Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview of CanL
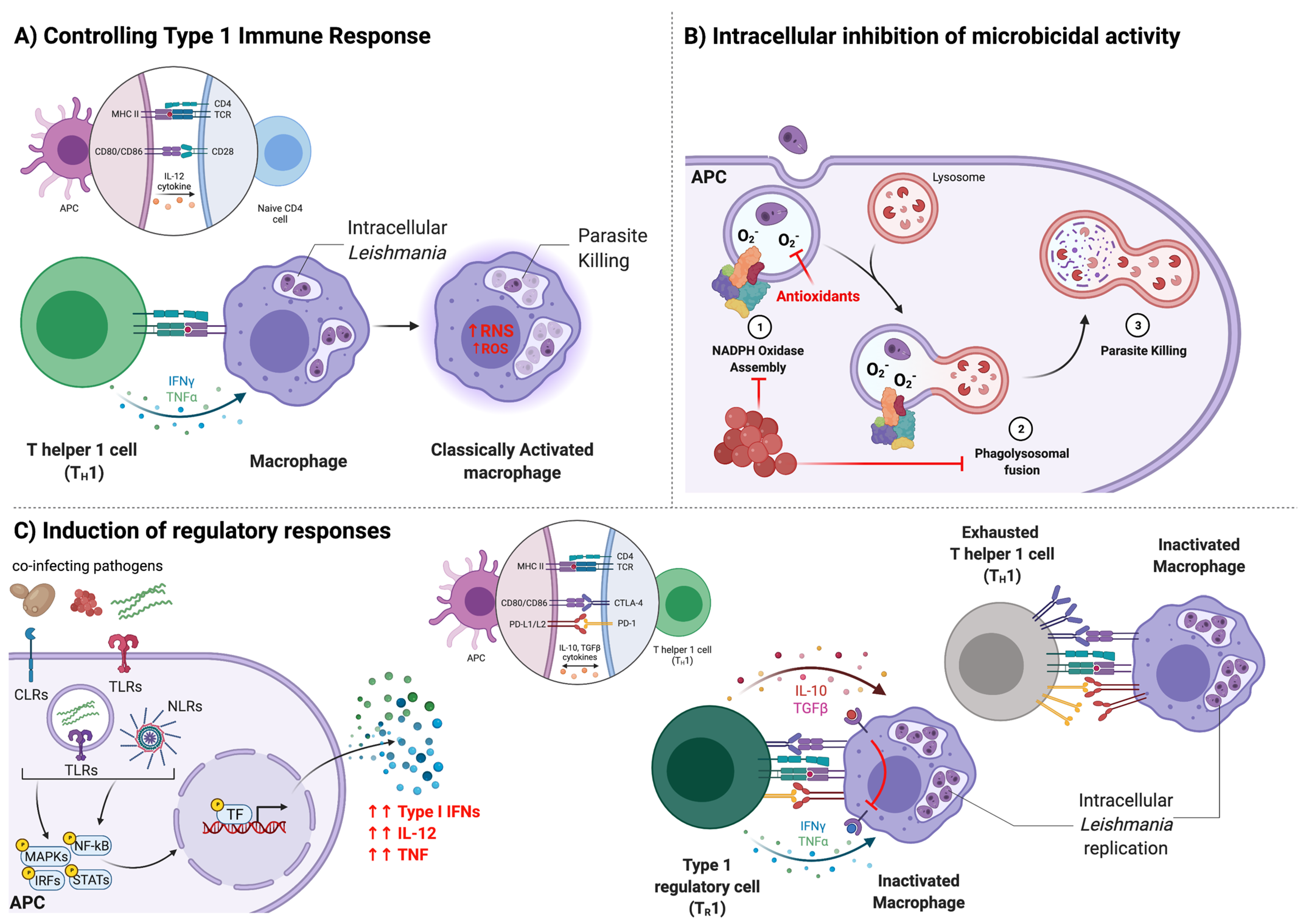
3. Immune Responses during CanL
4. Bacterial Co-Infections
4.1. Ehrlichia spp.
4.1.1. Microbe and Epidemiology
4.1.2. Clinical Disease and Biochemical Findings
4.1.3. Immunological Effects
4.2. Anaplasma spp.
4.2.1. Microbe and Epidemiology
4.2.2. Clinical Disease and Hematologic Findings
4.2.3. Immunological Effects
4.3. Borrelia spp.
4.3.1. Microbe and Epidemiology
4.3.2. Clinical Disease
4.3.3. Immunological Effects
5. Protozoal Co-Infections
5.1. Babesia spp.
5.1.1. Microbe and Epidemiology
5.1.2. Clinical Disease and Biochemical Findings
5.1.3. Hematologic and Immunological Effects
5.2. Trypanosoma cruzi
5.2.1. Microbe and Epidemiology
5.2.2. Diagnostic Challenges and Immunologic Effects
5.3. Toxoplasma gondii
5.3.1. Microbe and Epidemiology
5.3.2. Immunological Effects
6. Helminthic Co-Infections
6.1. Helminthes
6.2. Dirofilaria immitis
6.2.1. Epidemiology and Clinical Disease
6.2.2. Immunological Effects
7. Fungal Co-Infection
7.1. Paracoccidioides brasiliensis
7.1.1. Epidemiology and Clinical Disease
7.1.2. Immunological Effects
8. Effects on Diagnosis and Consideration of Cross-Reactions
9. Treatment Implications and Complexities
10. Prevention Strategies
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine Sandflies and the Spreading of Leishmaniases and Other Diseases of Public Health Concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Bourdeau, P.; Rowton, E.; Petersen, C. Impact of Different Leishmania Reservoirs on Sand Fly Transmission: Perspectives from Xenodiagnosis and Other One Health Observations. Vet. Parasitol. 2020, 287, 109237. [Google Scholar] [CrossRef]
- Coutinho-Abreu, I.V.; Sonoda, I.V.; Fonseca, J.A.; Melo, M.A.; Balbino, V.Q.; Ramalho-Ortigão, M. Lutzomyia longipalpis s.l. in Brazil and the Impact of the Sao Francisco River in the Speciation of This Sand Fly Vector. Parasites Vectors 2008, 1, 16. [Google Scholar] [CrossRef]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal Dynamics of Phlebotomine Sand Fly Species Proven Vectors of Mediterranean Leishmaniasis Caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef]
- Maia, C.; Cardoso, L. Spread of Leishmania infantum in Europe with Dog Travelling. Vet. Parasitol. 2015, 213, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Schaut, R.G.; Scott, B.D.; Mathur, D.; Berens, A.J.; Petersen, C.A. Leishmania Incidence and Prevalence in U.S. Hunting Hounds Maintained via Vertical Transmission. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Bennett, C.; Scott, B.; Senesac, R.; Oleson, J.J.; Petersen, C.A. Maternal Leishmania infantum Infection Status Has Significant Impact on Leishmaniasis in Offspring. PLoS Negl. Trop. Dis. 2019, 13, e0007058. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet Guidelines for the Practical Management of Canine Leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Attipa, C.; Solano-Gallego, L.; Papasouliotis, K.; Soutter, F.; Morris, D.; Helps, C.; Carver, S.; Tasker, S. Association between Canine Leishmaniosis and Ehrlichia canis Co-Infection: A Prospective Case-Control Study. Parasites Vectors 2018, 11, 184. [Google Scholar] [CrossRef]
- Toepp, A.J.; Monteiro, G.R.G.; Coutinho, J.F.V.; Lima, A.L.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Mahachi, K.; Anderson, B.; et al. Comorbid Infections Induce Progression of Visceral Leishmaniasis. Parasites Vectors 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Attipa, C.; Hicks, C.A.E.; Barker, E.N.; Christodoulou, V.; Neofytou, K.; Mylonakis, M.E.; Siarkou, V.I.; Vingopoulou, E.I.; Soutter, F.; Chochlakis, D.; et al. Canine Tick-Borne Pathogens in Cyprus and a Unique Canine Case of Multiple Co-Infections. Ticks Tick-Borne Dis. 2017, 8, 341–346. [Google Scholar] [CrossRef]
- Baxarias, M.; Álvarez-Fernández, A.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Ordeix, L.; Rojas, A.; Nachum-Biala, Y.; Baneth, G.; Solano-Gallego, L. Does Co-Infection with Vector-Borne Pathogens Play a Role in Clinical Canine Leishmaniosis? Parasites Vectors 2018, 11, 135. [Google Scholar] [CrossRef]
- Beall, M.J.; Alleman, A.R.; Breitschwerdt, E.B.; Cohn, L.A.; Couto, C.G.; Dryden, M.W.; Guptill, L.C.; Iazbik, C.; Kania, S.A.; Lathan, P.; et al. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in Dogs in North America. Parasites Vectors 2012, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Anziani, O.S.; Ewing, S.A.; Barker, R.W. Experimental Transmission of a Granulocytic Form of the Tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to Dogs. Am. J. Vet. Res. 1990, 51, 929–931. [Google Scholar] [PubMed]
- Groves, M.G.; Dennis, G.L.; Amyx, H.L.; Huxsoll, D.L. Transmission of Ehrlichia canis to Dogs by Ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 1975, 36, 937–940. [Google Scholar] [PubMed]
- Dantas-Torres, F. Canine Vector-Borne Diseases in Brazil. Parasites Vectors 2008, 1, 25. [Google Scholar] [CrossRef]
- Anderson, B.E.; Greene, C.E.; Jones, D.C.; Dawson, J.E. NOTES: Ehrlichia ewingii Sp. Nov., the Etiologic Agent of Canine Granulocytic Ehrlichiosis. Int. J. Syst. Bacteriol. 1992, 42, 299–302. [Google Scholar] [CrossRef]
- Carrade, D.D.; Foley, J.E.; Borjesson, D.L.; Sykes, J.E. Canine Granulocytic Anaplasmosis: A Review. J. Vet. Intern. Med. 2009, 23, 1129–1141. [Google Scholar] [CrossRef]
- Milutinović, M.; Masuzawa, T.; Tomanović, S.; Radulović, Ž.; Fukui, T.; Okamoto, Y. Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and Their Co-Infections in Host-Seeking Ixodes ricinus Ticks Collected in Serbia. Exp. Appl. Acarol. 2008, 45, 171–183. [Google Scholar] [CrossRef]
- Attipa, C.; Solano-Gallego, L.; Leutenegger, C.M.; Papasouliotis, K.; Soutter, F.; Balzer, J.; Carver, S.; Buch, J.S.; Tasker, S. Associations between Clinical Canine Leishmaniosis and Multiple Vector-Borne Co-Infections: A Case-Control Serological Study. BMC Vet. Res. 2019, 15, 331. [Google Scholar] [CrossRef]
