Wolf–Hunting Dog Interactions in a Biodiversity Hot Spot Area in Northern Greece: Preliminary Assessment and Implications for Conservation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
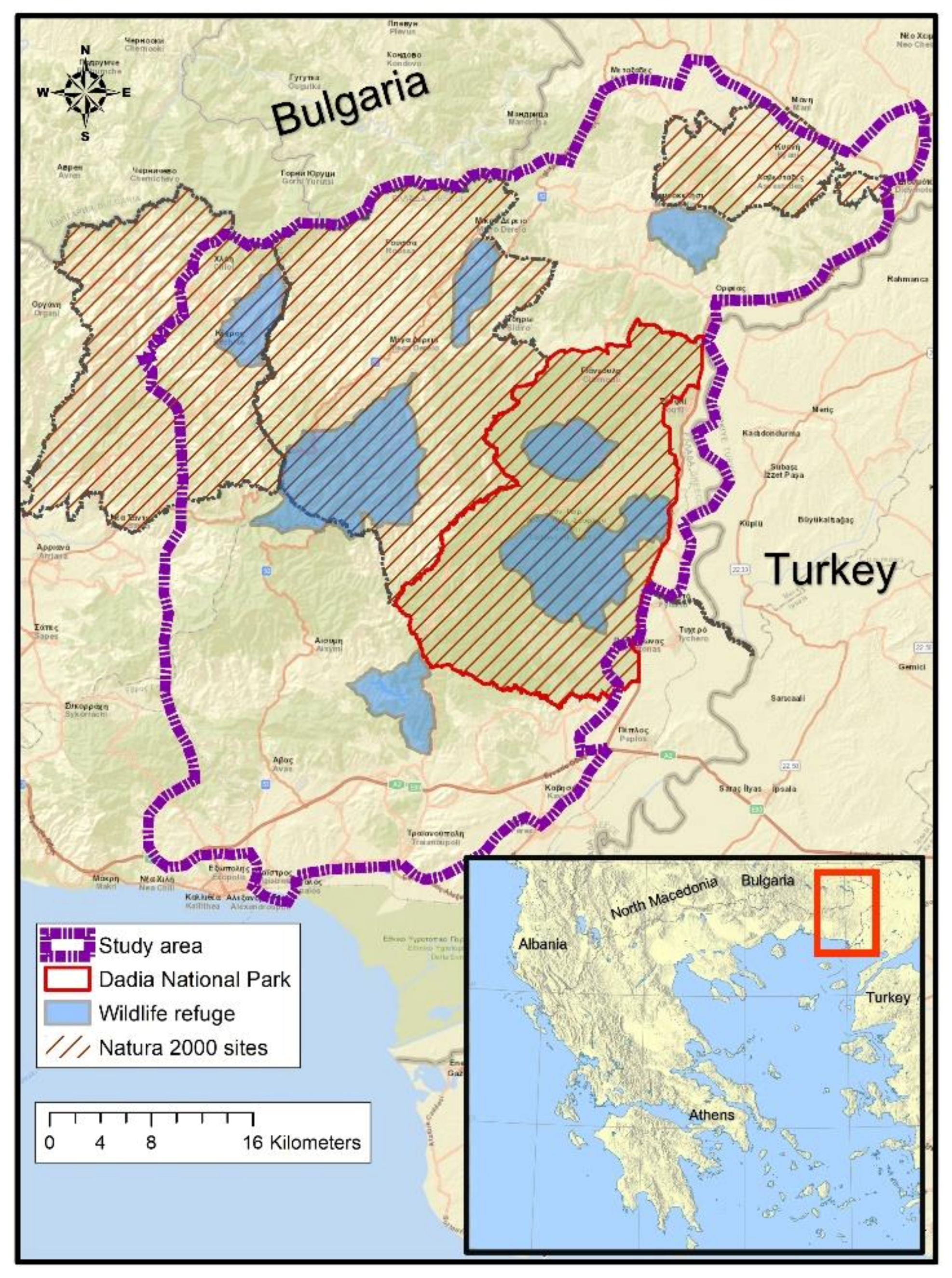
2.1. Study Area
2.2. Data Collection
2.2.1. Questionnaire Surveys to Hunters
2.2.2. Wolf Attacks on Livestock in the Study Area
2.2.3. Trophic Analysis of Wolf Scats
2.3. Statistical Analyses
2.3.1. Data Sets Used for Analyses
2.3.2. Distribution of Attacks in Relation to Wolf Annual Cycle
2.3.3. Hunting Dog Breed and Age Classes’ Selection
2.3.4. Dog Depredation Trends
2.3.5. Dog Depredation Risk Map—Maxent Analysis
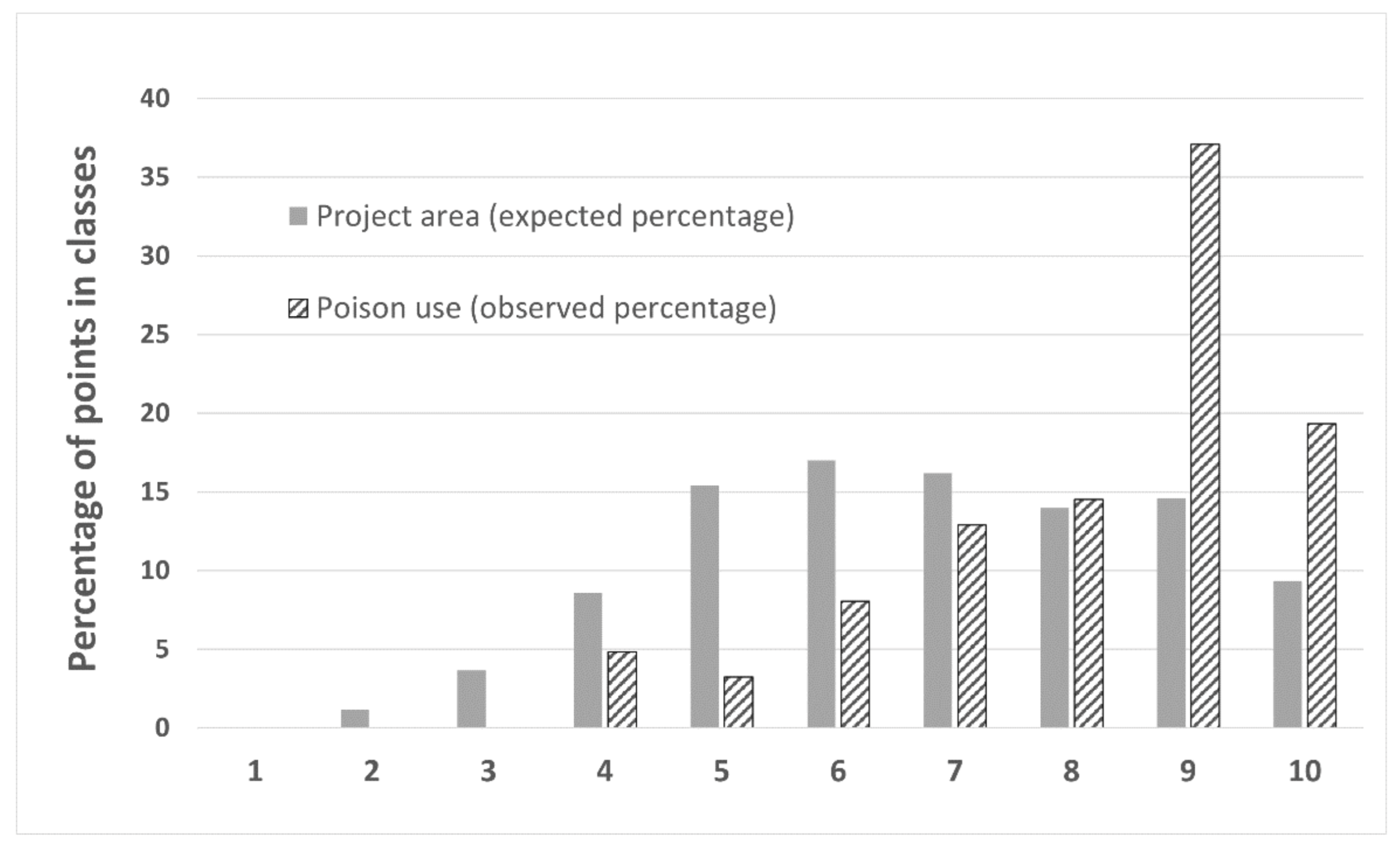
2.3.6. Association of Illegal Poison Bait Use in DNP with Dog Depredation Risk Map
2.4. Factors Related to Dog Depredation Levels
2.4.1. Severity of Attacks on Hunting Dogs
2.4.2. Levels of Hunting Dog Losses per Hunter
2.4.3. Levels of Hunting Dog Interactions per Village (Municipal District)
2.4.4. Severity of Losses per Wolf Social Unit for the Year 2019–2020
3. Results
3.1. Hunting Dog Depredation Interactions per Affected Hunter
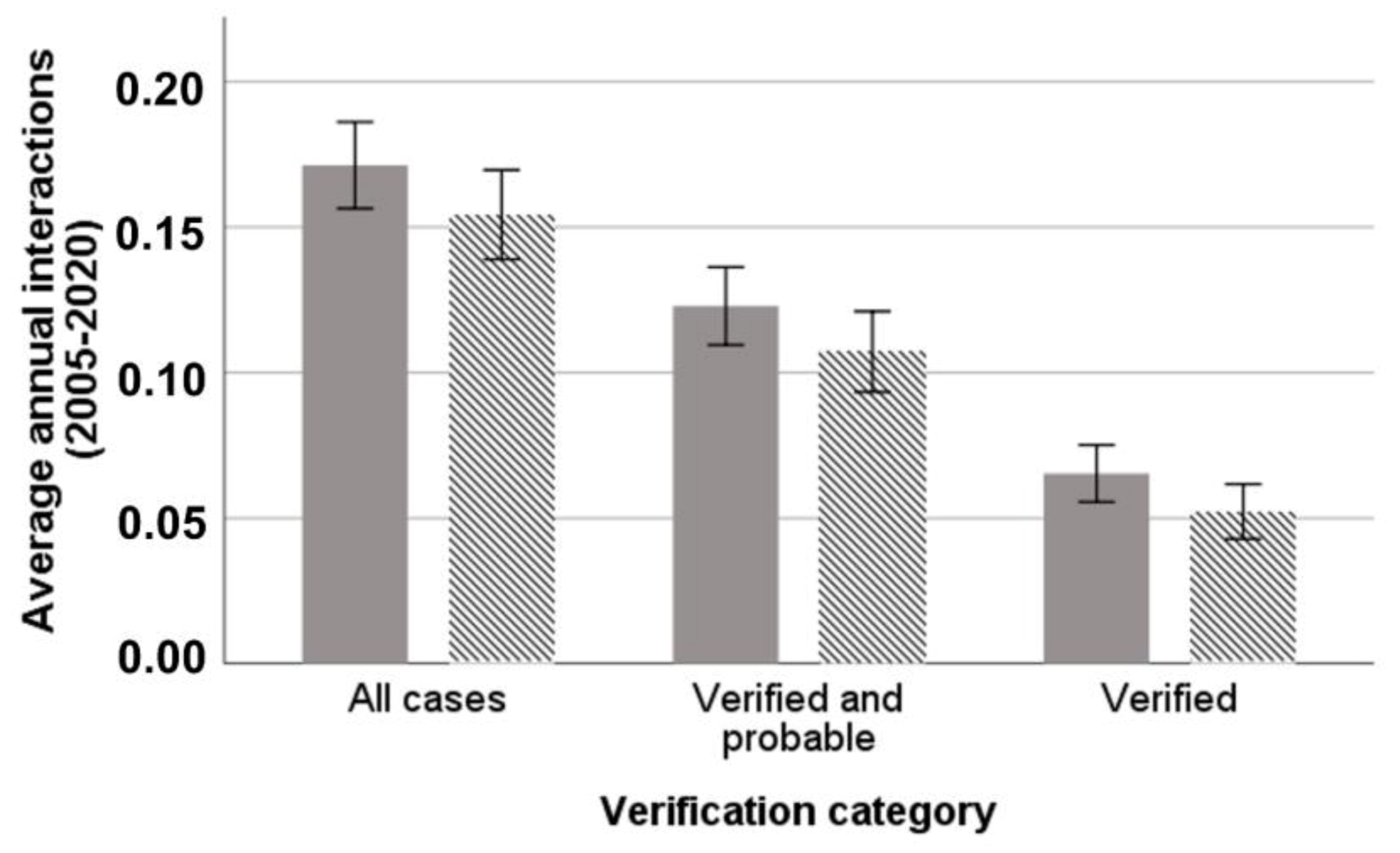
3.2. Monthly and Seasonal Variation of Wolf–Dog Interactions
3.3. Hunting Dog Breeds and Age Selection
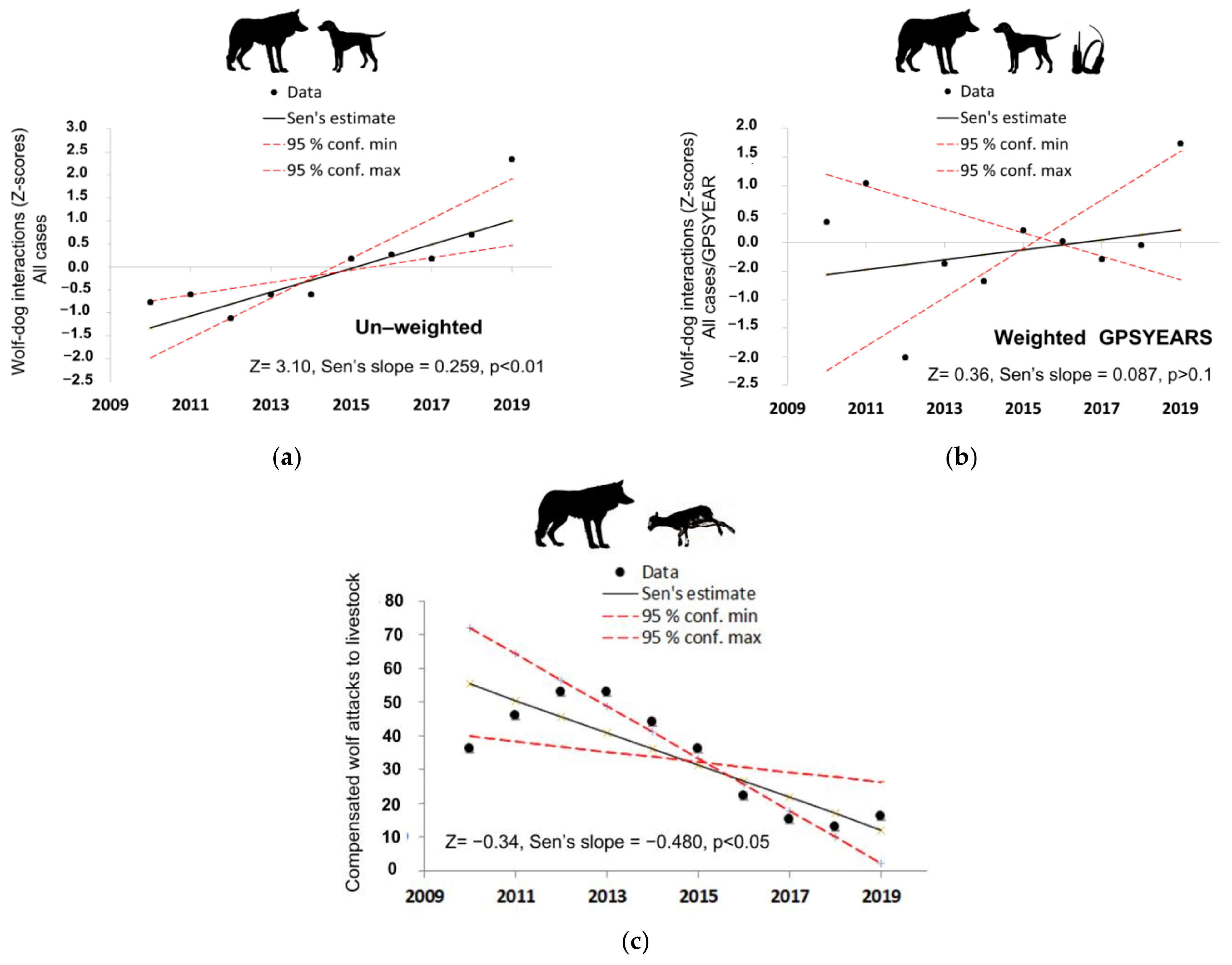
3.4. Trends of Wolf–Hunting Dog Interactions and Wolf Depredations on Livestock
3.5. Wild Boar and LGDs–Hunting Dog Interactions
3.6. Wolf Diet Analysis
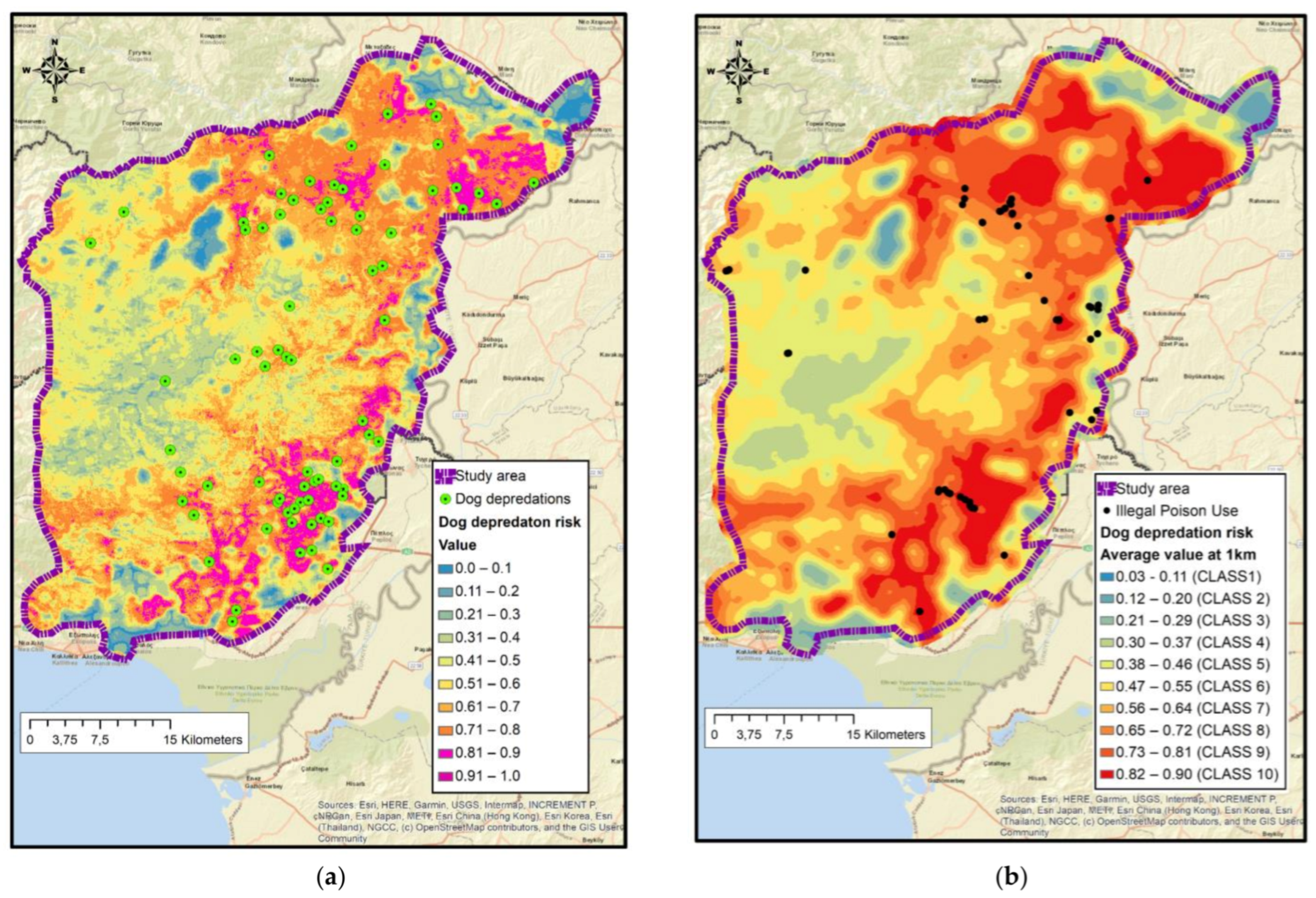
3.7. Maxent Dog Predation Risk Map Model Selection and Variables Involved
3.8. Factors Affecting Dog Depredation Levels
3.8.1. Attack Level
3.8.2. Hunter Level
3.8.3. Village Level
3.8.4. Wolf Social Unit Level
3.9. Hunter Opinions on Protective Measures and Other Information
3.9.1. Use of Protective Vests
3.9.2. Use of Risk Maps for Minimizing Wolf–Dog Encounter Rates
3.9.3. Hunter Practices
4. Discussion
4.1. Dog Depredation Levels and Trends
4.2. Predatory Character of Dog Fatalities—Percentage in Wolf Diet
4.3. Spatial Distribution of Attacks and Related Factors
4.4. Variation among Wolf Social Units, Villages, and Factors That May Relate to Trends Observed
4.5. Implications of the Use of Risk Maps and Other Protective Measures
5. Conclusions and Management Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Maxent Parametrization and Model Selection Procedure
References
- Butler, J.R.A.; Linnell, J.D.C.; Morrant, D.; Athreya, V.; Lescureux, N.; McKeown, A. Dog eat dog, cat eat dog: Social-ecological dimensions of dog predation by wild carnivores. In Free-Ranging Dogs and Wildlife Conservation; Gompper, M.E., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 117–143. [Google Scholar]
- Boitani, L. Wolf management in intensively used areas of Italy. In Wolves of the World: Perspectives of Behaviour, Ecology, and Conservation; Harrington, F.H., Paquet, P.C., Eds.; Noyes: Oceanville, NJ, USA, 1982; pp. 158–172. [Google Scholar]
- Sidorovich, V.E.; Tikhomirova, L.L.; Jędrzejewska, B. Wolf Canis lupus numbers, diet and damage to livestock in relation to hunting and ungulate abundance in northeastern Belarus during 1990–2000. Wildl. Biol. 2003, 9, 103–111. [Google Scholar] [CrossRef]
- Salvador, A.; Abad, P. Food habits of a wolf population (Canis lupus) in León province, Spain. Mammalia 1987, 51, 51. [Google Scholar] [CrossRef]
- Vos, J. Food habits and livestock depredation of two Iberian wolf packs (Canis lupus signatus) in the north of Portugal. J. Zool. 2000, 251, 457–462. [Google Scholar] [CrossRef]
- Barja, I. Prey and Prey-Age Preference by the Iberian Wolf Canis lupus signatus in a Multiple-Prey Ecosystem. Wildl. Biol. 2009, 15, 147–154. [Google Scholar] [CrossRef]
- Kojola, I.; Ronkainen, S.; Hakala, A.; Heikkinen, S.; Kokko, S. Interactions between wolves Canis lupusand dogs C. familiarisin Finland. Wildl. Biol. 2004, 10, 101–105. [Google Scholar] [CrossRef]
- Tikkunen, M.; Kojola, I. Does public information about wolf (Canis lupus) movements decrease wolf attacks on hunting dogs (C. familiaris)? Nat. Conserv. 2020, 42, 33–49. [Google Scholar] [CrossRef]
