Astragalus membranaceus Alters Rumen Bacteria to Enhance Fiber Digestion, Improves Antioxidant Capacity and Immunity Indices of Small Intestinal Mucosa, and Enhances Liver Metabolites for Energy Synthesis in Tibetan Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Preparation of Astragalus Membranaceus
2.2. Experimental Design
2.3. Data Collection and Sampling
2.4. Rumen Microbial DNA Extraction, Sequencing and Analysis
2.5. Meat and Intestinal Mucosa Analysis
2.6. Liver Metabolome Analysis
2.7. Statistical Analysis
3. Results
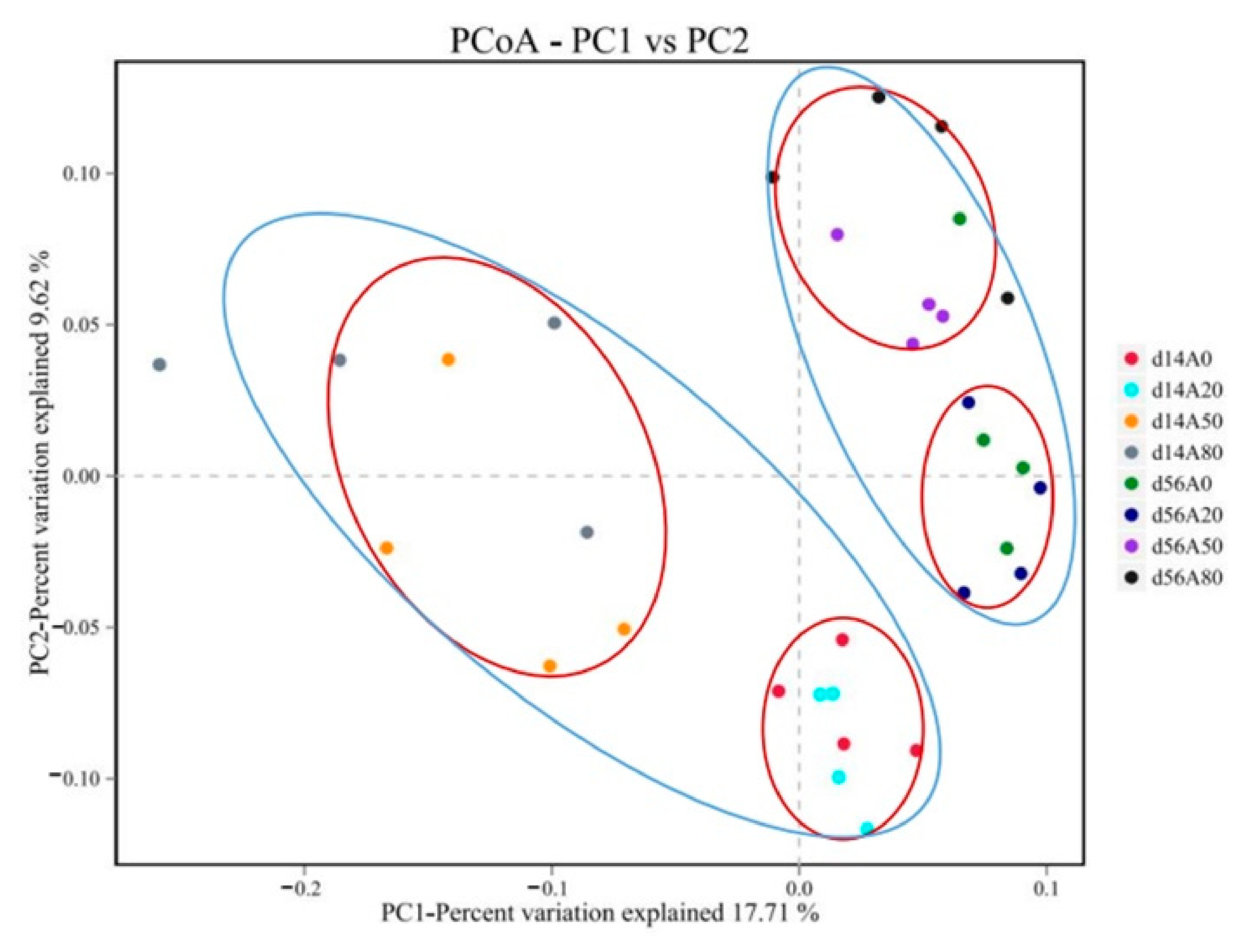
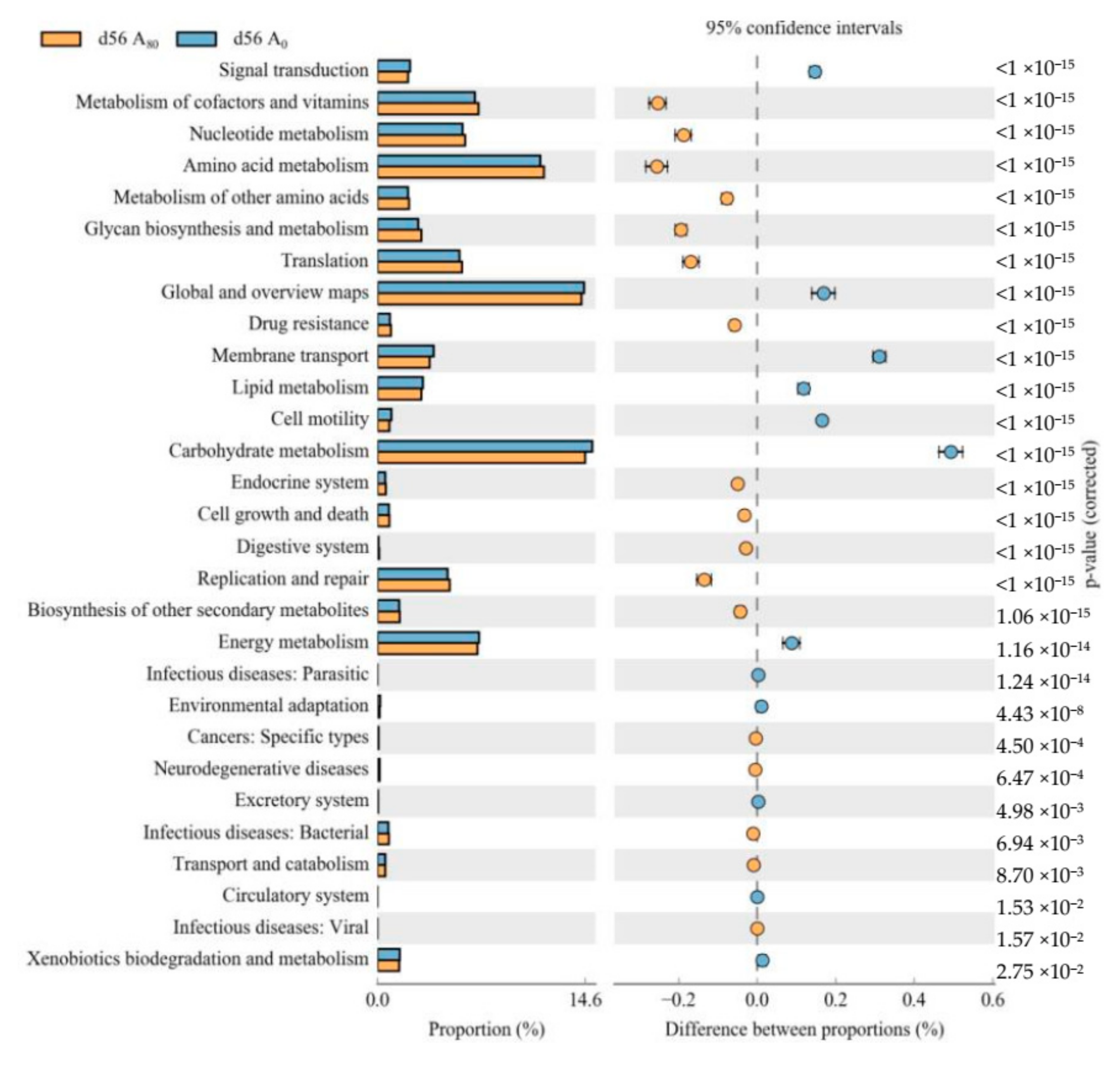
3.1. Ruminal Bacteria
3.2. Antioxidant Capacities and Immunity Indices of Meat Tissue and Small Intestinal Mucosa
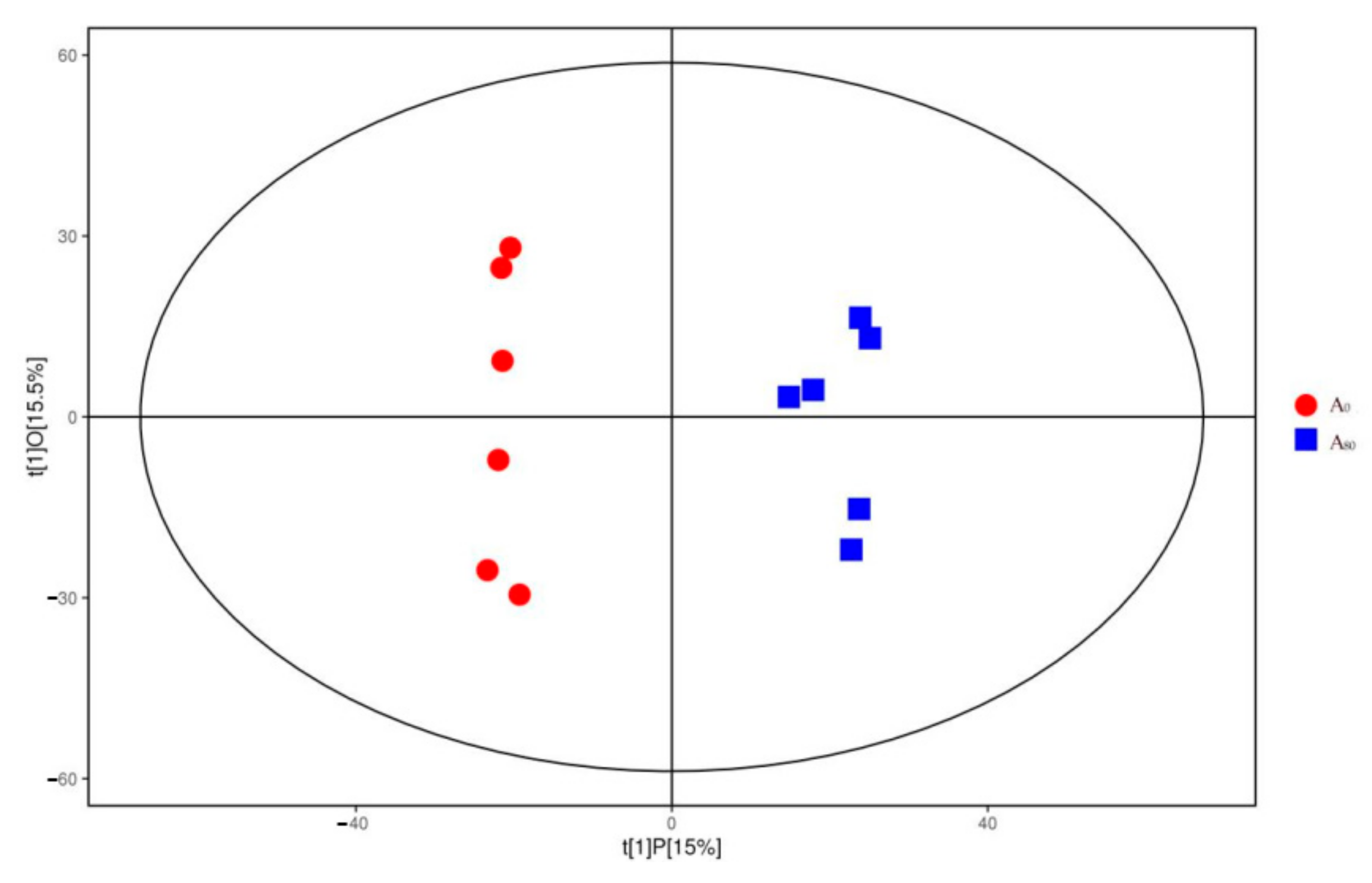
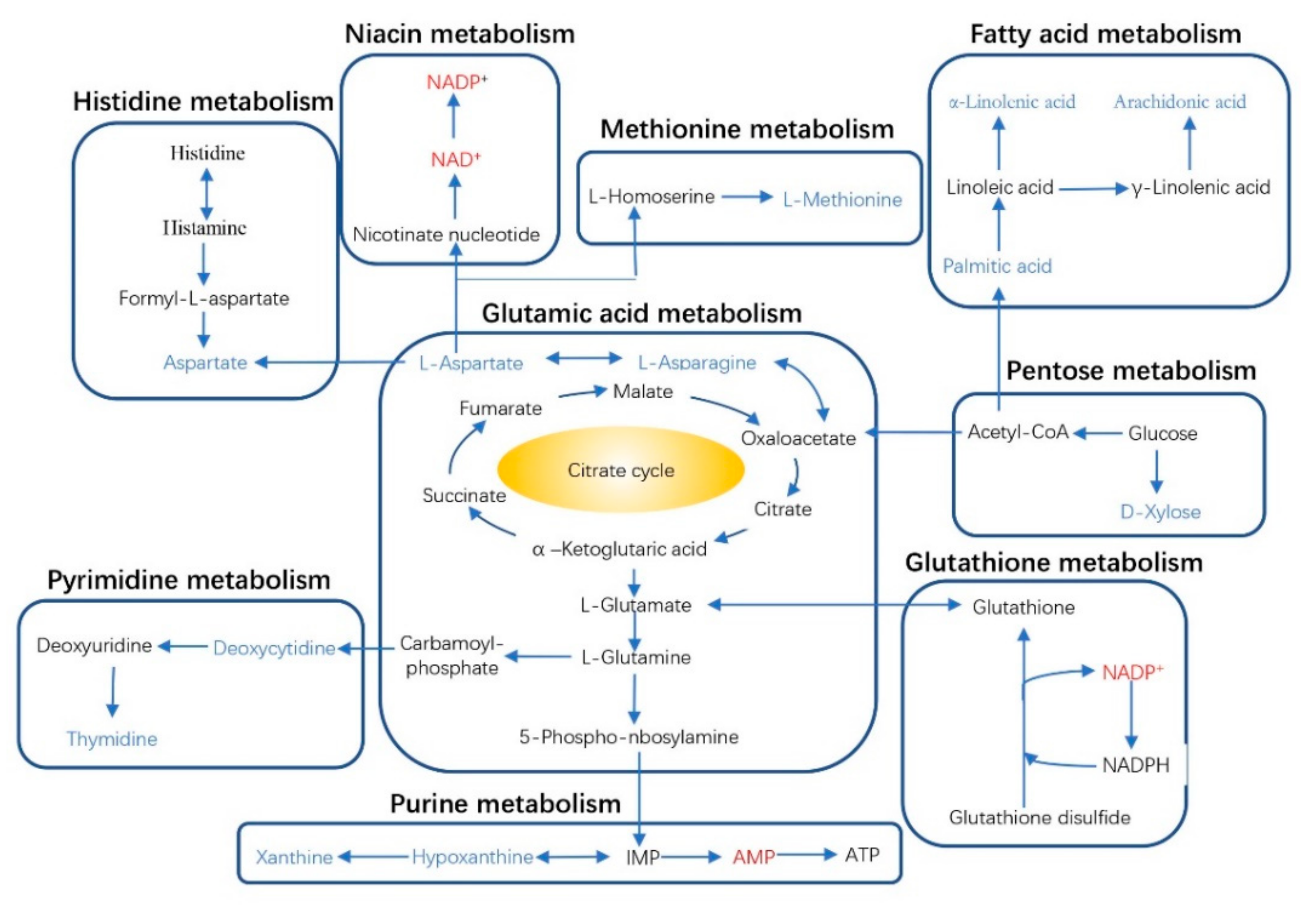
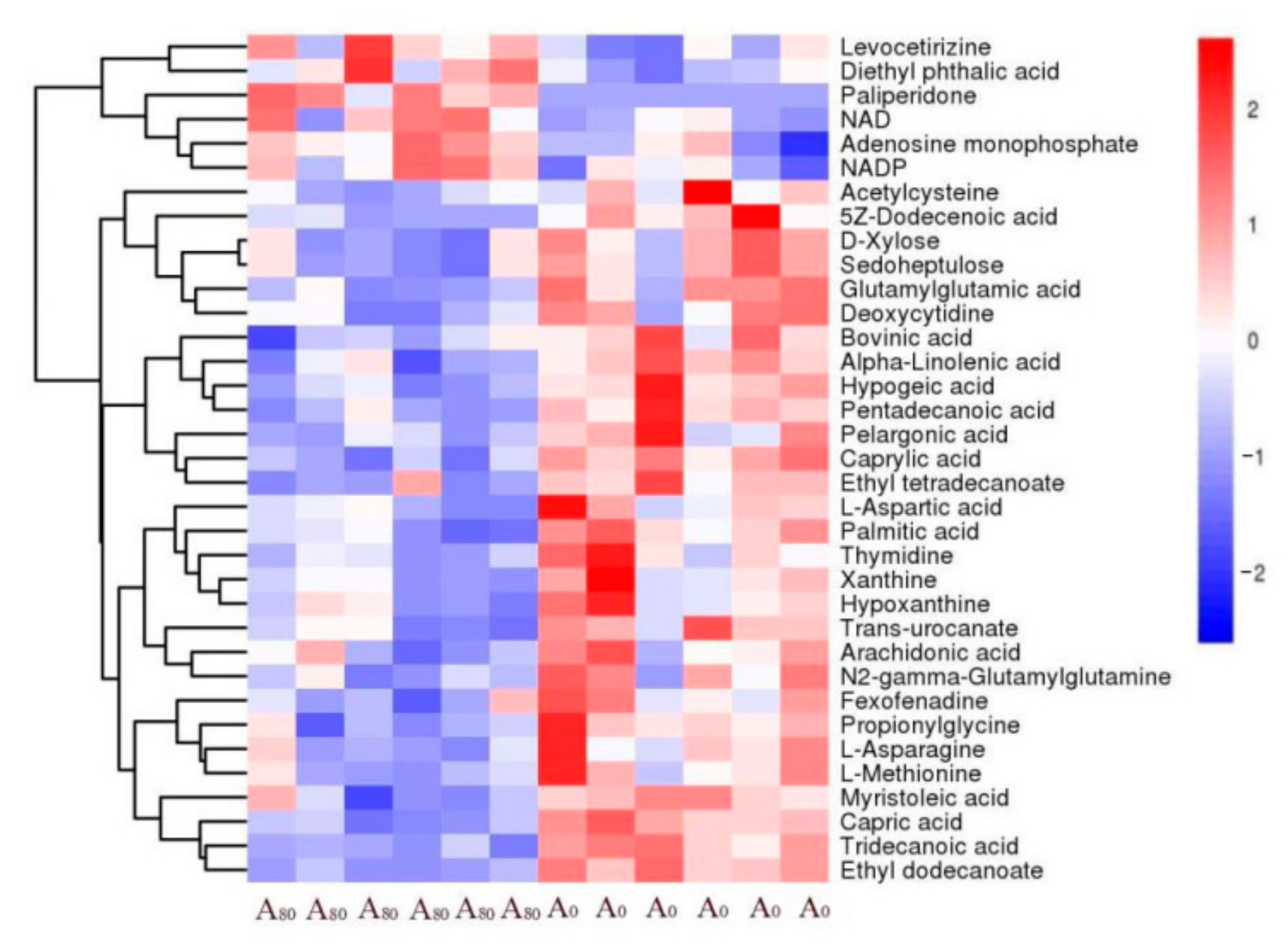
3.3. Liver Metabolome Responses
4. Discussion
4.1. Ruminal Bacteria
4.2. Antioxidant Capacities of Meat Tissue and Small Intestinal Mucosa and Secretory Immunoglobulin A (sIgA) of Small Intestinal Mucosa
4.3. Liver Metabolome Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Lehnert, L.W.; Holzapfel, M.; Schultz, R.; Heberling, G.; Görzen, E.; Meyer, H.; Seeber, E.; Pinkert, S.; Ritz, M.; et al. Multiple indicators yield diverging results on grazing degradation and climate controls across Tibetan pastures. Ecol. Indic. 2018, 93, 1199–1208. [Google Scholar] [CrossRef]
- Jing, X.P.; Wang, W.J.; Degen, A.A.; Guo, Y.M.; Kang, J.P.; Liu, P.P.; Ding, L.M.; Shang, Z.H.; Fievez, V.; Zhou, J.W.; et al. Tibetan sheep have a high capacity to absorb and to regulate metabolism of SCFA in the rumen epithelium to adapt to low energy intake. Br. J. Nutr. 2020, 123, 721–736. [Google Scholar] [CrossRef]
- Ma, Y.H.; Liu, C.Y.; Qu, D.; Chen, Y.; Huang, M.M.; Liu, Y.P. Antibacterial evaluation of sliver nanoparticles synthesized by polysaccharides from Astragalus membranaceus roots. Biomed. Pharmacother. 2017, 89, 351–357. [Google Scholar] [CrossRef]
- Qiu, C.J.; Cheng, Y.X. Effect of Astragalus membranaceus polysaccharide on the serum cytokine levels and spermatogenesis of mice. Int. J. Biol. Macromol. 2019, 140, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Hua, J.; Luan, Z.H.; Xue, P.Y.; Zhou, S.Q.; Wang, X.M.; Qin, N. Effects of the stems and leaves of Astragalus membranaceus on growth performance, immunological parameters, antioxidant status, and intestinal bacteria of quail. Anim. Sci. J. 2019, 90, 747–756. [Google Scholar] [CrossRef]