- Burgdorfer, W. Discovery of the Lyme Disease Spirochete and Its Relation to Tick Vectors. Yale J. Biol. Med. 1984, 57, 515–520. [Google Scholar] [PubMed]
- James, M.C.; Gilbert, L.; Bowman, A.S.; Forbes, K.J. The Heterogeneity, Distribution, and Environmental Associations of Borrelia burgdorferi sensu lato, the Agent of Lyme Borreliosis, in Scotland. Front. Public Health 2014, 2, 129. [Google Scholar] [CrossRef] [PubMed]
- Leschnik, M. Canine Borreliosis: Are We Facing the Facts? Vet. J. 2014, 199, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Zygner, W.; Jaros, S.; Wędrychowicz, H. Prevalence of Babesia canis, Borrelia afzelii, and Anaplasma phagocytophilum Infection in Hard Ticks Removed from Dogs in Warsaw (Central Poland). Vet. Parasitol. 2008, 153, 139–142. [Google Scholar] [CrossRef]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Cassini, R.; Zanutto, S.; di Regalbono, A.F.; Gabrielli, S.; Calderini, P.; Moretti, A.; Tampieri, M.P.; Pietrobelli, M. Canine Piroplasmosis in Italy: Epidemiological Aspects in Vertebrate and Invertebrate Hosts. Vet. Parasitol. 2009, 165, 30–35. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A Review of Canine Babesiosis: The European Perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Figueredo, L.A. Canine Babesiosis: A Brazilian Perspective. Vet. Parasitol. 2006, 141, 197–203. [Google Scholar] [CrossRef]
- Groves, M.G.; Dennis, G.L. Babesia gibsoni: Field and Laboratory Studies of Canine Infections. Exp. Parasitol. 1972, 31, 153–159. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Meyers, A.C.; Meinders, M.; Hamer, S.A. Widespread Trypanosoma cruzi Infection in Government Working Dogs along the Texas-Mexico Border: Discordant Serology, Parasite Genotyping and Associated Vectors. PLoS Negl. Trop. Dis. 2017, 11, e0005819. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas’ Disease in the United States. Clin. Microbiol. Rev. 2011, 24, 655–681. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Snowden, K.F.; Dominguez, B.; Dinges, L.; Rodgers, S.; Mays, G.; Hamer, S.A. Epidemiology and Molecular Typing of Trypanosoma cruzi in Naturally-Infected Hound Dogs and Associated Triatomine Vectors in Texas, USA. PLoS Negl. Trop. Dis. 2017, 11, e0005298. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Hamer, S.A.; Lane, S.; Levy, M.Z.; Hamer, G.L. Bionomics and Spatial Distribution of Triatomine Vectors of Trypanosoma cruzi in Texas and Other Southern States, USA. Am. J. Trop. Med. Hyg. 2018, 98, 113–121. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Auckland, L.D.; Snowden, K.F.; Hamer, G.L.; Hamer, S.A. Analysis of over 1500 Triatomine Vectors from across the US, Predominantly Texas, for Trypanosoma cruzi Infection and Discrete Typing Units. Infect. Genet. Evol. 2018, 58, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, E.; de Aguilar, A.V.G.; de Ramírez, M.A.H.; Chévez, J.E.R.; Nakagawa, J.; Cedillos, R.A.; Kita, K. Acute Chagas Disease in El Salvador 2000–2012—Need for Surveillance and Control. Memórias do Instituto Oswaldo Cruz 2014, 109, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, R.E.; Cécere, M.C.; Petersen, R.M.; Rubel, D.N.; Schweigmann, N.J. Chagas Disease in North-West Argentina: Association between Trypanosoma cruzi Parasitaemia in Dogs and Cats and Infection Rates in Domestic Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 12–15. [Google Scholar] [CrossRef]
- Alvedro, A.; Gaspe, M.S.; Milbourn, H.; Macchiaverna, N.P.; Laiño, M.A.; Enriquez, G.F.; Gürtler, R.E.; Cardinal, M.V. Trypanosoma cruzi Infection in Triatoma infestans and High Levels of Human–Vector Contact across a Rural-to-Urban Gradient in the Argentine Chaco. Parasites Vectors 2021, 14, 35. [Google Scholar] [CrossRef]
- Lehmann, T.; Marcet, P.L.; Graham, D.H.; Dahl, E.R.; Dubey, J.P. Globalization and the Population Structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 11423–11428. [Google Scholar] [CrossRef] [PubMed]
- Valadas, S.; Minervino, A.H.H.; Lima, V.M.F.; Soares, R.M.; Ortolani, E.L.; Gennari, S.M. Occurrence of Antibodies Anti-Neospora caninum, Anti-Toxoplasma gondii, and Anti-Leishmania chagasi in Serum of Dogs from Pará State, Amazon, Brazil. Parasitol. Res. 2010, 107, 453–457. [Google Scholar] [CrossRef]
- Tabar, M.D.; Altet, L.; Martínez, V.; Roura, X. Wolbachia, Filariae and Leishmania Coinfection in Dogs from a Mediterranean Area. J. Small Anim. Pract. 2013, 54, 174–178. [Google Scholar] [CrossRef]
- Maia, C.; Altet, L.; Serrano, L.; Cristóvão, J.M.; Tabar, M.D.; Francino, O.; Cardoso, L.; Campino, L.; Roura, X. Molecular Detection of Leishmania infantum, Filariae and Wolbachia spp. in Dogs from Southern Portugal. Parasites Vectors 2016, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nakagaki, K.; Nogami, S.; Harasawa, R.; Maeda, R.; Katae, H.; Hayashi, Y. Immunologic Protection against Canine Heartworm Infection. J. Vet. Med. Sci. 1997, 59, 1115–1121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ledesma, N.; Harrington, L. Mosquito Vectors of Dog Heartworm in the United States: Vector Status and Factors Influencing Transmission Efficiency. Top. Companion Anim. Med. 2011, 26, 178–185. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Dirofilariosis in the Americas: A More Virulent Dirofilaria Immitis? Parasites Vectors 2013, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm Disease (Dirofilaria Immitis) and Their Vectors in Europe—New Distribution Trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef]
- Ricci, G.; Mota, F.T.; Wakamatsu, A.; Serafim, R.C.; Borra, R.C.; Franco, M. Canine Paracoccidioidomycosis. Med. Mycol. 2004, 42, 379–383. [Google Scholar] [CrossRef]
- Toepp, A.J.; Petersen, C.A. The Balancing Act: Immunology of Leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing Canine Vector-Borne Diseases of Zoonotic Concern: Part One. Trends Parasitol. 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Llull, J.; Ramos, G.; Riera, C.; Arboix, M.; Alberola, J.; Ferrer, L. The Ibizian Hound Presents a Predominantly Cellular Immune Response against Natural Leishmania Infection. Vet. Parasitol. 2000, 90, 37–45. [Google Scholar] [CrossRef]