- Jethva, B.D.; Jhala, Y.V. Foraging ecology, economics and conservation of Indian wolves in the Bhal region of Gujarat, Western India. Biol. Conserv. 2004, 116, 351–357. [Google Scholar] [CrossRef]
- Fritts, S.H.; Paul, W.J. Interactions of Wolves and Dogs in Minnesota. Wildl. Soc. Bull. 1989, 17, 121–123. [Google Scholar] [CrossRef]
- Ruid, D.B.; Paul, W.J.; Roell, B.J.; Wydeven, A.P.; Willging, R.C.; Jurewicz, R.L.; Lonsway, D.H. Wolf-human confl icts and management in Minnesota, Wisconsin, and Michigan. In Recovery of Gray Wolves in the Great Lakes Region of the United States: An Endangered Species Success Story; Wydeven, A.P., Van Deelen, T.R., Heske, E.J., Eds.; Springer: New York, NY, USA, 2009; pp. 279–298. [Google Scholar]
- Edge, J.L.; Beyer, D.E.; Belant, J.L.; Jordan, M.J.; Roell, B.J. Livestock and Domestic Dog Predations by Wolves in Michigan. Hum.-Wildl. Interact. 2011, 5, 66–78. [Google Scholar] [CrossRef]
- Martins, I.; Krofel, M.; Mota, P.G.; Álvares, F. Consumption of Carnivores by Wolves: A Worldwide Analysis of Patterns and Drivers. Diversity 2020, 12, 470. [Google Scholar] [CrossRef]
- Bump, J.K.; Murawski, C.M.; Kartano, L.M.; Beyer, D.E.; Roell, B.J. Bear-Baiting May Exacerbate Wolf-Hunting Dog Conflict. PLoS ONE 2013, 8, e61708. [Google Scholar] [CrossRef]
- Tikkunen, M.; Kojola, I. Hunting dogs are at biggest risk to get attacked by wolves near wolves’ territory boundaries. Mammal Res. 2019, 64, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Kojola, I.; Kuittinen, J. Wolf Attacks on Dogs in Finland. Wildl. Soc. Bull. 2002, 30, 498–501. [Google Scholar]
- Backeryd, J. Wolf Attacks on Dogs in Scandinavia 1995–2005: Will Wolves in Scandinavia Go Extinct If Dog Owners Are Allowed to Kill a Wolf Attacking a Dog? Ph.D. Dissertation, Sveriges Lantbruksuniversitet, Uppsala, Sweden, 2007. [Google Scholar]
- Bassi, E.; Pervan, I.; Ugarković, D.; Kavčić, K.; Maksan, M.T.; Krofel, M.; Šprem, N. Attacks on hunting dogs: The case of wolf–dog interactions in Croatia. Eur. J. Wildl. Res. 2021, 67, 4. [Google Scholar] [CrossRef]
- Olson, E.R.; Treves, A.; Wydeven, A.P.; Ventura, S.J. Landscape predictors of wolf attacks on bear-hunting dogs in Wisconsin, USA. Wildl. Res. 2014, 41, 584–597. [Google Scholar] [CrossRef]
- Wierzbowska, I.A.; Hędrzak, M.; Popczyk, B.; Okarma, H.; Crooks, K.R. Predation of wildlife by free-ranging domestic dogs in Polish hunting grounds and potential competition with the grey wolf. Biol. Conserv. 2016, 201, 1–9. [Google Scholar] [CrossRef]
- Skordas, K.; Karampatzakis, T.; Margaritis, D.; Pagonis, K. Preliminary data incidents reports of wolf attacks on hunting dogs in Northern Greece. In Proceedings of the 2nd International Jackal Symposium Hellenic Zoological Archives, Attiki, Greece, 29 October–2 November 2018; p. 150. [Google Scholar]
- Lescureux, N.; Linnell, J.D. Warring brothers: The complex interactions between wolves (Canis lupus) and dogs (Canis familiaris) in a conservation context. Biol. Conserv. 2014, 171, 232–245. [Google Scholar] [CrossRef]
- Bisi, J.; Kurki, S.; Svensberg, M.; Liukkonen, T. Human dimensions of wolf (Canis lupus) conflicts in Finland. Eur. J. Wildl. Res. 2007, 53, 304–314. [Google Scholar] [CrossRef]
- Sjölander-Lindqvist, A. Social-Natural Landscape Reorganised: Swedish Forest-edge Farmers and Wolf Recovery. Conserv. Soc. 2009, 7, 130. [Google Scholar] [CrossRef]
- John, F.S.; Keane, A.; Edwards-Jones, G.; Jones, L.; Yarnell, R.; Jones, J.P.G. Identifying indicators of illegal behaviour: Carnivore killing in human-managed landscapes. Proc. R. Soc. B Biol. Sci. 2012, 279, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Parvanov, D.; Stoynov, E.; Vangelova, N.; Peshev, H.; Grozdanov, A.; Delov, V.; Iliev, Y. Vulture mortality resulting from illegal poisoning in the southern Balkan Peninsula. Environ. Sci. Pollut. Res. 2018, 25, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Aguilar, A.; Sanchez-Zapata, J.A.; Carrete, M.; Benítez, J.R.; Ávila, E.; Arenas, R.; Donázar, J.A. Action on multiple fronts, illegal poisoning and wind farm planning, is required to reverse the decline of the Egyptian vulture in southern Spain. Biol. Conserv. 2015, 187, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Skartsi, T.; Dobrev, V.; Oppel, S.; Kafetzis, A.; Kret, E.; Karampatsa, R.; Saravia, V.; Bounas, T.; Vavylis, D.; Sidiropoulos, L.; et al. Assessment of the Illegal Use of Poison in Natura 2000 Sites for the Egyptian Vulture in Greece and Bulgaria during the Period 2003–2012. Technical Report under Action A3 of the LIFE+ Project B. The Return of the Neophron (LIFE10 NAT/BG/000152); WWF Greece: Athens, Greece, 2014. [Google Scholar]