- Wang, X.J.; Ding, L.M.; Wei, H.Y.; Jiang, C.X.; Yan, Q.; Hu, C.S.; Jia, G.X.; Zhou, Y.Q.; Henkin, Z.; Degen, A.A. Astragalus membranaceus root supplementation improves average daily gain, rumen fermentation, serum immunity and antioxidant indices of Tibetan sheep. Animal 2021, 15, 100061. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewi, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Wei, H.Y.; Ding, L.M.; Wang, X.J.; Yan, Q.; Jiang, C.X.; Hu, C.S.; Wang, G.W.; Zhou, Y.Q.; Henkin, Z.; Degen, A.A. Astragalus root extract improved average daily gain, immunity, antioxidant status and ruminal microbiota of early weaned yak calves. J. Sci. Food Agric. 2021, 101, 82–90. [Google Scholar] [CrossRef]
- Du, H.X.; Erdene, K.; Chen, S.Y.; Qi, S.; Bao, Z.B.; Zhao, Y.X.; Wang, C.F.; Zhao, G.F.; Ao, C.J. Correlation of the rumen fluid microbiome and the average daily gain with a dietary supplementation of Allium mongolicum Regel extracts in sheep. J. Anim. Sci. 2019, 97, 2865–2877. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J. Appl. Microbiol. 2015, 119, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- Pitta, D.W.; Indugu, N.; Kumar, S.; Vecchiarelli, B.; Sinha, R.; Baker, L.D.; Bhukya, B.; Ferguson, J.D. Metagenomic assessment of the functional potential of the rumen microbiome in Holstein dairy cows. Anaerobe 2016, 38, 50–60. [Google Scholar] [CrossRef]
- Spring, S.; Bunk, B.; Sproeer, C.; Schumann, P.; Rohde, M.; Tindall, B.J.; Klenk, H.P. Characterization of the first cultured representative of Verrucomicrobia subdivision 5 indicates the proposal of a novel phylum. ISME J. 2016, 10, 2801–2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanGylswyk, N.O. Succiniclasticum ruminis gen. nov. sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Zhang, M.L.; Xue, C.X.; Zhu, W.Y.; Mao, S.Y. Characterization and comparison of the temporal dynamics of ruminal bacterial microbiota colonizing rice straw and alfalfa hay within ruminants. J. Dairy Sci. 2016, 99, 9668–9681. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Z.; Liu, K.Z.; Wang, Z.S.; Bai, X.; Peng, Q.H.; Jin, L. Bacterial community diversity associated with different utilization efficiencies of nitrogen in the gastrointestinal tract of goats. Front. Microbiol. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front. Microbiol. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Galvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.E.; Abut, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Ricke, S.C.; Martin, S.A.; Nisbet, D.J. Ecology, metabolism, and genetics of ruminal selenomonads. Crit. Rev. Microbiol. 