- Sanchez-Robert, E.; Altet, L.; Sanchez, A.; Francino, O. Polymorphism of Slc11a1 (Nramp1) Gene and Canine Leishmaniasis in a Case-Control Study. J. Hered. 2005, 96, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, P.; Oliva, G.; De Luna, R.; Ambrosio, R.; Cortese, L.; Persechino, A.; Gradoni, L.; Scalone, A. A Retrospective Clinical Study of Canine Leishmaniasis in 150 Dogs Naturally Infected by Leishmania infantum. Vet. Rec. 1997, 141, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Santos, R.; Oliveira, R.; Costa, L.; Prata, A.; Gonçalves, V.; Roquette, M.; Vala, H.; Santos-Gomes, G. Prognostic Factors and Life Expectancy in Canine Leishmaniosis. Vet. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic Challenges in the Era of Canine Leishmania infantum Vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef]
- Barbiéri, C.L. Immunology of Canine Leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on Adaptive and Innate Immunity in Canine Leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef]
- Thalhofer, C.J.; Chen, Y.; Sudan, B.; Love-Homan, L.; Wilson, M.E. Leukocytes Infiltrate the Skin and Draining Lymph Nodes in Response to the Protozoan Leishmania infantum chagasi. Infect. Immun. 2011, 79, 108–117. [Google Scholar] [CrossRef]
- Moradin, N.; Descoteaux, A. Leishmania Promastigotes: Building a Safe Niche within Macrophages. Front. Cell. Infect. Microbiol. 2012, 2, 121. [Google Scholar] [CrossRef]
- Liu, D.; Uzonna, J.E. The Early Interaction of Leishmania with Macrophages and Dendritic Cells and Its Influence on the Host Immune Response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- McConville, M.J.; de Souza, D.; Saunders, E.; Likic, V.A.; Naderer, T. Living in a Phagolysosome; Metabolism of Leishmania Amastigotes. Trends Parasitol. 2007, 23, 368–375. [Google Scholar] [CrossRef]
- Van Assche, T.; Deschacht, M.; da Luz, R.A.I.; Maes, L.; Cos, P. Leishmania-Macrophage Interactions: Insights into the Redox Biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, E.; Gebhard, D.; Mommaas, A.M.; van Hoeij, M.; Langermans, J.A.; Ruitenberg, E.J.; Rutten, V.P. Infection of a Canine Macrophage Cell Line with Leishmania infantum: Determination of Nitric Oxide Production and Anti-Leishmanial Activity. Vet. Parasitol. 2000, 92, 181–189. [Google Scholar] [CrossRef]
- Panaro, M.A.; Brandonisio, O.; de Caprariis, D.; Cavallo, P.; Cianciulli, A.; Mitolo, V.; Otranto, D. Canine Leishmaniasis in Southern Italy: A Role for Nitric Oxide Released from Activated Macrophages in Asymptomatic Infection? Parasites Vectors 2008, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Mechanisms and Consequences of Persistence of Intracellular Pathogens: Leishmaniasis as an Example. Cell. Microbiol. 2008, 10, 1221–1234. [Google Scholar] [CrossRef]
- Gupta, G.; Oghumu, S.; Satoskar, A.R. Mechanisms of Immune Evasion in Leishmaniasis. Adv. Appl. Microbiol. 2013, 82, 155–184. [Google Scholar] [CrossRef]
- De Freitas, E.O.; de Souza Leoratti, F.M.; Freire-de-Lima, C.G.; Morrot, A.; Feijó, D.F. The Contribution of Immune Evasive Mechanisms to Parasite Persistence in Visceral Leishmaniasis. Front. Immunol. 2016, 7, 153. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to Master the Host Immune System? Leishmania Parasites Have the Solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef]
- Ueno, N.; Bratt, C.L.; Rodriguez, N.E.; Wilson, M.E. Differences in Human Macrophage Receptor Usage, Lysosomal Fusion Kinetics and Survival between Logarithmic and Metacyclic Leishmania infantum Chagasi Promastigotes. Cell. Microbiol. 2009, 11, 1827–1841. [Google Scholar] [CrossRef]
- Rodríguez, N.E.; Gaur, U.; Wilson, M.E. Role of Caveolae in Leishmania chagasi Phagocytosis and Intracellular Survival in Macrophages. Cell. Microbiol. 2006, 8, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.D.; Gedamu, L. Role of Peroxidoxins in Leishmania chagasi Survival. Evidence of an Enzymatic Defense against Nitrosative Stress. J. Biol. Chem. 2003, 278, 10816–10823. [Google Scholar] [CrossRef]
- Plewes, K.A.; Barr, S.D.; Gedamu, L. Iron Superoxide Dismutases Targeted to the Glycosomes of Leishmania chagasi Are Important for Survival. Infect. Immun. 2003, 71, 5910–5920. [Google Scholar] [CrossRef] [PubMed]
- Longoni, S.S.; Sánchez-Moreno, M.; López, J.E.R.; Marín, C. Leishmania infantum Secreted Iron Superoxide Dismutase Purification and Its Application to the Diagnosis of Canine Leishmaniasis. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nylén, S. Immunobiology of Visceral Leishmaniasis. Front. Immunol. 2012, 3, 251. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Juelsgaard, R.; Martinez, P.A.; Jones, D.E.; Petersen, C.A. Programmed Death 1-Mediated T Cell Exhaustion during Visceral Leishmaniasis Impairs Phagocyte Function. J. Immunol. 2013, 191, 5542–5550. [Google Scholar] [CrossRef]
- Lage, R.S.; Oliveira, G.C.; Busek, S.U.; Guerra, L.L.; Giunchetti, R.C.; Corrêa-Oliveira, R.; Reis, A.B. Analysis of the Cytokine Profile in Spleen Cells from Dogs Naturally Infected by Leishmania chagasi. Vet. Immunol. Immunopathol. 2007, 115, 135–145. [Google Scholar] [CrossRef]
- Turchetti, A.P.; da Costa, L.F.; de Lima Romão, E.; Fujiwara, R.T.; da Paixão, T.A.; Santos, R.L. Transcription of Innate Immunity Genes and Cytokine Secretion by Canine Macrophages Resistant or Susceptible to Intracellular Survival of Leishmania infantum. Vet. Immunol. Immunopathol. 2015, 163, 67–76. [Google Scholar] [CrossRef]
- Strauss-Ayali, D.; Baneth, G.; Shor, S.; Okano, F.; Jaffe, C.L. Interleukin-12 Augments a Th1-Type Immune Response Manifested as Lymphocyte Proliferation and Interferon Gamma Production in Leishmania infantum-Infected Dogs. Int. J. Parasitol. 2005, 35, 63–73. [Google Scholar] [CrossRef]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; del Real, G.; Ruitenberg, J. Cellular and Humoral Immune Responses in Dogs Experimentally and Naturally Infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [CrossRef]