- Mateo-Tomás, P.; Olea, P.P.; Sánchez-Barbudo, I.S.; Mateo, R. Alleviating human-wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 2012, 49, 376–385. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Hristov, H.; Dobrev, D.; Angelov, I.; Kurtev, M. Long-term population status, breeding parameters and limiting factors of the Griffon vulture (Gyps fulvus Hablizl, 1783) population in the Eastern Rhodopes, Bulgaria. Acta Zool. Bulg. 2014, 66, 373–384. [Google Scholar]
- Velevski, M.; Nikolov, S.C.; Hallmann, B.; Dobrev, V.; Sidiropoulos, L.; Saravia, V.; Tsiakiris, R.; Arkumarev, V.; Galanaki, A.; Kominos, T.; et al. Population decline and range contraction of the Egyptian Vulture Neophron percnopterus in the Balkan Peninsula. Bird Conserv. Int. 2015, 25, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Oppel, S.; Dobrev, V.; Arkumarev, V.; Saravia, V.; Bounas, A.; Kret, E.; Skartsi, T.; Velevski, M.; Stoychev, S.; Nikolov, S.C. Assessing the effectiveness of intensive conservation actions: Does guarding and feeding increase productivity and survival of Egyptian Vultures in the Balkans? Biol. Conserv. 2016, 198, 157–164. [Google Scholar] [CrossRef]
- Sakellari, M.; Xirouchakis, S.; Baxevani, K.; Probonas, M. Wildlife poisoning in Crete and the intentions of local interest groups to engage in anti-poisoning actions. Biodiversity 2016, 17, 79–89. [Google Scholar] [CrossRef]
- Ntemiri, K.; Saravia, V.; Angelidis, C.; Baxevani, K.; Probonas, M.; Kret, E.; Mertzanis, Y.; Iliopoulos, Y.; Georgiadis, L.; Skartsi, D.; et al. Animal mortality and illegal poison bait use in Greece. Environ. Monit. Assess. 2018, 190, 488. [Google Scholar] [CrossRef]
- Vasilakis, D.P.; Whitfield, D.P.; Schindler, S.; Poirazidis, K.S.; Kati, V. Reconciling endangered species conservation with wind farm development: Cinereous vultures (Aegypius monachus) in south-eastern Europe. Biol. Conserv. 2016, 196, 10–17. [Google Scholar] [CrossRef]
- Skartsi, T.; Vasilakis, D.; Elorriaga, J. Population trends and conservation of vultures in the National Park of Dadia—Lefkimi—Soufli forest. In The Dadia—Lefkimi—Soufli Forest National Park, Greece: Biodiversity, Management and Conservation; Catsadorakis, G., Källander, H., Eds.; WWF Greece: Athens, Greece, 2010; pp. 183–193. [Google Scholar]
- Poirazidis, K.; Schindler, S.; Kakalis, E.; Ruiz, C.; Bakaloudis, D.E.; Scandolara, C.; Eastham, C.; Hristov, H.; Catsadorakis, G. Population Estimates for the Diverse Raptor Assemblage of Dadia National Park, Greece. Ardeola 2011, 58, 3–17. [Google Scholar] [CrossRef]
- Liarikos, C. Development trajectories and prospects in the Dadia-Lefkimi-Soufli Forest National Park. In The Dadia—Lefkimi—Soufli Forest National Park, Greece: Biodiversity, Management and Conservation; Catsadorakis, G., Källander, H., Eds.; WWF Greece: Athens, Greece, 2010; pp. 47–62. [Google Scholar]
- Hellenic National Statistical Authority. Data on Livestock Availability per Local Community in the Project Area for the Year 2012; Hellenic National Statistical Authority: Athens, Greece, 2012.
- Zakkak, S.; Mola, M.; De Gooijer, T.; Dijk, P.; Halivelentzios, A.; Giannakidis, G. Population Estimates of Roe Deer in the Dadia-Lefkimi-Soufli National Park. In Proceedings of the 14th International Congress on the Zoogeography and Ecology of Greece and Adjacent Regions, Thessaloniki, Greece, 27–30 June 2017. [Google Scholar]
- Reinhardt, I.; Kluth, G.; Nowak, S.; Mysłajek, R.W. Standards for the Monitoring of the Central European Wolf Population in Germany and Poland; Federal Agency for Nature Conservation: Bonn, Germany, 2015.