1996, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.J.; Conant, G.C.; Lamberson, W.R.; Cockrum, R.R.; Austin, K.J.; Rule, D.C.; Cammack, K.M. Diet and feed efficiency status affect rumen microbial profiles of sheep. Small Rumin. Res. 2017, 156, 12–19. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, M.X.; Huang, H.; Wang, S.J.; Ma, L.L.; Wang, H.N.; Hu, L.P.; Wei, K.; Zhu, R.L. The dynamic distribution of Small-Tail Han sheep microbiota across different intestinal segments. Front. Microbiol. 2018, 9, 32. [Google Scholar] [CrossRef]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.B.; Bi, Y.L.; Tu, Y.; Ma, T.; Dong, L.F.; Du, H.C.; Diao, Q.Y. Sanguinarine and resveratrol affected rumen fermentation parameters and bacterial community in calves. Anim. Feed. Sci. Technol. 2019, 251, 64–75. [Google Scholar] [CrossRef]
- Newbold, C.J.; López, S.; Nelson, N.; Ouda, J.O.; Wallace, R.J.; Moss, A.R. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br. J. Nutr. 2005, 94, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Emerson, E.L.; Weimer, P.J. Fermentation of model hemicelluloses by Prevotella strains and Butyrivibrio fibrisolvens in pure culture and in ruminal enrichment cultures. Appl. Microbiol. Biotechnol. 2017, 101, 4269–4278. [Google Scholar] [CrossRef] [PubMed]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, R.; Sweeney, T.; Vigors, S.; Thornton, K.; Rajauria, G.; O’Doherty, J.V. The effect of increasing inclusion levels of a fucoidan-rich extract derived from Ascophyllum nodosum on growth performance and aspects of intestinal health of pigs post-weaning. Mar. Drugs 2019, 17, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef] [Green Version]
- Petri, R.M.; Schwaiger, T.; Penner, G.B.; Beauchemin, K.A.; Forster, R.J.; McKinnon, J.J.; McAllister, T.A. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLoS ONE 2013, 8, e83424. [Google Scholar]
- Andrés, S.; Jaramillo, E.; Bodas, R.; Blanco, C.; Benavides, J.; Fernández, P.; Gonzalez, E.P.; Frutos, J.; Belenguer, A.; Lopez, S. Grain grinding size of cereals in complete pelleted diets for growing lambs: Effects on ruminal microbiota and fermentation. Small Rumin. Res. 2018, 159, 38–44. [Google Scholar] [CrossRef]
- Wu, S.R.; Ren, X.C.; Li, Y.L.; Guo, W.; Lei, X.Y.; Yao, J.H.; Yang, X.J. Effect of dietary Astragalus Polysaccharide supplements on testicular miRNA expression profiles and enzymatic changes of breeder cocks. Sci. Rep. 2017, 7, 38864. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.G.; Liu, Y.L.; Yin, Y.L.; Kong, X.F.; Huang, R.L.; Li, T.J.; Wu, G.Y.; Hou, Y.Q. Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids. 2009, 37, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, J.; Zhao, C.; Wang, X.F.; Yao, J.H.; Gong, Y.S.; Yang, X.J. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015, 72, 624–632. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liu, Y.J.; Yin, Y.Y.; Jin, W.; Mao, S.Y.; Liu, J.H. Response of rumen microbiota, and metabolic profiles of rumen fluid, liver and serum of goats to high-grain diets. Animal 2019, 13, 1855–1864. [Google Scholar] [CrossRef]
- Liu, M.; Qin, J.; Hao, Y.R.; Liu, M.; Luo, J.; Luo, T.; Wei, L. Astragalus polysaccharide suppresses skeletal muscle myostatin expression in diabetes: Involvement of ROS-ERK and NF-kappa B pathways. Oxid. Med. Cell. Longev. 2013, 2013, 782497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef]
- Deng, K.P.; Fan, Y.X.; Ma, T.W.; Wang, Z.; TanTai, W.J.; Nie, H.T.; Guo, Y.X.; Yu, X.Q.; Sun, L.W.; Wang, F. Carcass traits, meat quality, antioxidant status and antioxidant gene expression in muscle and liver of Hu lambs fed perilla seed. J. Anim. Physiol. Anim. Nutr. 2018, 102, E828–E837. [Google Scholar] [CrossRef]
- Salvi, M.; Battaglia, V.; Brunati, A.M.; La Rocca, N.; Tibaldi, E.; Pietrangeli, P.; Marcocci, L.; Mondovi, B.; Rossi, C.A.; Toninello, A. Catalase takes part in rat liver mitochondria oxidative stress defense. J. Biol. Chem. 2007, 282, 24407–24415. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.Z.; Yu, M.; Liu, H.W.; Sun, H.X.; Cao, Y.; Zhou, D.W. Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim. Feed. Sci. Technol. 2012, 174, 60–67. [Google Scholar] [CrossRef]
- Suzuki, T.; Ainai, A.; Hasegawa, H. Functional and structural characteristics of secretory IgA antibodies elicited by mucosal vaccines against influenza virus. Vaccine 2017, 35, 5297–5302. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.Y.; Guo, Y.R.; Yang, L.; Zhang, X.; Shen, W.Q.; Wang, Z.; Wen, S.; Zhao, D.D.; Wu, H.P.; Chen, J. Effects of oral florfenicol on intestinal structure, function and microbiota in mice. Arch. Microbiol. 2020, 202, 161–169. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Tao, Y.H.; Lai, C.H.; Huang, C.X.; Zhou, Y.M.; Yong, Q. Effects of mannanoligosaccharide supplementation on the growth performance, immunity, and oxidative status of Partridge Shank chickens. Animals 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Nie, T.; Zhao, S.T.; Mao, L.F.; Yang, Y.T.; Sun, W.; Lin, X.L.; Liu, S.; Li, K.; Sun, Y.R.; Li, P.; et al. The natural compound, formononetin, extracted from Astragalus membranaceus increases adipocyte thermogenesis by modulating PPAR gamma activity. Br. J. Pharmacol. 2018, 175, 1439–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.Y.; Fan, Y.; Kong, J.L.; Wu, D.Z.; Hu, Z.B. Effects of components isolated from Astragalus membranaceus Bunge on cardiac function injured by myocardial ischemia reperfusion in rats. China J. Chin. Mater. Med. 2000, 25, 300–302. (In Chinese) [Google Scholar]