- Pinelli, E.; van der Kaaij, S.Y.; Slappendel, R.; Fragio, C.; Ruitenberg, E.J.; Bernadina, W.; Rutten, V.P. Detection of Canine Cytokine Gene Expression by Reverse Transcription-Polymerase Chain Reaction. Vet. Immunol. Immunopathol. 1999, 69, 121–126. [Google Scholar] [CrossRef]
- Park, A.Y.; Hondowicz, B.D.; Scott, P. IL-12 Is Required to Maintain a Th1 Response during Leishmania major Infection. J. Immunol. 2000, 165, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kima, P.E.; Soong, L. Interferon Gamma in Leishmaniasis. Front. Immunol. 2013, 4, 156. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Maia, C.; Campino, L. Methods for Diagnosis of Canine Leishmaniasis and Immune Response to Infection. Vet. Parasitol. 2008, 158, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Montserrrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-Specific Production of IFN-γ and IL-10 in Stimulated Blood from Dogs with Clinical Leishmaniosis. Parasites Vectors 2016, 9, 317. [Google Scholar] [CrossRef]
- Nylén, S.; Sacks, D. Interleukin-10 and the Pathogenesis of Human Visceral Leishmaniasis. Trends Immunol. 2007, 28, 378–384. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-Corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic Indicators of Clinical Progression during Canine Leishmania infantum Infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of Immunity during Visceral Leishmania Infection. Parasites Vectors 2016, 9, 118. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef]
- Da Costa Vieira, R.F.; Biondo, A.W.; Guimarães, A.M.S.; dos Santos, A.P.; dos Santos, R.P.; Dutra, L.H.; de Paiva Diniz, P.P.V.; de Morais, H.A.; Messick, J.B.; Labruna, M.B.; et al. Ehrlichiosis in Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for Veterinary Practitioners on Canine Ehrlichiosis and Anaplasmosis in Europe. Parasites Vectors 2015, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Gettings, J.R.; Self, S.C.W.; McMahan, C.S.; Brown, D.A.; Nordone, S.K.; Yabsley, M.J. Local and Regional Temporal Trends (2013–2019) of Canine Ehrlichia spp. Seroprevalence in the USA. Parasites Vectors 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Laidoudi, Y.; Athias, E.; Bouam, A.; Dizoé, S.; Davoust, B.; Mediannikov, O. Molecular and Serological Detection of Animal and Human Vector-Borne Pathogens in the Blood of Dogs from Côte d’Ivoire. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101412. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, A.P.S.; Vieira, V.B.D.; Batalini, L.S.; do Carmo, S.B.; Friozi, E.; de Arruda, E.J.; da Costa Lima Junior, M.S.; Neitzke-Abreu, H.C. PCR Sensitivity of Peripheral Blood of Dogs Co-Infected with Leishmania spp. and Ehrlichia spp. in Endemic Area of Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 843–847. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Agulla, B.; Piya, B.; Fernández-Ruiz, N.; Villaescusa, A.; García-Sancho, M.; Rodríguez-Franco, F.; Sainz, Á. Stray Dogs in Nepal Have High Prevalence of Vector-Borne Pathogens: A Molecular Survey. Parasites Vectors 2020, 13, 174. [Google Scholar] [CrossRef]
- Vrhovec, M.G.; Pantchev, N.; Failing, K.; Bauer, C.; Travers-Martin, N.; Zahner, H. Retrospective Analysis of Canine Vector-Borne Diseases (CVBD) in Germany with Emphasis on the Endemicity and Risk Factors of Leishmaniosis. Parasitol. Res. 2017, 116, 131–144. [Google Scholar] [CrossRef][Green Version]
- Procajło, A.; Skupień, E.M.; Bladowski, M.; Lew, S. Monocytic Ehrlichiosis in Dogs. Pol. J. Vet. Sci. 2011, 14, 515–520. [Google Scholar] [CrossRef]
- Cardinot, C.B.; Silva, J.E.S.; Yamatogi, R.S.; Nunes, C.M.; Biondo, A.W.; Vieira, R.F.C.; Junior, J.P.A.; Marcondes, M. Detection of Ehrlichia canis, Babesia vogeli, and Toxoplasma gondii DNA in the Brain of Dogs Naturally Infected with Leishmania infantum. J. Parasitol. 2016, 102, 275–279. [Google Scholar] [CrossRef]
- De Tommasi, A.S.; Otranto, D.; Dantas-Torres, F.; Capelli, G.; Breitschwerdt, E.B.; de Caprariis, D. Are Vector-Borne Pathogen Co-Infections Complicating the Clinical Presentation in Dogs? Parasites Vectors 2013, 6, 97. [Google Scholar] [CrossRef]
- Andrade, G.B.; Barreto, W.T.G.; dos Santos, L.L.; Ribeiro, L.R.R.; de Macedo, G.C.; de Sousa, K.C.M.; André, M.R.; Machado, R.Z.; Herrera, H.M. Pathology of Dogs in Campo Grande, MS, Brazil Naturally Co-Infected with Leishmania infantum and Ehrlichia canis. Rev. Bras. Parasitol. Vet. 2014, 23, 509–515. [Google Scholar] [CrossRef]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Mastellone, V.; Manco, A.; Lombardi, P.; Ciaramella, P.; Avallone, L. Platelet Aggregation and Haemostatic Response in Dogs Naturally Co-Infected by Leishmania infantum and Ehrlichia canis: Platelet Aggregation in Canine Leishmaniasis and Ehrlichiosis. J. Vet. Med. Ser. A 2006, 53, 546–548. [Google Scholar] [CrossRef]
- Crossley, E.C.; Jordan, J.M.; Walker, D.H. Rickettsia. In International Encyclopedia of Public Health; Heggenhougen, H.K., Quah, S.R., Eds.; Academic Press: Oxford, UK, 2008; pp. 582–590. ISBN 978-0-12-373960-5. [Google Scholar]
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A Prototypical Emerging Pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Rikihisa, Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum Lack Genes for Lipid A Biosynthesis and Incorporate Cholesterol for Their Survival. Infect. Immun. 2003, 71, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Mavromatis, K.; Doyle, C.K.; Lykidis, A.; Ivanova, N.; Francino, M.P.; Chain, P.; Shin, M.; Malfatti, S.; Larimer, F.; Copeland, A.; et al. The Genome of the Obligately Intracellular Bacterium Ehrlichia canis Reveals Themes of Complex Membrane Structure and Immune Evasion Strategies. J. Bacteriol. 2006, 188, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of Microorganisms and Activation of the Immune Response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Ishii, K.J.; Koyama, S.; Nakagawa, A.; Coban, C.; Akira, S. Host Innate Immune Receptors and beyond: Making Sense of Microbial Infections. Cell Host Microbe 2008, 3, 352–363. [Google Scholar] [CrossRef]
