the inclusion of quebracho in the fattening concentrate. J. Anim. Sci. 2017, 95, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Larraín, R.E.; Schaefer, D.M.; Richards, M.P.; Reed, J.D. Finishing steers with diets based on corn, high-tannin sorghum or a mix of both: Color and lipid oxidation in beef. Meat Sci. 2008, 79, 656–665. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Fang, Y.; Wang, Y.Q.; Sun, H.X.; Zhou, D.W. Effects of substituting finely ground sorghum for finely ground corn on feed digestion and meat quality in lambs infected with Haemonchus contortus. Anim. Feed. Sci. Technol. 2016, 211, 31–40. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed. Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob pulp: A nutritional and functional by-product worldwide spread in the formulation of different food products and beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Jerónimo, E.; Dentinho, M.T.; Guerreiro, O.; Francisco, A.; Soldado, D.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J.B. Cistus ladanifer L. in ruminant diets–A sustainable approach to improve the feed nutritional value and the quality of edible products. In Advances in Animal Health, Medicine and Production; Springer International Publishing AG: Cham, Switzerland, 2020; pp. 128–160. ISBN 978-3-030-61980-0. [Google Scholar]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Skogsmyr, I.; Fagerström, T. The cost of anti-herbivory defence: An evaluation of some ecological and physiological factors. Oikos 1992, 64, 451–457. [Google Scholar] [CrossRef]
- Guerreiro, O.; Dentinho, M.T.P.; Moreira, O.C.; Guerra, A.R.; Ramos, P.A.B.; Bessa, R.J.B.; Duarte, M.F.; Jerónimo, E. Potential of Cistus ladanifer L. (rockrose) in small ruminant diets–Effect of season and plant age on chemical composition, in vitro digestibility and antioxidant activity. Grass Forage Sci. 2016, 71, 437–447. [Google Scholar] [CrossRef]
- Lobón, S.; Sanz, A.; Blanco, M.; Ripoll, G.; Joy, M. The type of forage and condensed tannins in dams’ diet: Influence on meat shelf life of their suckling lambs. Small Rumin. Res. 2017, 154, 115–122. [Google Scholar] [CrossRef]
- Brogna, D.M.R.; Tansawat, R.; Cornforth, D.; Ward, R.; Bella, M.; Luciano, G.; Priolo, A.; Villalba, J. The quality of meat from sheep treated with tannin- and saponin-based remedies as a natural strategy for parasite control. Meat Sci. 2014, 96, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Biondi, L.; Lanza, M.; Priolo, A. Dietary tannins improve lamb meat colour stability. Meat Sci. 2009, 81, 120–125. [Google Scholar] [CrossRef]
- Luciano, G.; Vasta, V.; Monahan, F.J.; López-Andrés, P.; Biondi, L.; Lanza, M.; Priolo, A. Antioxidant status, colour stability and myoglobin resistance to oxidation of Longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem. 2011, 124, 1036–1042. [Google Scholar] [CrossRef]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Minieri, S.; Roscini, V.; Mannelli, F.; Rapaccini, S.; Lupi, P.; Conte, G.; Serra, A.; Cappucci, A.; et al. Chestnut or quebracho tannins in the diet of grazing ewes supplemented with soybean oil: Effects on animal performances, blood parameters and fatty acid composition of plasma and milk lipids. Small Rumin. Res. 2017, 153, 23–30. [Google Scholar] [CrossRef]