- Iliopoulos, Y.; Zakkak, S.; Skartsi, D. Summer Wolf Population Size Estimation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas by Using an Hierarchical Multimethod Approach (Habitat Modelling, Sign Surveys and Camera Trapping); VCF; WWF Greece: Callisto, Greece, 2021. [Google Scholar]
- Catsadorakis, G. The Dadia-Lefkimi-Soufli National Park, Greece: Biodiversity, Management and Conservation-Introduction. In The Dadia-Lefkimi-Soufli National Park, Greece: Biodiversity, Management and Conservation; Catsadorakis, G., Källander, H., Eds.; WWF Greece: Athens, Greece, 2010; pp. 17–29. [Google Scholar]
- Zografou, K.; Kati, V.; Grill, A.; Wilson, R.J.; Tzirkalli, E.; Pamperis, L.N.; Halley, J.M. Signals of Climate Change in Butterfly Communities in a Mediterranean Protected Area. PLoS ONE 2014, 9, e87245. [Google Scholar] [CrossRef] [Green Version]
- Rigg, R.; Finďo, S.; Wechselberger, M.; Gorman, M.L.; Sillero-Zubiri, C.; Macdonald, D.W. Mitigating carnivore–livestock conflict in Europe: Lessons from Slovakia. Oryx 2011, 45, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Dondina, O.; Meriggi, A.; Dagradi, V.; Perversi, M.; Milanesi, P. Wolf predation on livestock in an area of northern Italy and prediction of damage risk. Ethol. Ecol. Evol. 2015, 27, 200–219. [Google Scholar] [CrossRef]
- Khorozyan, I.; Soofi, M.; Ghoddousi, A.; Hamidi, A.K.; Waltert, M. The relationship between climate, diseases of domestic animals and human-carnivore conflicts. Basic Appl. Ecol. 2015, 16, 703–713. [Google Scholar] [CrossRef]
- Khorozyan, I.; Ghoddousi, S.; Soufi, M.; Soofi, M.; Waltert, M. Cattle selectivity by leopards suggests ways to mitigate human–leopard conflict. Ecol. Evol. 2018, 8, 8011–8018. [Google Scholar] [CrossRef]
- Khan, T.U.; Luan, X.; Ahmad, S.; Mannan, A.; Khan, W.; Khan, A.A.; Khan, B.U.; Din, E.U.; Bhattarai, S.; Shah, S.; et al. Status and Magnitude of Grey Wolf Conflict with Pastoral Communities in the Foothills of the Hindu Kush Region of Pakistan. Animals 2019, 9, 787. [Google Scholar] [CrossRef] [Green Version]
- Haidt, A.; Gawryś, R.; Szewczyk, M. Human Decision-Making as a Key Factor in the Risk of Wolf–Dog Interactions during Outdoor Activities. Animals 2021, 11, 2497. [Google Scholar] [CrossRef]
- Salganik, M.J.; Heckathorn, D.D. 5. Sampling and Estimation in Hidden Populations Using Respondent-Driven Sampling. Sociol. Methodol. 2004, 34, 193–240. [Google Scholar] [CrossRef]
- Mariacher, A.; Fanelli, R.; Garofalo, L.; Perfetti, G.; Lorenzini, R.; Fico, R. Who is the killer? Barking up the wrong tree. Mammalia 2019, 83, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Wilmers, C.C.; Stahler, D.R. Constraints on active-consumption rates in gray wolves, coyotes, and grizzly bears. Can. J. Zool. 2002, 80, 1256–1261. [Google Scholar] [CrossRef]
- ELGA. Confirmed Cases of Bear and Wolf Attacks on Livestock in Greece for the Years 2010–2019; ELGA: Athens, Greece, 2020. [Google Scholar]
- Bassi, E.; Donaggio, E.; Marcon, A.; Scandura, M.; Apollonio, M. Trophic niche overlap and wild ungulate consumption by red fox and wolf in a mountain area in Italy. Mamm. Biol. 2012, 77, 369–376. [Google Scholar] [CrossRef]
- Petridou, M.; Youlatos, D.; Lazarou, Y.; Selinides, K.; Pylidis, C.; Giannakopoulos, A.; Kati, V.; Iliopoulos, Y. Wolf diet and livestock selection in central Greece. Mammalia 2019, 83, 530–538. [Google Scholar] [CrossRef]
- Vila, C.; Urios, V.; Castroviejo, J. Observations on the daily activity patterns in the Iberian wolf. In Ecology and Conservation of Wolves in a Changing World; Carbyn, L.N., Fritts, S.H., Seip, D.R., Eds.; Canadian Circumpolar Institute: Alberta, BC, Canada, 1995; pp. 335–340. [Google Scholar]
- Meriggi, A.; Lovari, S. A Review of Wolf Predation in Southern Europe: Does the Wolf Prefer Wild Prey to Livestock? J. Appl. Ecol. 1996, 33, 1561. [Google Scholar] [CrossRef]
- Iliopoulos, Y.; Sgardelis, S.; Koutis, V.; Savaris, D. Wolf depredation on livestock in central Greece. Mammal Res. 2009, 54, 11–22. [Google Scholar] [CrossRef]
- Jacobs, J. Quantitative measurement of food selection. Oecologia 1974, 14, 413–417. [Google Scholar] [CrossRef]