- Kumagai, Y.; Cheng, Z.; Lin, M.; Rikihisa, Y. Biochemical Activities of Three Pairs of Ehrlichia chaffeensis Two-Component Regulatory System Proteins Involved in Inhibition of Lysosomal Fusion. Infect. Immun. 2006, 74, 5014–5022. [Google Scholar] [CrossRef][Green Version]
- Rikihisa, Y. Ehrlichia Subversion of Host Innate Responses. Curr. Opin. Microbiol. 2006, 9, 95–101. [Google Scholar] [CrossRef]
- Harrus, S.; Waner, T.; Friedmann-Morvinski, D.; Fishman, Z.; Bark, H.; Harmelin, A. Down-Regulation of MHC Class II Receptors of DH82 Cells, Following Infection with Ehrlichia canis. Vet. Immunol. Immunopathol. 2003, 96, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Rikihisa, Y. Ehrlichia chaffeensis Downregulates Surface Toll-like Receptors 2/4, CD14 and Transcription Factors PU.1 and Inhibits Lipopolysaccharide Activation of NF-Kappa B, ERK 1/2 and P38 MAPK in Host Monocytes. Cell. Microbiol. 2004, 6, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Dotsika, E.; Frydas, S.; Karagouni, E. Toll-like Receptor 4 Promotes Control of Leishmania infantum Infection through Inducement of Leishmanicidal Activity in Host Macrophages: Role of Mitogen Activated Kinases. J. Biol. Regul. Homeost. Agents 2014, 28, 41–52. [Google Scholar] [PubMed]
- Zhang, J.; Sinha, M.; Luxon, B.A.; Yu, X. Survival Strategy of Obligately Intracellular Ehrlichia chaffeensis: Novel Modulation of Immune Response and Host Cell Cycles. Infect. Immun. 2004, 72, 498–507. [Google Scholar] [CrossRef]
- Yang, Q.; Stevenson, H.L.; Scott, M.J.; Ismail, N. Type I Interferon Contributes to Noncanonical Inflammasome Activation, Mediates Immunopathology, and Impairs Protective Immunity during Fatal Infection with Lipopolysaccharide-Negative Ehrlichiae. Am. J. Pathol. 2015, 185, 446–461. [Google Scholar] [CrossRef]
- Kumar, R.; Bunn, P.T.; Singh, S.S.; Ng, S.S.; Montes de Oca, M.; De Labastida Rivera, F.; Chauhan, S.B.; Singh, N.; Faleiro, R.J.; Edwards, C.L.; et al. Type I Interferons Suppress Anti-Parasitic Immunity and Can Be Targeted to Improve Treatment of Visceral Leishmaniasis. Cell Rep. 2020, 30, 2512–2525. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, L.A.; Benevides, L.; Maruyama, S.R.; Tavares, L.; Fukutani, K.F.; Francozo, M.; Sparwasser, T.; Cunha, F.Q.; Almeida, R.P.; da Silva, J.S.; et al. TLR4 Abrogates the Th1 Immune Response through IRF1 and IFN-β to Prevent Immunopathology during L. Infantum Infection. PLoS Pathog. 2020, 16, e1008435. [Google Scholar] [CrossRef]
- Dunning Hotopp, J.C.; Lin, M.; Madupu, R.; Crabtree, J.; Angiuoli, S.V.; Eisen, J.A.; Eisen, J.; Seshadri, R.; Ren, Q.; Wu, M.; et al. Comparative Genomics of Emerging Human Ehrlichiosis Agents. PLoS Genet. 2006, 2, e21. [Google Scholar] [CrossRef]
- Lin, M.; Rikihisa, Y. Degradation of P22phox and Inhibition of Superoxide Generation by Ehrlichia chaffeensis in Human Monocytes. Cell. Microbiol. 2007, 9, 861–874. [Google Scholar] [CrossRef]
- Liu, H.; Bao, W.; Lin, M.; Niu, H.; Rikihisa, Y. Ehrlichia Type IV Secretion Effector ECH0825 Is Translocated to Mitochondria and Curbs ROS and Apoptosis by Upregulating Host MnSOD. Cell. Microbiol. 2012, 14, 1037–1050. [Google Scholar] [CrossRef]
- Xiong, Q.; Bao, W.; Ge, Y.; Rikihisa, Y. Ehrlichia ewingii Infection Delays Spontaneous Neutrophil Apoptosis through Stabilization of Mitochondria. J. Infect. Dis. 2008, 197, 1110–1118. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Jiang, Y.; Zhang, L.; Popov, V.L.; Zhang, J.; Walker, D.H.; Yu, X. Obligate Intracellular Bacterium Ehrlichia Inhibiting Mitochondrial Activity. Microbes Infect. 2011, 13, 232–238. [Google Scholar] [CrossRef][Green Version]
- Bitsaktsis, C.; Huntington, J.; Winslow, G. Production of IFN-Gamma by CD4 T Cells Is Essential for Resolving Ehrlichia Infection. J. Immunol. 2004, 172, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Barnewall, R.E.; Rikihisa, Y. Abrogation of Gamma Interferon-Induced Inhibition of Ehrlichia chaffeensis Infection in Human Monocytes with Iron-Transferrin. Infect. Immun. 1994, 62, 4804–4810. [Google Scholar] [CrossRef]
- Lee, E.H.; Rikihisa, Y. Protein Kinase A-Mediated Inhibition of Gamma Interferon-Induced Tyrosine Phosphorylation of Janus Kinases and Latent Cytoplasmic Transcription Factors in Human Monocytes by Ehrlichia chaffeensis. Infect. Immun. 1998, 66, 2514–2520. [Google Scholar] [CrossRef]
- Bichiou, H.; Bouabid, C.; Rabhi, I.; Guizani-Tabbane, L. Transcription Factors Interplay Orchestrates the Immune-Metabolic Response of Leishmania Infected Macrophages. Front. Cell. Infect. Microbiol. 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A.; Grenda, S. Bacteria of the Genus Anaplasma—Characteristics of Anaplasma and Their Vectors: A Review. Vet. Med. 2008, 53, 573–584. [Google Scholar] [CrossRef]
- Miró, G.; Montoya, A.; Roura, X.; Gálvez, R.; Sainz, A. Seropositivity Rates for Agents of Canine Vector-Borne Diseases in Spain: A Multicentre Study. Parasites Vectors 2013, 6, 117. [Google Scholar] [CrossRef]
- Da Costa Oliveira, V.; Mendes, A.A.V., Jr.; Ferreira, L.C.; Calvet, T.M.Q.; dos Santos, S.A.; Figueiredo, F.B.; Campos, M.P.; de Carvalho Rodrigues, F.d.C.; de Oliveira, R.d.V.C.; de Lemos, E.R.S.; et al. Frequency of Co-Seropositivities for Certain Pathogens and Their Relationship with Clinical and Histopathological Changes and Parasite Load in Dogs Infected with Leishmania infantum. PLoS ONE 2021, 16, e0247560. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of Obligatory Intracellular Infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Egenvall, A.; Lilliehöök, I.; Bjöersdorff, A.; Engvall, E.O.; Karlstam, E.; Artursson, K.; Heldtander, M.; Gunnarsson, A. Detection of Granulocytic Ehrlichia Species DNA by PCR in Persistently Infected Dogs. Vet. Rec. 2000, 146, 186–190. [Google Scholar] [CrossRef]