- Mu, C.; Yang, W.; Wang, P.; Zhao, J.; Hao, X.; Zhang, J. Effects of high-concentrate diet supplemented with grape seed proanthocyanidins on growth performance, liver function, meat quality, and antioxidant activity in finishing lambs. Anim. Feed. Sci. Technol. 2020, 266, 114518. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Leparmarai, P.T.; Sinz, S.; Kunz, C.; Liesegang, A.; Ortmann, S.; Kreuzer, M.; Marquardt, S. Transfer of total phenols from a grapeseed-supplemented diet to dairy sheep and goat milk, and effects on performance and milk quality. J. Anim. Sci. 2019, 97, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Muíño, I.; Apeleo, E.; de la Fuente, J.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Díaz, M.T.; Cañeque, V.; Lauzurica, S. Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Sci. 2014, 98, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Biondi, L.; Randazzo, C.L.; Russo, N.; Pino, A.; Natalello, A.; Van Hoorde, K.; Caggia, C. Dietary supplementation of tannin-extracts to lambs: Effects on meat fatty acids aomposition and stability and on microbial characteristics. Foods 2019, 8, 469. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: Comparison between mimosa, chestnut and tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef]
- Gesteira, S.M.; Oliveira, R.L.; Trajano, J.; Ribeiro, C.; de Sousa Costa, E.I.; Ribeiro, R.D.X.; Pereira, E.S.; Bezerra, L.R. Fatty acid profile, physicochemical composition and sensorial attributes of salted and sun-dried meat from young Nellore bulls supplemented with condensed tannins. PLoS ONE 2019, 14, e0219581. [Google Scholar]
- Staerfl, S.M.; Soliva, C.R.; Leiber, F.; Kreuzer, M. Fatty acid profile and oxidative stability of the perirenal fat of bulls fattened on grass silage and maize silage supplemented with tannins, garlic, maca and lupines. Meat Sci. 2011, 89, 98–104. [Google Scholar] [CrossRef]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Werle, C.H.; Almeida, A.R.E.; Schneider, C.R.; Grunevald, D.G.; Faciola, A.P. Black Wattle (Acacia mearnsii) condensed tannins as feed additives to lactating dairy cows. Animals 2020, 10, 662. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Torres, J.L.; Medina, I. Antioxidant mechanism of grape procyanidins in muscle tissues: Redox interactions with endogenous ascorbic acid and α-tocopherol. Food Chem. 2012, 134, 1767–1774. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free. Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef]
- Abia, R.; Fry, S.C. Degradation and metabolism of 14C-labelled proanthocyanidins from carob (Ceratonia siliqua) pods in the gastrointestinal tract of the rat. J. Sci. Food Agric. 2001, 81, 1156–1165. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Bruni, R.; Del Rio, D. Bioactivation of high-molecular-weight polyphenols by the gut microbiome. In Diet-Microbe Interactions in the Gut; Tuohy, K., Del Rio, D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 73–101. ISBN 978-0-12-407825-3. [Google Scholar]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Berktaş, I.; Özçelik, B. Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. LWT-Food Sci. Technol. 2016, 72, 559–565. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Wang, X.; Dai, T.; McClements, D.J.; Liu, J. Impact of in vitro simulated digestion on the potential health benefits of proanthocyanidins from Choerospondias axillaris peels. Food Res. Int. 2015, 78, 378–387. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The gastrointestinal tract as a key target organ for the health-promoting effects of dietary proanthocyanidins. Front. Nutr. 2017, 3, 57. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Jaggers, G.K.; Oteiza, P.I.; Waterhouse, A.L. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J. Agric. Food Chem. 2013, 61, 121–127. [Google Scholar] [CrossRef]