- Salmi, T.; Määttä, A.; Anttila, P.; Ruoho-Airola, T.; Amnell, T. Detecting Trends of Annual Values of Atmospheric Pollutants by the Mann-Kendall Test and Sen’s Slope Estimates—The Excel Template Application MAKESENS; Science Open: Helsinki, Finland, 2002. [Google Scholar]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Rostro-García, S.; Tharchen, L.; Abade, L.; Astaras, C.; Cushman, S.A.; Macdonald, D.W. Scale dependence of felid predation risk: Identifying predictors of livestock kills by tiger and leopard in Bhutan. Landsc. Ecol. 2016, 31, 1277–1298. [Google Scholar] [CrossRef]
- Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu/ (accessed on 30 August 2021).
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps; Computer Software Program Produced by the Authors at the University of Massachusetts; University of Massachusetts: Amherst, MA, USA, 2012. [Google Scholar]
- Kret, E.; Vavylis, D.; Saravia, V.; Ntemiri, Κ. Poison Bait Detection with Specially Trained Dogs in Thrace and Central Greece Annual Report 2014 Life+ Project “The Return of the Neophron” Life10 nat/bg/000152 July 2015; WWF Greece: Athens, Greece, 2015. [Google Scholar]
- Vavylis, D.; Kret, E.; Saravia, V.; Ntemiri, Κ. Poison Bait Detection with Specially Trained Dogs in Thrace and Central Greece, Annual Report 2015; Conservation Action C1; Hellenic Ornithological Society & WWF Greece: Athens, Greece, 2016; 53p. (In Greek) [Google Scholar]
- Vavylis, D.; Kret, E.; Saravia, V.; Ntemiri, Κ. Poison Bait Detection with Specially Trained Dogs in Thrace and Central Greece, Annual Report 2016; Conservation Action C1; Hellenic Ornithological Society & WWF Greece: Athens, Greece, 2016; 66p. (In Greek) [Google Scholar]
- Kret, E. Poison Bait Detection with Specially Trained Dog, Annual Report 2017; WWF Greece: Athens, Greece, 2017; 35p. (In Greek) [Google Scholar]
- Kret, E.; Vavylis, D.; Saravia, V. Poison Bait Detection with Specially Trained Dogs in Thrace and Central Greece, Annual Report 2018; Hellenic Ornithological Society & WWF Greece: Athens, Greece, 2019; 61p. (In Greek) [Google Scholar]
- Kret, E.; Vavylis, D.; Saravia, V. Poison Bait Detection with Specially Trained Dogs in Thrace and Central Greece and Epirus, Annual Report 2020; Hellenic Ornithological Society & WWF Greece: Athens, Greece, 2021; 43p. (In Greek) [Google Scholar]
- Johnston, R.; Jones, K.; Manley, D. Confounding and collinearity in regression analysis: A cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual. Quant. 2018, 52, 1957–1976. [Google Scholar] [CrossRef] [Green Version]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Elith, J. Quantitative methods for modeling species habitat: Comparative performance and an application to Australian plants. In Quantitative Methods for Conservation Biology; Ferson, S., Burgham, M., Eds.; Springer: New York, NY, USA, 2000; pp. 39–58. [Google Scholar]
- Delibes-Mateos, M. Wolf Media Coverage in the Region of Castilla y León (Spain): Variations over Time and in Two Contrasting Socio-Ecological Settings. Animals 2020, 10, 736. [Google Scholar] [CrossRef]
- Mengüllüoğlu, D.; Ilaslan, E.; Emir, H.; Berger, A. Diet and wild ungulate preferences of wolves in northwestern Anatolia during winter. PeerJ 2019, 7, e7446. [Google Scholar] [CrossRef]
- Carricondo-Sanchez, D.; Zimmermann, B.; Wabakken, P.; Eriksen, A.; Milleret, C.; Ordiz, A.; Sanz-Pérez, A.; Wikenros, C. Wolves at the door? Factors influencing the individual behavior of wolves in relation to anthropogenic features. Biol. Conserv. 2020, 244, 108514. [Google Scholar] [CrossRef]
- Migli, D.; Youlatos, D.; Iliopoulos, Y. Winter food habits of wolves in central Greece. J. Biol. Res. 2005, 4, 217–220. [Google Scholar]
- Mohammadi, A.; Kaboli, M.; Sazatornil, V.; López-Bao, J.V. Anthropogenic food resources sustain wolves in conflict scenarios of Western Iran. PLoS ONE 2019, 14, e0218345. [Google Scholar] [CrossRef]