- Johns, J.L.; MacNamara, K.C.; Walker, N.J.; Winslow, G.M.; Borjesson, D.L. Infection with Anaplasma phagocytophilum Induces Multilineage Alterations in Hematopoietic Progenitor Cells and Peripheral Blood Cells. Infect. Immun. 2009, 77, 4070–4080. [Google Scholar] [CrossRef] [PubMed]
- Woldehiwet, Z. The Natural History of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Rikihisa, Y. Roles of P38 Mitogen-Activated Protein Kinase, NF-KappaB, and Protein Kinase C in Proinflammatory Cytokine MRNA Expression by Human Peripheral Blood Leukocytes, Monocytes, and Neutrophils in Response to Anaplasma phagocytophila. Infect. Immun. 2002, 70, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Carlyon, J.A.; Chan, W.-T.; Galán, J.; Roos, D.; Fikrig, E. Repression of Rac2 MRNA Expression by Anaplasma phagocytophila Is Essential to the Inhibition of Superoxide Production and Bacterial Proliferation. J. Immunol. 2002, 169, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Gokce, H.I.; Woldehiwet, Z. Lymphocyte Responses to Mitogens and Rickettsial Antigens in Sheep Experimentally Infected with Ehrlichia (Cytoecetes) phagocytophila. Vet. Parasitol. 1999, 83, 55–64. [Google Scholar] [CrossRef]
- Whist, S.K.; Storset, A.K.; Johansen, G.M.; Larsen, H.J.S. Modulation of Leukocyte Populations and Immune Responses in Sheep Experimentally Infected with Anaplasma (Formerly Ehrlichia) phagocytophilum. Vet. Immunol. Immunopathol. 2003, 94, 163–175. [Google Scholar] [CrossRef]
- Bussmeyer, U.; Sarkar, A.; Broszat, K.; Lüdemann, T.; Möller, S.; van Zandbergen, G.; Bogdan, C.; Behnen, M.; Dumler, J.S.; von Loewenich, F.D.; et al. Impairment of Gamma Interferon Signaling in Human Neutrophils Infected with Anaplasma phagocytophilum. Infect. Immun. 2010, 78, 358–363. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barat, N.C.; Barat, C.E.; Bakken, J.S. Human Granulocytic Anaplasmosis and Macrophage Activation. Clin. Infect. Dis. 2007, 45, 199–204. [Google Scholar] [CrossRef]
- Radolf, J.D.; Caimano, M.J.; Stevenson, B.; Hu, L.T. Of Ticks, Mice and Men: Understanding the Dual-Host Lifestyle of Lyme Disease Spirochaetes. Nat. Rev. Microbiol. 2012, 10, 87–99. [Google Scholar] [CrossRef]
- Little, S.; Braff, J.; Place, J.; Buch, J.; Dewage, B.G.; Knupp, A.; Beall, M. Canine Infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013-2019. Parasites Vectors 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Mahachi, K.; Kontowicz, E.; Anderson, B.; Toepp, A.J.; Lima, A.L.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Ozanne, M.; et al. Predominant Risk Factors for Tick-Borne Co-Infections in Hunting Dogs from the USA. Parasites Vectors 2020, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Littman, M.P.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM Small Animal Consensus Statement on Lyme Disease in Dogs: Diagnosis, Treatment, and Prevention. J. Vet. Intern. Med. 2006, 20, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Littman, M.P.; Gerber, B.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM Consensus Update on Lyme Borreliosis in Dogs and Cats. J. Vet. Intern. Med. 2018, 32, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Rothenberg, R.J.; Barbour, A.G. Absence of Lipopolysaccharide in the Lyme Disease Spirochete, Borrelia burgdorferi. Infect. Immun. 1987, 55, 2311–2313. [Google Scholar] [CrossRef]
- Singh, S.K.; Girschick, H.J. Toll-like Receptors in Borrelia burgdorferi-Induced Inflammation. Clin. Microbiol. Infect. 2006, 12, 705–717. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Hawley, K.L.; Benjamin, S.J.; Weinerman, B.; Luu, S.M.; Salazar, J.C. Phagosomal TLR Signaling upon Borrelia burgdorferi Infection. Front. Cell. Infect. Microbiol. 2014, 4, 55. [Google Scholar] [CrossRef]
- Strle, K.; Sulka, K.B.; Pianta, A.; Crowley, J.T.; Arvikar, S.L.; Anselmo, A.; Sadreyev, R.; Steere, A.C. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate With Borrelia burgdorferi Antibodies During Early Infection and With Autoantibodies Late in the Illness in Patients With Antibiotic-Refractory Lyme Arthritis. Clin. Infect. Dis. 2017, 64, 930–938. [Google Scholar] [CrossRef]
- Oosting, M.; Berende, A.; Sturm, P.; ter Hofstede, H.J.M.; de Jong, D.J.; Kanneganti, T.; van der Meer, J.W.M.; Kullberg, B.; Netea, M.G.; Joosten, L.A.B. Recognition of Borrelia burgdorferi by NOD2 Is Central for the Induction of an Inflammatory Reaction. J. Infect. Dis. 2010, 201, 1849–1858. [Google Scholar] [CrossRef]
- Jutras, B.L.; Lochhead, R.B.; Kloos, Z.A.; Biboy, J.; Strle, K.; Booth, C.J.; Govers, S.K.; Gray, J.; Schumann, P.; Vollmer, W.; et al. Borrelia burgdorferi Peptidoglycan Is a Persistent Antigen in Patients with Lyme Arthritis. Proc. Natl. Acad. Sci. USA 2019, 116, 13498–13507. [Google Scholar] [CrossRef]
- Petzke, M.M.; Brooks, A.; Krupna, M.A.; Mordue, D.; Schwartz, I. Recognition of Borrelia burgdorferi, the Lyme Disease Spirochete, by TLR7 and TLR9 Induces a Type I IFN Response by Human Immune Cells. J. Immunol. 2009, 183, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.C.; Duhnam-Ems, S.; Vake, C.L.; Cruz, A.R.; Moore, M.W.; Caimano, M.J.; Velez-Climent, L.; Shupe, J.; Krueger, W.; Radolf, J.D. Activation of Human Monocytes by Live Borrelia burgdorferi Generates TLR2-Dependent and -Independent Responses Which Include Induction of IFN-β. PLoS Pathog. 2009, 5, e1000444. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Dunham-Ems, S.M.; La Vake, C.J.; Petzke, M.M.; Sahay, B.; Sellati, T.J.; Radolf, J.D.; Salazar, J.C. Phagosomal Signaling by Borrelia burgdorferi in Human Monocytes Involves Toll-like Receptor (TLR) 2 and TLR8 Cooperativity and TLR8-Mediated Induction of IFN-Beta. Proc. Natl. Acad. Sci. USA 2011, 108, 3683–3688. [Google Scholar] [CrossRef] [PubMed]
- Petnicki-Ocwieja, T.; DeFrancesco, A.S.; Chung, E.; Darcy, C.T.; Bronson, R.T.; Kobayashi, K.S.; Hu, L.T. Nod2 Suppresses Borrelia burgdorferi Mediated Murine Lyme Arthritis and Carditis through the Induction of Tolerance. PLoS ONE 2011, 6, e17414. [Google Scholar] [CrossRef] [PubMed]