- Jimenez-Ramsey, L.M.; Rogler, J.C.; Housley, T.L.; Butler, L.G.; Elkin, R.G. Absorption and distribution of 14C-labeled condensed tannins and related sorghum phenolics in chickens. J. Agric. Food Chem. 1994, 42, 963–967. [Google Scholar] [CrossRef]
- Quijada, J.; Drake, C.; Gaudin, E.; El-Korso, R.; Hoste, H.; Mueller-Harvey, I. Condensed tannin changes along the digestive tract in lambs fed with sainfoin pellets or hazelnut skins. J. Agric. Food Chem. 2018, 66, 2136–2142. [Google Scholar] [CrossRef]
- Perez-Maldonado, R.A.; Norton, B.W. Digestion of 14C-labelled condensed tannins from Desmodium intortum in sheep and goats. Br. J. Nutr. 1996, 76, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Waghorn, G.C.; Woolley, D.J.; Mcnabb, W.C.; Barry, T.N. Assay and digestion of 14C-labelled condensed tannins in the gastrointestinal tract of sheep. Br. J. Nutr. 1994, 72, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Becker, K.; Abel, H.; Szegletti, C. Degradation of condensed tannins by rumen microbes exposed to quebracho tannins (QT) in rumen simulation technique (RUSITEC) and effects of QT on fermentative processes in the RUSITEC. J. Sci. Food Agric. 1995, 69, 495–500. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Becker, K. In vitro effects of and interactions between tannins and saponins and fate of tannins in the rumen. J. Sci. Food Agric. 1995, 69, 481–493. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Jones, W.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef]
- Kerem, Z.; Chetrit, D.; Shoseyov, O.; Regev-Shoshani, G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. J. Agric. Food Chem. 2006, 54, 10288–10293. [Google Scholar] [CrossRef]
- Yamamoto, M.; Miyamoto, S.; Moon, J.-H.; Murota, K.; Hara, Y.; Terao, J. Effect of dietary green tea catechin preparation on oxidative stress parameters in large intestinal mucosa of rats. Biosci. Biotechnol. Biochem. 2006, 70, 286–289. [Google Scholar] [CrossRef][Green Version]
- Gladine, C.; Morand, C.; Rock, E.; Gruffat, D.; Bauchart, D.; Durand, D. The antioxidative effect of plant extracts rich in polyphenols differs between liver and muscle tissues in rats fed n-3 PUFA rich diets. Anim. Feed. Sci. Technol. 2007, 139, 257–272. [Google Scholar] [CrossRef]
- Frank, J. Beyond Vitamin E supplementation: An alternative strategy to improve vitamin E status. J. Plant Physiol. 2005, 162, 834–843. [Google Scholar] [CrossRef]
- Frank, J.; Eliasson, C.; Leroy-Nivard, D.; Budek, A.; Lundh, T.; Vessby, B.; Aman, P.; Kamal-Eldin, A. Dietary secoisolariciresinol diglucoside and its oligomers with 3-hydroxy-3-methyl glutaric acid decrease vitamin E levels in rats. Br. J. Nutr. 2004, 92, 169–176. [Google Scholar] [CrossRef]
- Facino, R.M.; Carini, M.; Aldini, G.; Calloni, M.T.; Bombardelli, E.; Morazzoni, P. Sparing effect of procyanidins from Vitis vinifera on vitamin E: In vitro studies. Planta Med. 1998, 64, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Sgorlon, S.; Stradaioli, G.; Zanin, D.; Stefanon, B. Biochemical and molecular responses to antioxidant supplementation in sheep. Small Rumin. Res. 2006, 64, 143–151. [Google Scholar] [CrossRef]
- Puiggros, F.; Llópiz, N.; Ardévol, A.; Bladé, C.; Arola, L.; Salvadó, M.J. Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J. Agric. Food Chem. 2005, 53, 6080–6086. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free. Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by olive oil and wine polyphenols and neuroprotection. Antioxidants (Basel) 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