- Newsome, T.; Fleming, P.; Dickman, C.R.; Doherty, T.S.; Ripple, W.J.; Ritchie, E.G.; Wirsing, A.J. Making a New Dog? Bioscience 2017, 67, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, E.; Meriggi, A.; Brangi, A.; Vidus-Rosin, A. Effects of arboriculture stands on European hare Lepus europaeus spring habitat use in an agricultural area of northern Italy. Acta Thériol. 2011, 56, 229–238. [Google Scholar] [CrossRef]
- Thurfjell, H.; Ball, J.P.; Åhlén, P.-A.; Kornacher, P.; Dettki, H.; Sjöberg, K. Habitat use and spatial patterns of wild boar Sus scrofa (L.): Agricultural fields and edges. Eur. J. Wildl. Res. 2009, 55, 517–523. [Google Scholar] [CrossRef]
- Fonseca, C. Winter habitat selection by wild boar Sus scrofa in southeastern Poland. Eur. J. Wildl. Res. 2008, 54, 361–366. [Google Scholar] [CrossRef]
- Iliopoulos, Y.; Youlatos, D.; Sgardelis, S. Wolf pack rendezvous site selection in Greece is mainly affected by anthropogenic landscape features. Eur. J. Wildl. Res. 2013, 60, 23–34. [Google Scholar] [CrossRef]
- Sazatornil, V.; Rodríguez, A.; Klaczek, M.; Ahmadi, M.; Álvares, F.; Arthur, S.; Blanco, J.C.; Borg, B.L.; Cluff, D.; Cortés, Y.; et al. The role of human-related risk in breeding site selection by wolves. Biol. Conserv. 2016, 201, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Herrero, J.; Rodrigues, P.; García-Serrano, A.; Prada, C.; Giménez-Anaya, A.; Ayala, R.; Fernández-Arberas, O.; Fonseca, C. Habitat use by wild boar Sus scrofa in Moncayo Nature Park, Spain. Pirineos 2016, 171, e023. [Google Scholar] [CrossRef]
- Oftedal, O.T.; Gittleman, J.L. Patterns of energy output during reproduction in carnivores. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Cornell University Press: Ithaca, NY, USA, 1989; pp. 355–378. [Google Scholar]
- MacNulty, D.R.; Smith, D.W.; Mech, L.D.; Vucetich, J.A.; Packer, C. Nonlinear effects of group size on the success of wolves hunting elk. Behav. Ecol. 2012, 23, 75–82. [Google Scholar] [CrossRef]
- Treves, A.; Jurewicz, R.R.; Naughton-Treves, L.; Rose, R.A.; Willging, R.C.; Wydeven, A.P. Wolf depredation on domestic animals in Wisconsin, 1976–2000. Wildl. Soc. Bull. 2002, 30, 231–241. [Google Scholar] [CrossRef]
- Iliopoulos, Y. Distribution and Population Estimates of the Wolf in Greece, Project Life “Lycos” NAT97-GR04249: Conservation of the Wolf (Canis lupus L.) and Its Habitats in Greece; Arcturos: Thessaloniki, Greece, 1999; 86p. (In Greek) [Google Scholar]
- Garrott, R.A.; Bruggeman, J.E.; Becker, M.S.; Kalinowski, S.T.; White, P.J. Evaluating prey switching in wolf–ungulate systems. Ecol. Appl. 2007, 17, 1588–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, T.; Gazzola, A.; Chiriac, S.; Rîșnoveanu, G. Wolf diet and prey selection in the South-Eastern Carpathian Mountains, Romania. PLoS ONE 2019, 14, e0225424. [Google Scholar] [CrossRef] [PubMed]
- Poirazidis, K.; Kapsalis, L.; Kret, E.; Korakis, G.; Vassilakis, D.; Skartsi, D. Diachronic Recording and Mapping of the Change of Grazing Capacity in the National Park Dadia-Lefkimmi-Soufli. In Proceedings of the 9th Conference of the Hellenic Range and Pasture Society, Larissa, Greece, 9–12 October 2018; pp. 310–316. [Google Scholar]
- Janeiro-Otero, A.; Newsome, T.M.; Van Eeden, L.M.; Ripple, W.J.; Dormann, C.F. Grey wolf (Canis lupus) predation on livestock in relation to prey availability. Biol. Conserv. 2020, 243, 108433. [Google Scholar] [CrossRef]
- Mattioli, L.; Capitani, C.; Avanzinelli, E.; Bertelli, I.; Gazzola, A.; Apollonio, M. Predation by wolves (Canis lupus) on roe deer (Capreolus capreolus) in north-eastern Apennine, Italy. J. Zool. 2004, 264, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Torretta, E.; Serafini, M.; Imbert, C.; Milanesi, P.; Meriggi, A. Wolves and wild ungulates in the Ligurian Alps (Western Italy): Prey selection and spatial-temporal interactions. Mammalia 2017, 81, 81. [Google Scholar] [CrossRef]
- Holzapfel, M.; Wagner, C.; Kluth, G.; Reinhardt, I.; Ansorge, H. Feeding ecology of the wolf Canis lupus in Germany—Results from the last ten years. Beiträge Jagd. Wildforsch. 2011, 36, 117–128. [Google Scholar]
- Sand, H.; Eklund, A.; Zimmermann, B.; Wikenros, C.; Wabakken, P. Prey Selection of Scandinavian Wolves: Single Large or Several Small? PLoS ONE 2016, 11, e0168062. [Google Scholar] [CrossRef]