- Dagenais-Lussier, X.; Loucif, H.; Murira, A.; Laulhé, X.; Stäger, S.; Lamarre, A.; van Grevenynghe, J. Sustained IFN-I Expression during Established Persistent Viral Infection: A “Bad Seed” for Protective Immunity. Viruses 2017, 10, 12. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.F.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B.A. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef]
- Cavalcanti, A.S.; Ribeiro-Alves, M.; Pereira, L.D.O.; Mestre, G.L.; Ferreira, A.B.R.; Morgado, F.N.; Boité, M.C.; Cupolillo, E.; Moraes, M.O.; Porrozzi, R. Parasite Load Induces Progressive Spleen Architecture Breakage and Impairs Cytokine MRNA Expression in Leishmania infantum-Naturally Infected Dogs. PLoS ONE 2015, 10, e0123009. [Google Scholar] [CrossRef]
- Strle, F.; Nadelman, R.B.; Cimperman, J.; Nowakowski, J.; Picken, R.N.; Schwartz, I.; Maraspin, V.; Aguero-Rosenfeld, M.E.; Varde, S.; Lotric-Furlan, S.; et al. Comparison of Culture-Confirmed Erythema Migrans Caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann. Intern. Med. 1999, 130, 32–36. [Google Scholar] [CrossRef]
- Jones, K.L.; Muellegger, R.R.; Means, T.K.; Lee, M.; Glickstein, L.J.; Damle, N.; Sikand, V.K.; Luster, A.D.; Steere, A.C. Higher MRNA Levels of Chemokines and Cytokines Associated with Macrophage Activation in Erythema Migrans Skin Lesions in Patients from the United States than in Patients from Austria with Lyme Borreliosis. Clin. Infect. Dis. 2008, 46, 85–92. [Google Scholar] [CrossRef]
- Strle, K.; Drouin, E.E.; Shen, S.; El Khoury, J.; McHugh, G.; Ruzic-Sabljic, E.; Strle, F.; Steere, A.C. Borrelia burgdorferi Stimulates Macrophages to Secrete Higher Levels of Cytokines and Chemokines than Borrelia afzelii or Borrelia garinii. J. Infect. Dis. 2009, 200, 1936–1943. [Google Scholar] [CrossRef]
- Grygorczuk, S.; Świerzbińska, R.; Moniuszko, A.; Kondrusik, M.; Zajkowska, J.; Czupryna, P.; Dunaj, J.; Pancewicz, S. Synthesis of Th17 Cytokines in the Culture of Peripheral Blood Mononuclear Cells Stimulated with Borrelia burgdorferi sensu lato. Ann. Agric. Environ. Med. 2016, 23, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Rodríguez-Cortés, A.; Blake, D.P.; Allenspach, K.; Alberola, J.; Solano-Gallego, L. Transcription of Toll-Like Receptors 2, 3, 4 and 9, FoxP3 and Th17 Cytokines in a Susceptible Experimental Model of Canine Leishmania infantum Infection. PLoS ONE 2015, 10, e0140325. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.L.; Albuquerque, T.D.R.; Do-Valle-Matta, M.A.; Caldas, I.S.; Diniz, L.F.; Talvani, A.; Bahia, M.T.; Andrade, C.M.; Galvão, L.M.C.; Câmara, A.C.J.; et al. Naturally Leishmania infantum-Infected Dogs Display an Overall Impairment of Chemokine and Chemokine Receptor Expression during Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2013, 153, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lopez Kostka, S.; Dinges, S.; Griewank, K.; Iwakura, Y.; Udey, M.C.; von Stebut, E. IL-17 Promotes Progression of Cutaneous Leishmaniasis in Susceptible Mice. J. Immunol. 2009, 182, 3039–3046. [Google Scholar] [CrossRef]
- Gonzalez-Lombana, C.; Gimblet, C.; Bacellar, O.; Oliveira, W.W.; Passos, S.; Carvalho, L.P.; Goldschmidt, M.; Carvalho, E.M.; Scott, P. IL-17 Mediates Immunopathology in the Absence of IL-10 Following Leishmania major Infection. PLoS Pathog. 2013, 9, e1003243. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Yisaschar-Mekuzas, Y.; Jaffe, C.L.; Pastor, J.; Cardoso, L.; Baneth, G. Identification of Babesia Species Infecting Dogs Using Reverse Line Blot Hybridization for Six Canine Piroplasms, and Evaluation of Co-Infection by Other Vector-Borne Pathogens. Vet. Parasitol. 2013, 191, 367–373. [Google Scholar] [CrossRef]
- Cardoso, L.; Yisaschar-Mekuzas, Y.; Rodrigues, F.T.; Costa, Á.; Machado, J.; Diz-Lopes, D.; Baneth, G. Canine Babesiosis in Northern Portugal and Molecular Characterization of Vector-Borne Co-Infections. Parasites Vectors 2010, 3, 27. [Google Scholar] [CrossRef]
- De Sousa, K.C.M.; André, M.R.; Herrera, H.M.; de Andrade, G.B.; Jusi, M.M.G.; dos Santos, L.L.; Barreto, W.T.G.; Machado, R.Z.; de Oliveira, G.P. Molecular and Serological Detection of Tick-Borne Pathogens in Dogs from an Area Endemic for Leishmania infantum in Mato Grosso Do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 525–531. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Nandini, M.K. Overview of Canine Babesiosis. In Veterinary Medicine and Pharmaceuticals; IntechOpen: London, UK, 2019; pp. 1–17. ISBN 978-1-78985-440-4. [Google Scholar]
- Köster, L.S.; Lobetti, R.G.; Kelly, P. Canine Babesiosis: A Perspective on Clinical Complications, Biomarkers, and Treatment. Vet. Med. Res. Rep. 2015, 6, 119–128. [Google Scholar] [CrossRef]
- Carli, E.; Tasca, S.; Trotta, M.; Furlanello, T.; Caldin, M.; Solano-Gallego, L. Detection of Erythrocyte Binding IgM and IgG by Flow Cytometry in Sick Dogs with Babesia canis canis or Babesia canis vogeli Infection. Vet. Parasitol. 2009, 162, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. The Immunopathology of Canine Vector-Borne Diseases. Parasites Vectors 2011, 4, 48. [Google Scholar] [CrossRef]
- Da Silva Krawczak, F.; Reis, I.A.; da Silveira, J.A.; Avelar, D.M.; Marcelino, A.P.; Werneck, G.L.; Labruna, M.B.; Paz, G.F. Leishmania, Babesia and Ehrlichia in Urban Pet Dogs: Co-Infection or Cross-Reaction in Serological Methods? Rev. Soc. Bras. Med. Trop. 2015, 48, 64–68. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Oliveira, T.M.F.; Furuta, P.I.; de Carvalho, D.; Machado, R.Z. Study of Cross-Reactivity in Serum Samples from Dogs Positive for Leishmania sp., Babesia canis and Ehrlichia canis in Enzyme-Linked Immunosorbent Assay and Indirect Fluorescent Antibody Test. Rev. Bras. Parasitol. Vet. 2008, 17, 7–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zanette, M.F.; de Lima, V.M.F.; Laurenti, M.D.; Rossi, C.N.; Vides, J.P.; da Coata Vieira, R.F.; Biondo, A.W.; Marcondes, M. Serological Cross-Reactivity of Trypanosoma Cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi Tests in Dogs. Rev. Soc. Bras. Med. Trop. 2014, 47, 105–107. [Google Scholar] [CrossRef]