| Animal | Source | CT Level in Diets (g/kg DM) | Basal Diet | Sample | Effect | References | |
|---|---|---|---|---|---|---|---|
| Plant | Level in Diet (g/kg DM) | ||||||
| Lamb | Grape pomace | 50 and 100 1 | - | Corn, soybean meal, wheat bran, oil cake of flax seed, naked oat straw and potato rattan | muscle and testes | ↓ROS and MDA levels; ↑Total antioxidant capacity; ↑ activity of GPx4 and SOD in muscle and testes; ↑activity of CAT in testes | [49,50] |
| Lamb | Grape pomace | 51.7 and 103 | 2.21 and 4.41 | Forage:concentrate (40:60) supplemented with 2.7% of linseed oil supplied to dams | muscle | ↓MDA levels and MMb % in suckling lambs | [51] |
| Lamb | Grape pomace | 50 | 2.23 4 | Barley straw and concentrate ad libitum; grape pomace included in concentrate | muscle | =MDA levels and MMb % | [52] |
| Lamb | Grape pomace | 50, 100, 150 and 200 2 | 2.7; 3.5; 4.9 and 6.0 5 0.8; 1.2; 1.8 and 2.6 6 | Concentrate: lucerne meal (80:20) | muscle | ↑ FRAP values from day 1 to day 3 in the diets with 150 and 200 g/kg grape pomace; ↓ MDA levels from day 5 onward in the diets with 200 g/kg grape pomace; ↓ carbonyl content at days 5 and 7 of storage | [53] |
| Steers | Grape pomace | 150 2 | 50.7 5 24.1 7 | Wheat straw: concentrate: lucerne (9:80:10) | muscle | ↑ FRAP values; ↓ MDA and carbonyl levels | [54] |
| Cow | Grape pomace silage | 50, 75 and 100 | - | Silage: concentrate (60:40); grape pomace silage replace partially the corn silage in forage | milk | ↑ reducing power; =production of conjugated diene hydroperoxides | [55] |
| Lamb | Carob pulp | 240 and 350 2 | 3.4 and 4.5 8 | Concentrate: dehydrated lucerne (80:20); carob pulp replaces partially barley of the concentrate | muscle | =MDA levels, free thiol and carbonyl levels and MMb % | [56] |
| Goat | Pine bark | - | 130 4 | Rye grass pasture supplemented with mixture of pine bark with molasses (6% w/w) and alfalfa (5% w/w) containing 130 g/kg DM of CT | muscle | =MDA levels | [57] |
| Goat | Peanut skin | 250; 500 and 7502 | 39; 78 and 117 2 | Concentrate (cracked corn, soybean meal, soy hull and molasses) and peanut skin | muscle | =MDA levels in diets with 500 and 750 g/kg peanut skin | [58] |
| Lamb | Cistus ladanifer L. | 250 | 20.9 9 | Dehydrated lucerne supplemented with 0 or 6% of a blend of sunflower and linseed oils (1:2, v/v) | muscle | ↓ MDA levels after lipid oxidation induction | [59] |
| Lamb | Cistus ladanifer L. | 50, 100 and 200 | 2.7; 6.9 and 15.6 9 | Concentrate: dehydrated lucerne (50:50); supplemented with 0, 4 and 8% of a blend of soybean and linseed oils (1:2, v/v); Cistus ladanifer replaces partially the forage | muscle | ↓ MDA levels; ↓ MDA levels after lipid oxidation induction; ↑ α-tocopherol content; =total phenolic content, FRAP and TEAC values | [60,61] |
| Lamb | Cistus ladanifer L. | 150 | 3.5–5.6 9 | Concentrate: dehydrated lucerne (50:50); supplemented with 5–6% of soybean oil; Cistus ladanifer replaces partially the forage | muscle | =MDA levels | [62] |
| Goat | Larrean divaricata | 125 2 | 1.74 | Alfalfa hay, corn and soybean meal | muscle | =DPPH values; =total phenolic content; ↓ MDA levels in meat stored at 4 °C over 6 days, at 26 °C for 6 h and at −18 °C for 30 days | [63] |
| Acacia aroma | 125 2 | 5.63 | |||||
| Goat | Acacia farnesiana | 100, 200 and 300 | - | Lucerne hay and concentrate | milk | ↑ total phenolic content; =catechin concentration; ↑ ORAC and FRAP values | [64] |