- Song, Y.; Wang, H.X.; Zhang, J.; Yu, X.C.; Lu, M.L.; Zhao, S.L.; Zhou, Z.H. Inhibitory effect of Astragalus polysaccharide on energy metabolism of myocardia hypertrophy induced abdominal aorta constriction in rats. Chin. J. Pharmacol. Toxicol. 2012, 26, 177–182. (In Chinese) [Google Scholar]
- Yu, Y.; Wang, S.R.; Nie, B.; Sun, Y.K.; Yan, Y.F.; Zhu, L.Q. Effect of Astragali radix injection on myocardial cell mitochondrial structure and function in process of reversing myocardial cell hypertrophy. China J. Chin. Mater. Med. 2012, 37, 979–984. (In Chinese) [Google Scholar]
- Zhang, S.P.; Luan, A.N.; Lu, M.L.; Han, R.H.; Hu, J.; Wang, H.X. Effect of astragaloside IV on myocardial hypertrophy and PGC-1 α induced by isoproterenol in rats. Chin. Pharmacol. Clin. 2014, 30, 65–69. (In Chinese) [Google Scholar]
- Xie, Z.; Zhang, J.; Ma, S.; Huang, X.; Huang, Y. Effect of Chinese herbal medicine treatment on plasma lipid profile and hepatic lipid metabolism in Hetian broiler. Poult. Sci. 2017, 96, 1918–1924. [Google Scholar] [CrossRef]
- Luan, A.N.; Tang, F.T.; Yang, Y.H.; Lu, M.L.; Wang, H.X.; Zhang, Y.J. Astragalus polysaccharide attenuates isoproterenol-induced cardiac hypertrophy by regulating TNF-α/PGC-1α signaling mediated energy biosynthesis. Environ. Toxicol. Pharmacol. 2015, 39, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lai, Y.N.; Wang, L.Y.; Xia, Y.P.; Chen, W.J.; Zhao, X.L.; Yu, M.H.; Li, Y.M.; Zhang, Y.; Ye, H.Y. Astragalus polysaccharides repress myocardial lipotoxicity in a PPARalpha-dependent manner in vitro and in vivo in mice. J. Diabetes Complicat. 2015, 29, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Sirithanakorn, C.; Adina-Zada, A.; Wallace, J.C.; Jitrapakdee, S.; Attwood, P.V. Mechanisms of inhibition of rhizobium etli pyruvate carboxylase by L-Aspartate. Biochemistry 2014, 53, 7100–7106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Y.; Ying, S.; Shao, C.; Zhu, H.; Yan, J.; Shi, Z. Metabolomic profiling of goslings with visceral gout reveals a distinct metabolic signature. Br. Poult. Sci. 2020, 61, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.A.; Smith, A.M.; Gryshkova, V.; Donley, E.L.R.; Valentin, J.P.; Burrier, R.E. A targeted metabolomics-based assay using human induced pluripotent stem cell-derived cardiomyocytes identifies structural and functional cardiotoxicity potential. Toxicol. Sci. 2020, 174, 218–240. [Google Scholar] [CrossRef]