- Tsachalidis, E.; Hadjisterkotis, E. Current distribution and population status of wild boar (Sus scrofa L.) in Greece. Acta Silv. Lignaria Hung. 2009, 5, 153–157. [Google Scholar]
- Meriggi, A.; Dagradi, V.; Dondina, O.; Perversi, M.; Milanesi, P.; Lombardini, M.; Raviglione, S.; Repossi, A. Short-term responses of wolf feeding habits to changes of wild and domestic ungulate abundance in Northern Italy. Ethol. Ecol. Evol. 2015, 27, 389–411. [Google Scholar] [CrossRef]
- Imbert, C.; Caniglia, R.; Fabbri, E.; Milanesi, P.; Randi, E.; Serafini, M.; Torretta, E.; Meriggi, A. Why do wolves eat livestock? Factors influencing wolf diet in northern Italy. Biol. Conserv. 2016, 195, 156–168. [Google Scholar] [CrossRef]
- Ballard, W.B.; Carbyn, L.N.; Smith, D.W. Wolf Interactions with Non-Prey. In Wolves: Ecology, Behavior and Conservation; Mech, L.D., Boitani, L., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 259–271. [Google Scholar]
- Galaverni, M.; Palumbo, D.; Fabbri, E.; Caniglia, R.; Greco, C.; Randi, E. Monitoring wolves (Canis lupus) by non-invasive genetics and camera trapping: A small-scale pilot study. Eur. J. Wildl. Res. 2012, 58, 47–58. [Google Scholar] [CrossRef]
- Viola, P.; Adriani, S.; Rossi, C.; Franceschini, C.; Primi, R.; Apollonio, M.; Amici, A. Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season. Animals 2021, 11, 1895. [Google Scholar] [CrossRef]
- Kauffman, M.J.; Varley, N.; Smith, D.W.; Stahler, D.R.; MacNulty, D.R.; Boyce, M. Landscape heterogeneity shapes predation in a newly restored predator?prey system. Ecol. Lett. 2007, 10, 690–700. [Google Scholar] [CrossRef]
- Triantakonstantis, D.P.; Kollias, V.J.; Kalivas, D.P. Forest Re-growth Since 1945 in the Dadia Forest Nature Reserve in Northern Greece. New For. 2006, 32, 51–69. [Google Scholar] [CrossRef]
- Pavlov, P.; HoneB, J. The Behaviour of Feral Pigs, Sus scrofa, in Flocks of Lambing Ewes. Wildl. Res. 1982, 9, 101–109. [Google Scholar] [CrossRef]
- Choquenot, D.; Lukins, B.; Curran, G. Assessing Lamb Predation by Feral Pigs in Australia’s Semi-Arid Rangelands. J. Appl. Ecol. 1997, 34, 1445. [Google Scholar] [CrossRef]
- Ingendaay, C.; Burger, M.; Linzmann, H.; Brunnberg, L. Injuries in the dog due to wild boar. Kleintierpraxis 2008, 53, 13–23. [Google Scholar]
- Mayer, J. Wild Pig Attacks on Humans. In Wildlife Damage Management Conferences—Proceedings; Armstrong, J.B., Gallagher, G.R., Eds.; The Internet Center for Wildlife Damage Management: Ft. Collins, Co, USA, 2013; pp. 17–35. [Google Scholar]
- Wilson, C.J. Feral Wild Boar in England: Status, Impact and Management; Department for Environment, Food and Rural Affairs: London, UK, 2008. [Google Scholar]
- Thurfjell, H.; Spong, G.; Ericsson, G. Effects of hunting on wild boarSus scrofabehaviour. Wildl. Biol. 2013, 19, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Selva, N.; Jędrzejewska, B.; Jędrzejewski, W.; Wajrak, A. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can. J. Zool. 2005, 83, 1590–1601. [Google Scholar] [CrossRef]
- Ballari, A.S.; Barrios-Garcia, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mamm. Rev. 2013, 44, 124–134. [Google Scholar] [CrossRef]
- López-Bao, J.V.; Frank, J.; Svensson, L.; Åkesson, M.; Langefors, Å. Building public trust in compensation programs through accuracy assessments of damage verification protocols. Biol. Conserv. 2017, 213, 36–41. [Google Scholar] [CrossRef]
- Mikkelsen, L.; Rigét, F.F.; Kyhn, L.A.; Sveegaard, S.; Dietz, R.; Tougaard, J.; Carlström, J.A.K.; Carlén, I.; Koblitz, J.C.; Teilmann, J. Comparing Distribution of Harbour Porpoises (Phocoena phocoena) Derived from Satellite Telemetry and Passive Acoustic Monitoring. PLoS ONE 2016, 11, e0158788. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized Maxent Model Predictions of Climate Change Impacts on the Suitable Distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Low, B.W.; Yeo, D.C. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, B.W.; Zeng, Y.; Tan, H.H.; Yeo, D.C. Predictor complexity and feature selection affect Maxent model transferability: Evidence from global freshwater invasive species. Divers. Distrib. 2021, 27, 497–511. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions, Monographs in Population Biology 49; Princeton University Press: Princeton, NJ, USA, 2011; ISBN 9780691136882. [Google Scholar]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