| Cow | Ficus infectoria | 119 2 | 154 | Rice straw, maize green and concentrate (maize, mustard cake and rice bran); Ficus infectoria leaves included in concentrate replacing rice bran | erythrocytes | ↑ SOD and CAT activity; ↑ GSH levels; ↓ MDA levels; ↑ total thiol levels | [65] |
| Lamb | Ficus infectoria | 106, 159 and 212 2 | 10; 15 and 20 4 | Wheat straw, green fodder, and concentrate; Ficus infectoria leaves replacing partially the wheat bran of the concentrate | erythrocytes | ↑ SOD and CAT activity; ↑GSH levels; ↓ MDA levels; ↑ total thiol and protein thiol levels | [66] |
| Lamb | Ficus infectoria and Psidium guajava (70:30) | 96; 144 and 192 3 | 10; 15 and 20 4 | Wheat straw, oat hay and concentrate (maize, wheat bran, deoiled soybean meal); leaf meal mixture replace the concentrate | erythrocytes | ↑ SOD, GPx and CAT activity; ↑ GSH and GST levels; ↑ total thiol and protein thiol levels; =MDA levels | [67] |
| Goat | Oak (Quercus leucotrichophora) | - | 33.5 4 | Concentrate: oak leaves as roughage | erythrocytes | ↑ SOD and CAT activity; ↑ GSH levels | [68] |
| Lamb | Sainfoin (Onobrychis viccifolia) | - | 5.59–6.71 4,8 | Silage mixture of timothy and sainfoin (50:50) ad libitum, straw (60–80g/d) and barley (229 g/d) | muscle | =MDA levels in raw meat; ↓ MDA levels under pro-oxidant conditions (cooking and incubation with pro-oxidant catalysts) | [69] |
| Lamb | Sainfoin (Onobrychis viccifolia) | - | 21.9 10 | Sainfoin pasture supplied to dams | muscle | ↓ MDA levels suckling lambs | [70] |
| Steers | High-tannin sorghum | 383–765 | 17.3–34.6 11 | Silage:concentrate (10:90); high-tannin sorghum replace partially the corn of the concentrate | muscle | ↓ MDA levels in vacuum-packaged beef; ↑ MDA levels in displayed beef over 6, 10 and 15 days; =SOD, CAT and GPx activity | [71] |
| Lamb | Sorghum grain | 100; 200 and 400 | 8.2; 16.4 and 24.5 11 | Forage (Aneurolepidium Chinense hay and alfalfa hay):concentrate (corn grain and soybean meal) (42:58). Stepwise replacement of corn grain by sorghum grain | muscle | =MDA levels; ↑ tannin levels | [72] |
| Animal | Source | CT Level (g/kg DM) | Basal Diet | Sample | Effect | References | ||
|---|---|---|---|---|---|---|---|---|
| Plant | CT Levels of Extract (g/kg DM) | CT Extract Levels (g/kg DM) | ||||||
| Lamb | Quebracho | - | 50 1 | 3.7 6 | Concentrate (corn, soybean meal, wheat and barley) + straw ad libitum. Quebracho extract included in concentrate | muscle | =MDA and MMb levels | [70] |
| Lamb | Quebracho | 750 1 | 100 1 | 75 1,5 | Dietary treatments supplied to dams. Pasture vs. forage diets supplemented with concentrate. Quebracho extract included in concentrate | muscle | ↓ MDA levels; ↑ α-tocopherol levels in muscle of suckling lambs; =MMb levels | [80] |
| Lamb | Quebracho (Schinopsis lorentzii) | - | 89 | 40.4 2 | High-concentrate diet (barley and soyabean meal) and lucerne hay; quebracho extract included in concentrate and forage mixture | muscle | =MDA levels; ↑ total phenols levels; ↑ FRAP and TEAC values; ↓ MMb % | [82,83] |
| Lamb | Quebracho (Schinopsis lorentzii) | - | 95.7 | 64 | High-concentrate diet (barley and soyabean meal) and lucerne hay; quebracho extract included in concentrate and forage mixture | liver | =total phenolic content; ↑ FRAP values in raw samples; =total phenolic content and FRAP values in SPE samples | [84] |
| plasma | ↑ total phenolic content and FRAP values in raw samples; =total phenolic content and FRAP values in SPE samples | |||||||