| Ingredient (g/kg DM) | Concentrate 1 | Oat Hay | AMT |
|---|---|---|---|
| Crude protein | 201 | 84 | 194 |
| Neutral detergent fiber | - 2 | 485 | 523 |
| Acid detergent fiber | - | 392 | 416 |
| Polysaccharides | - | - | 125.8 |
| Flavonoids | - | - | 0.12 |
| Astragaloside | - | - | 0.87 |
| Items | Day | Treatment 1 | SEM | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| A0 | A20 | A50 | A80 | T | L | Q | |||
| OTUs | 14 | 866 a | 890 a | 786 b | 802 b | 12 | <0.001 | 0.004 | 0.012 |
| 56 | 867 a | 885 a | 882 a | 826 b | 8 | 0.016 | 0.541 | 0.162 | |
| Chao1 index | 14 | 951 a | 978 a | 889 b | 887 b | 46 | 0.003 | 0.003 | 0.010 |
| 56 | 968 | 964 | 967 | 936 | 30 | 0.665 | 0.548 | 0.238 | |
| Shannon index | 14 | 5.046 a | 5.319 a | 4.678 b | 4.688 b | 0.343 | 0.003 | 0.016 | 0.041 |
| 56 | 4.998 | 5.152 | 4.989 | 4.900 | 0.227 | 0.391 | 0.878 | 0.307 | |
| Simpson index | 14 | 0.019 b | 0.013 b | 0.031 a | 0.030 a | 0.010 | 0.014 | 0.019 | 0.063 |
| 56 | 0.022 | 0.019 | 0.024 | 0.027 | 0.004 | 0.705 | 0.646 | 0.432 | |
| Items (%) | Day | Treatment 1 | SEM | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| A0 | A20 | A50 | A80 | T | L | Q | |||
| Firmicutes | 14 | 39.79 b | 41.38 b | 48.90 b | 58.88 a | 2.427 | 0.006 | 0.001 | 0.002 |
| 56 | 37.22 b | 39.07 b | 42.73 a | 46.28 a | 1.334 | 0.043 | 0.013 | 0.054 | |
| Bacteroidetes | 14 | 46.48 a | 47.85 a | 32.97 b | 29.13 b | 3.192 | 0.047 | 0.010 | 0.039 |
| 56 | 50.91 a | 46.18 b | 45.11 b | 36.59 c | 2.176 | 0.003 | 0.001 | 0.004 | |
| Patescibacteria | 14 | 3.52 | 4.04 | 3.70 | 3.34 | 0.435 | 0.965 | 0.242 | 0.208 |
| 56 | 3.37 | 4.17 | 4.17 | 4.81 | 0.375 | 0.666 | 0.053 | 0.142 | |
| Proteobacteria | 14 | 3.19 | 2.46 | 3.50 | 2.46 | 0.421 | 0.812 | 0.775 | 0.949 |
| 56 | 1.67 | 2.90 | 2.28 | 5.81 | 0.714 | 0.168 | 0.060 | 0.126 | |
| Kiritimatiellaeota | 14 | 0.70 b | 1.34 b | 4.57 a | 3.80 a | 0.601 | 0.027 | 0.011 | 0.035 |
| 56 | 1.01 b | 1.56 b | 2.62 a | 0.99 b | 0.262 | 0.033 | 0.686 | 0.093 | |
| Synergistetes | 14 | 0.44 | 0.69 | 1.03 | 0.51 | 0.151 | 0.585 | 0.247 | 0.494 |
| 56 | 2.17 | 2.09 | 1.26 | 1.09 | 0.435 | 0.801 | 0.211 | 0.421 | |
| Actinobacteria | 14 | 0.92 | 1.07 | 1.80 | 0.82 | 0.185 | 0.242 | 0.251 | 0.363 |
| 56 | 0.35 | 0.11 | 0.32 | 0.15 | 0.055 | 0.334 | 0.446 | 0.203 | |
| Tenericutes | 14 | 1.22 | 0.64 | 0.93 | 0.96 | 0.225 | 0.882 | 0.818 | 0.805 |