| Number of Dogs | ||||
|---|---|---|---|---|
| Interaction | “Verified” | “Probable” | “Alleged-Claimed” | Total |
| Only approach and attack | 10 | 2 | 1 | 13 |
| Injury | 6 | 1 | 1 | 8 |
| Death | 34 | 41 | 35 | 110 |
| Total sum | 50 | 44 | 37 | 131 |
| B | Standard Error | 95% Wald C.I | ||||
|---|---|---|---|---|---|---|
| Lower | Upper | Wald X2 | Sig. | |||
| Gunshots as reaction | −2.719 | 0.743 | −4.176 | −1.262 | 13.383 | 0.000 |
| Other hunter reaction | −3.167 | 1.051 | −5.227 | −1.106 | 9.075 | 0.003 |
| Hunting hare × Distance from hunter (km) | 3.032 | 1.217 | 0.646 | 5.417 | 6.205 | 0.013 |
| Number of wolves appeared | 0.272 | 0.1293 | 0.019 | 0.525 | 4.430 | 0.035 |
| GPS use on dogs | −0.829 | 0.491 | −1.792 | 0.133 | 2.850 | 0.091 |
| Parameter | B | Std. Error | 95% Wald CI | Exp(B) | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald X2 | Sig. | ||||
| (Intercept) | 0.942 | 0.198 | 0.554 | 1.330 | 22.610 | 0.000 | 2.565 |
| Hunting and training effort (days) | −0.007 | 0.002 | −0.011 | −0.003 | 12.193 | 0.000 | 0.993 |
| Hare hunting | 0.226 | 0.093 | 0.043 | 0.409 | 5.879 | 0.015 | 1.254 |
| Hunting experience (years) | −0.009 | 0.004 | −0.016 | −0.001 | 5.494 | 0.019 | 0.991 |
| Participation in teams | −0.172 | 0.116 | −0.401 | 0.056 | 2.187 | 0.139 | 0.842 |
| Wolf Attacks to Livestock | Livestock Density | Average Dog Predation Risk | Cumulative Dog Predation Risk | Cumulative Hunting Effort | ||
|---|---|---|---|---|---|---|
| Correlation | −0.454 * | −0.235 | 0.374 ** | 0.737 ** | 0.704 ** | |
| Significance (two-tailed) | 0.017 | 0.239 | 0.050 | 0.000 | 0.000 | |
| Bootstrap | Bias | 0.061 | 0.022 | 0.015 | −0.018 | −0.025 |
| Std. Error | 0.264 | 0.195 | 0.131 | 0.113 | 0.165 | |
| 95% C.I | Lower | −0.745 | −0.525 | 0.112 | 0.442 | 0.222 |
| Upper | 0.300 | 0.235 | 0.628 | 0.884 | 0.882 | |
| Parameter | B | Std. Error | 95% Wald C.I | |||
|---|---|---|---|---|---|---|
| Lower | Upper | Wald X2 | Sig. | |||
| “Verified-Probable” interactions | ||||||
| (Intercept) | −0.693 | 0.866 | −2.391 | 1.004 | 0.641 | 0.423 |
| Wolf reproduction present | 2.079 | 1.000 | 0.119 | 4.039 | 4.324 | 0.038 |
| “Verified” interactions | ||||||
| (Intercept) | −18.767 | 9.1812 | −36.762 | −0.772 | 4.178 | 0.041 |
| Number of adult wolves | 2.334 | 1.046 | 0.283 | 4.385 | 4.975 | 0.026 |
| Maxent average predation risk | 27.68 | 14.13 | −0.015 | 55.38 | 3.837 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliopoulos, Y.; Antoniadi, E.; Kret, E.; Zakkak, S.; Skartsi, T. Wolf–Hunting Dog Interactions in a Biodiversity Hot Spot Area in Northern Greece: Preliminary Assessment and Implications for Conservation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas. Animals 2021, 11, 3235. https://doi.org/10.3390/ani11113235
Iliopoulos Y, Antoniadi E, Kret E, Zakkak S, Skartsi T. Wolf–Hunting Dog Interactions in a Biodiversity Hot Spot Area in Northern Greece: Preliminary Assessment and Implications for Conservation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas. Animals. 2021; 11(11):3235. https://doi.org/10.3390/ani11113235
Chicago/Turabian StyleIliopoulos, Yorgos, Eirini Antoniadi, Elzbieta Kret, Sylvia Zakkak, and Theodora Skartsi. 2021. "Wolf–Hunting Dog Interactions in a Biodiversity Hot Spot Area in Northern Greece: Preliminary Assessment and Implications for Conservation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas" Animals 11, no. 11: 3235. https://doi.org/10.3390/ani11113235
APA StyleIliopoulos, Y., Antoniadi, E., Kret, E., Zakkak, S., & Skartsi, T. (2021). Wolf–Hunting Dog Interactions in a Biodiversity Hot Spot Area in Northern Greece: Preliminary Assessment and Implications for Conservation in the Dadia-Lefkimi-Soufli Forest National Park and Adjacent Areas. Animals, 11(11), 3235. https://doi.org/10.3390/ani11113235