| Sheep | Quebracho (Schinopsis lorentzii) | 456 2 | 52.8 5 | 16 g/kg DM intake | 250 g Chopped grass hay + 800 g concentrate/day | plasma | =MDA levels | [85] |
| Sheep | Quebracho (Aspidosperma quebracho) | - | 80 1 | - | Dried beet pulp supplemented with 2% of vegetable oil | muscle | =MDA levels | [81] |
| Lamb | Grape seed (Vinis vitifera) | 950 | 25 | 14.1 7 | Dehydrated lucerne supplemented with 0 or 6% of a mixture of sunflower and linseed oils (1:2, v/v) | muscle | ↓ MDA levels after lipid oxidation induction | [59] |
| Sheep | Grape peel and seed (Vinis vitifera) | >800 | 10% DM intake | - | Concentrate (barley, beet pulp, soybean meal, molasses): meadow hay (30:70) | plasma | ↑ TEAC values; ↑ the length of the lag phase of conjugated dienes generation; presence of five different phenolic compounds, including epicatechin | [87] |
| Lamb | Grape seed | - | 10, 20 and 40 mg/kg BW/day | - | Total mixed feed with concentrate (corn, soybean meal, cottonseed meal, wheat bran): forage (corn and millet straw) (70:30) | muscle | ↑ total antioxidant capacity, ↑ activity of CAT, SOD and GPx4; ↓ MDA levels | [86] |
| Lamb | Grape seed | 413 | 50 mg extract/kg DM 5 | - | Barley straw and concentrate (barley, soya and molasses) ad libitum, grape seed extract included in concentrate | muscle | =MDA levels and MMb %; | [52] |
| Sheep Goat | Grape seed | - | 74 5 | 7.3–7.5 4 | Forage:concentrate:dried sugar beet pulp (51:46:3), grape seed included in concentrate | milk plasma | =FRAP values in plasma; ↑ total phenol concentration in plasma and milk | [88] |
| Lamb | Red wine extract | - | 900 mg extract/kg feed 5 | - | Barley + concentrate (corn meal, barley, wheat, soybean meal, sunflower meal) supplemented with extruded linseed and deodorized fish oil; red wine extract included in concentrate | muscle | =MDA and protein carbonyl levels; =total phenols content | [89] |
| Lamb | Mimosa (Acacia mearnsii) | - | 40 1 | 22.3 2 | Concentrate (barley, wheat bran, soybean meal, molasses): dehydrated lucerne (85:15) | muscle | =MDA levels; ↓ MMb % | [90] |
| Lamb | Mimosa (Acacia mearnsii) | 881 3 | 40 1 | 22.3 2 | Concentrate (barley, wheat bran, soybean meal, molasses): dehydrated lucerne (85:15) | muscle | =MDA levels in raw and cooked meat and in meat homogenates with Fe3+/Asc; did not after color stability | [91] |
| Bull | Mimosa (Acacia mearnsii) | 720 3 | 10, 30 and 50 | - | Concentrate (corn, soybean meal, soybean oil (4.3%)): Tifton-85 hay (60:40) | muscle | =MDA levels in salted and sun-dried meat | [92] |
| Bull | Mimosa (Acacia mearnsii) | 700 | 141 1,5 | - | Maize silage:concentrate; mimosa extract included in concentrate | perirenal fat | =oxidative stability evaluated by rancimat test | [93] |
| Cow | Mimosa (Acacia mearnsii) | 805 1 | 6.1; 12.2; 18.4 and 24.6 | 5; 10; 15 and 20 | Concentrate: forage (80:20) | milk | =MDA levels and reducing power; ↑ diene conjugates concentration | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. https://doi.org/10.3390/ani11113243
Soldado D, Bessa RJB, Jerónimo E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals. 2021; 11(11):3243. https://doi.org/10.3390/ani11113243
Chicago/Turabian StyleSoldado, David, Rui J. B. Bessa, and Eliana Jerónimo. 2021. "Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products" Animals 11, no. 11: 3243. https://doi.org/10.3390/ani11113243
APA StyleSoldado, D., Bessa, R. J. B., & Jerónimo, E. (2021). Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals, 11(11), 3243. https://doi.org/10.3390/ani11113243