| 56 | 0.52 | 0.95 | 0.64 | 1.06 | 0.115 | 0.326 | 0.210 | 0.476 | |
| Cyanobacteria | 14 | 0.34 | 0.18 | 1.46 | 0.27 | 0.229 | 0.149 | 0.630 | 0.512 |
| 56 | 0.07 | 0.03 | 0.24 | 0.18 | 0.041 | 0.268 | 0.158 | 0.386 | |
| Spirochaetes | 14 | 0.74 | 0.16 | 0.66 | 0.07 | 0.171 | 0.439 | 0.350 | 0.661 |
| 56 | 0.15 | 0.20 | 0.44 | 0.11 | 0.075 | 0.467 | 0.299 | 0.178 | |
| Items (%) | Day | Treatment 1 | SEM | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| A0 | A20 | A50 | A80 | T | L | Q | |||
| Rikenellaceae_RC9_gut_group | 14 | 15.19 | 15.00 | 15.59 | 15.39 | 0.573 | 0.989 | 0.823 | 0.976 |
| 56 | 12.93 b | 13.09 b | 15.61 a | 14.36 b | 0.350 | 0.006 | 0.024 | 0.044 | |
| uncultured_bacterium_f_F082 | 14 | 10.43 | 12.80 | 11.92 | 13.39 | 0.823 | 0.653 | 0.292 | 0.566 |
| 56 | 16.70 | 16.66 | 17.51 | 18.44 | 0.429 | 0.448 | 0.116 | 0.257 | |
| Christensenellaceae_R-7_group | 14 | 10.64 b | 10.12 b | 12.78 b | 15.55 a | 0.763 | 0.026 | 0.006 | 0.010 |
| 56 | 3.73 | 3.71 | 3.96 | 3.64 | 0.206 | 0.964 | 0.995 | 0.943 | |
| Prevotella_1 | 14 | 3.55 | 3.35 | 3.04 | 3.41 | 0.140 | 0.665 | 0.578 | 0.539 |
| 56 | 7.67 a | 5.95 b | 4.64 b | 4.50 b | 0.402 | 0.003 | <0.001 | 0.001 | |
| uncultured_bacterium_f_ Muribaculaceae | 14 | 4.87 b | 5.02 b | 5.24 b | 8.28 a | 0.434 | 0.002 | 0.003 | 0.001 |
| 56 | 3.74 | 4.03 | 3.18 | 3.04 | 0.238 | 0.604 | 0.002 | 0.001 | |
| Ruminococcaceae_NK4A214_ group | 14 | 5.25 | 5.84 | 6.84 | 4.98 | 0.376 | 0.332 | 0.955 | 0.283 |
| 56 | 2.06 | 3.29 | 3.87 | 2.63 | 0.293 | 0.133 | 0.400 | 0.063 | |
| Succiniclasticum | 14 | 1.32 | 2.55 | 2.30 | 3.15 | 0.366 | 0.380 | 0.110 | 0.283 |
| 56 | 5.33 | 5.10 | 6.37 | 5.06 | 0.376 | 0.615 | 0.896 | 0.790 | |
| Quinella | 14 | 1.71 | 1.84 | 2.41 | 3.78 | 0.583 | 0.625 | 0.205 | 0.402 |
| 56 | 3.40 a | 3.52 a | 1.95 b | 2.15 b | 0.208 | <0.001 | 0.001 | 0.603 | |
| Ruminococcus_2 | 14 | 2.13 | 1.94 | 1.68 | 3.50 | 0.342 | 0.245 | 0.221 | 0.154 |
| 56 | 1.01 b | 3.45 a | 2.08 b | 0.88 b | 0.339 | 0.008 | 0.582 | 0.011 | |
| uncultured_bacterium_ o_WCHB1-41 | 14 | 1.37 b | 1.87 b | 4.30 a | 1.88 b | 0.371 | 0.006 | 0.247 | 0.059 |
| 56 | 0.93 b | 1.37 b | 2.62 a | 1.09 b | 0.216 | 0.007 | 0.395 | 0.037 | |
| Veillonellaceae_UCG-001 | 14 | 0.11 | 0.27 | 0.10 | 0.56 | 0.071 | 0.051 | 0.053 | 0.085 |
| 56 | 1.96 | 2.52 | 2.69 | 2.90 | 0.209 | 0.463 | 0.111 | 0.269 | |
| Desulfovibrio | 14 | 0.93 b | 1.17 b | 2.68 a | 1.78 b | 0.212 | 0.003 | 0.027 | 0.028 |
| 56 | 1.21 | 1.85 | 1.17 | 2.42 | 0.328 | 0.528 | 0.335 | 0.579 | |
| Selenomonas_3 | 14 | 0.38 b | 0.49 b | 0.42 b | 0.85 a | 0.054 | <0.001 | 0.002 | 0.001 |
| 56 | 1.04 | 1.87 | 1.07 | 1.58 | 0.198 | 0.403 | 0.660 | 0.845 | |
| Items 1 | Treatment 2 | SEM | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|
| A0 | A20 | A50 | A80 | T | L | Q | ||
| Meat | ||||||||
| CAT (U·mg−1) | 6.43 b | 6.05 b | 7.84 a | 6.85 ab | 0.239 | 0.031 | 0.429 | 0.365 |
| T-AOC (U·mg−1) | 0.91 | 1.30 | 1.20 | 1.13 | 0.061 | 0.162 | 0.014 | 0.051 |
| SOD (U·mg−1) | 8.7 b | 10.1 ab | 10.8 a | 10.9 a | 0.278 | 0.033 | 0.337 | 0.624 |
| MDA (nmol·mg−1) | 0.65 | 0.67 | 0.62 | 0.63 | 0.011 | 0.401 | 0.914 | 0.620 |
| Small intestinal mucosa | ||||||||
| CAT (U·mg−1) | 5.75 | 7.53 | 7.36 | 8.21 | 0.381 | 0.703 | 0.353 | 0.310 |
| T-AOC (U·mg−1) | 2.49 b | 2.34 b | 2.82 a | 2.58 ab | 0.062 | 0.041 | 0.617 | 0.689 |
| SOD (U·mg−1) | 10.2 b | 16.0 a | 15.5 a | 19.4 a | 1.066 | 0.002 | 0.097 | 0.004 |
| MDA (nmol·mg−1) | 0.74 | 0.81 | 0.69 | 0.69 | 0.021 | 0.199 | 0.230 | 0.126 |
| sIgA (μg/mg) | 0.37 b | 0.53 b | 1.14 a | 1.14 a | 0.110 | 0.001 | 0.164 | 0.395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, C.; Ding, L.; Tang, Y.; Wei, H.; Jiang, C.; Yan, Q.; Dong, Q.; Degen, A.A. Astragalus membranaceus Alters Rumen Bacteria to Enhance Fiber Digestion, Improves Antioxidant Capacity and Immunity Indices of Small Intestinal Mucosa, and Enhances Liver Metabolites for Energy Synthesis in Tibetan Sheep. Animals 2021, 11, 3236. https://doi.org/10.3390/ani11113236
Wang X, Hu C, Ding L, Tang Y, Wei H, Jiang C, Yan Q, Dong Q, Degen AA. Astragalus membranaceus Alters Rumen Bacteria to Enhance Fiber Digestion, Improves Antioxidant Capacity and Immunity Indices of Small Intestinal Mucosa, and Enhances Liver Metabolites for Energy Synthesis in Tibetan Sheep. Animals. 2021; 11(11):3236. https://doi.org/10.3390/ani11113236
Chicago/Turabian StyleWang, Xianju, Changsheng Hu, Luming Ding, Yiguo Tang, Haiyan Wei, Cuixia Jiang, Qi Yan, Quanmin Dong, and Abraham Allan Degen. 2021. "Astragalus membranaceus Alters Rumen Bacteria to Enhance Fiber Digestion, Improves Antioxidant Capacity and Immunity Indices of Small Intestinal Mucosa, and Enhances Liver Metabolites for Energy Synthesis in Tibetan Sheep" Animals 11, no. 11: 3236. https://doi.org/10.3390/ani11113236
APA StyleWang, X., Hu, C., Ding, L., Tang, Y., Wei, H., Jiang, C., Yan, Q., Dong, Q., & Degen, A. A. (2021). Astragalus membranaceus Alters Rumen Bacteria to Enhance Fiber Digestion, Improves Antioxidant Capacity and Immunity Indices of Small Intestinal Mucosa, and Enhances Liver Metabolites for Energy Synthesis in Tibetan Sheep. Animals, 11(11), 3236. https://doi.org/10.3390/ani11113236