- Troncarelli, M.Z.; Camargo, J.B.; Machado, J.G.; Lucheis, S.B.; Langoni, H. Leishmania spp. and/or Trypanosoma cruzi Diagnosis in Dogs from Endemic and Nonendemic Areas for Canine Visceral Leishmaniasis. Vet. Parasitol. 2009, 164, 118–123. [Google Scholar] [CrossRef]
- De Castro Ferreira, E.; de Lana, M.; Carneiro, M.; Reis, A.B.; Paes, D.V.; da Silva, E.S.; Schallig, H.; Gontijo, C.M.F. Comparison of Serological Assays for the Diagnosis of Canine Visceral Leishmaniasis in Animals Presenting Different Clinical Manifestations. Vet. Parasitol. 2007, 146, 235–241. [Google Scholar] [CrossRef]
- Ordóñez, D.; Fernández-Soto, P.; Fernández-Martín, A.M.; Crego-Vicente, B.; Febrer-Sendra, B.; Diego, J.G.-B.; Vicente, B.; López-Abán, J.; Belhassen-García, M.; Muro, A.; et al. A Trypanosoma cruzi Genome Tandem Repetitive Satellite DNA Sequence as a Molecular Marker for a LAMP Assay for Diagnosing Chagas’ Disease. Dis. Markers 2020, 2020, 8074314. [Google Scholar] [CrossRef]
- Silber, A.M.; Búa, J.; Porcel, B.M.; Segura, E.L.; Ruiz, A.M. Trypanosoma Cruzi: Specific Detection of Parasites by PCR in Infected Humans and Vectors Using a Set of Primers (BP1/BP2) Targeted to a Nuclear DNA Sequence. Exp. Parasitol. 1997, 85, 225–232. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-Time PCR Applications for Diagnosis of Leishmaniasis. Parasites Vectors 2018, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Maia, C.; Pereira, A.; Arfuso, F.; Gaglio, G.; Rizzo, M.; Caracappa, G.; Marino, G.; Pollmeier, M.; Giannetto, S.; et al. Identification of Trypanosomatids and Blood Feeding Preferences of Phlebotomine Sand Fly Species Common in Sicily, Southern Italy. PLoS ONE 2020, 15, e0229536. [Google Scholar] [CrossRef] [PubMed]
- Bastrenta, B.; Mita, N.; Buitrago, R.; Vargas, F.; Flores, M.; Machane, M.; Yacsik, N.; Torrez, M.; Le Pont, F.; Brenière, F. Human Mixed Infections of Leishmania spp. and Leishmania-Trypanosoma cruzi in a Sub Andean Bolivian Area: Identification by Polymerase Chain Reaction/Hybridization and Isoenzyme. Memórias do Instituto Oswaldo Cruz 2003, 98, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.G.; Lauria-Pires, L.; Nitz, N.; Lozzi, S.P.; Nascimento, R.J.; Monteiro, P.S.; Rebelo, M.M.; de Cássia Rosa, A.; Santana, J.M.; Teixeira, A.R.L. Exposure to Mixed Asymptomatic Infections with Trypanosoma cruzi, Leishmania braziliensis and Leishmania chagasi in the Human Population of the Greater Amazon. Trop. Med. Int. Health 2007, 12, 629–636. [Google Scholar] [CrossRef]
- Viettri, M.; Herrera, L.; Aguilar, C.M.; Morocoima, A.; Reyes, J.; Lares, M.; Lozano-Arias, D.; García-Alzate, R.; Chacón, T.; Feliciangeli, M.D.; et al. Molecular Diagnosis of Trypanosoma cruzi/Leishmania spp. Coinfection in Domestic, Peridomestic and Wild Mammals of Venezuelan Co-Endemic Areas. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 123–130. [Google Scholar] [CrossRef]
- Duprey, Z.H.; Steurer, F.J.; Rooney, J.A.; Kirchhoff, L.V.; Jackson, J.E.; Rowton, E.D.; Schantz, P.M. Canine Visceral Leishmaniasis, United States and Canada, 2000–2003. Emerg. Infect. Dis. 2006, 12, 440–446. [Google Scholar] [CrossRef]
- Meyers, A.C.; Edwards, E.E.; Sanders, J.P.; Saunders, A.B.; Hamer, S.A. Fatal Chagas Myocarditis in Government Working Dogs in the Southern United States: Cross-Reactivity and Differential Diagnoses in Five Cases across Six Months. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100545. [Google Scholar] [CrossRef]
- Denkers, E.Y.; Gazzinelli, R.T. Regulation and Function of T-Cell-Mediated Immunity during Toxoplasma gondii Infection. Clin. Microbiol. Rev. 1998, 11, 569–588. [Google Scholar] [CrossRef]
- Rios, L.E.; Vázquez-Chagoyán, J.C.; Pacheco, A.O.; Zago, M.P.; Garg, N.J. Immunity and Vaccine Development Efforts against Trypanosoma cruzi. Acta Trop. 2019, 200, 105168. [Google Scholar] [CrossRef]
- Krautz, G.M.; Kissinger, J.C.; Krettli, A.U. The Targets of the Lytic Antibody Response against Trypanosoma cruzi. Parasitol. Today 2000, 16, 31–34. [Google Scholar] [CrossRef]
- Avila, J.L.; Rojas, M.; Galili, U. Immunogenic Gal Alpha 1----3Gal Carbohydrate Epitopes Are Present on Pathogenic American Trypanosoma and Leishmania. J. Immunol. 1989, 142, 2828–2834. [Google Scholar]
- Mateus, J.; Guerrero, P.; Lasso, P.; Cuervo, C.; González, J.M.; Puerta, C.J.; Cuéllar, A. An Animal Model of Acute and Chronic Chagas Disease With the Reticulotropic Y Strain of Trypanosoma cruzi That Depicts the Multifunctionality and Dysfunctionality of T Cells. Front. Immunol. 2019, 10, 918. [Google Scholar] [CrossRef]
- Da Costa Oliveira, V.; Boechat, V.C.; Mendes, A.A.V., Jr.; de Fátima Madeira, M.; Ferreira, L.C.; Figueiredo, F.B.; Campos, M.P.; de Carvalho Rodrigues, F.d.C.; de Oliveira, R.d.V.C.; Amendoeira, M.R.R.; et al. Occurrence of Leishmania infantum in the Central Nervous System of Naturally Infected Dogs: Parasite Load, Viability, Co-Infections and Histological Alterations. PLoS ONE 2017, 12, e0175588. [Google Scholar] [CrossRef]
- De Cássia Paulan, S.; de Souza Lins, A.G.; da Silva Tenório, M.; da Silva, D.T.; de Jesus Pena, H.F.; Machado, R.Z.; Gennari, S.M.; Buzetti, W.A.S. Seroprevalence Rates of Antibodies Against Leishmania infantum and Other Protozoan and Rickettsial Parasites in Dogs. Rev. Bras. Parasitol. Vet. 2013, 22, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Cabezón, O.; Millán, J.; Gomis, M.; Dubey, J.P.; Ferroglio, E.; Almería, S. Kennel Dogs as Sentinels of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Majorca Island, Spain. Parasitol. Res. 2010, 107, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Gennari, S.M. Clinical Toxoplasmosis in Dogs and Cats: An Update. Front. Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.R.; Cadieu, J.; Kiupel, M.; Lim, A.; Bolin, S.R.; Mansell, J. Cutaneous Toxoplasmosis in Two Dogs. J. Vet. Diagn. Investig. 2012, 24, 636–640. [Google Scholar] [CrossRef]
- Pepper, A.; Mansfield, C.; Stent, A.; Johnstone, T. Toxoplasmosis as a Cause of Life-Threatening Respiratory Distress in a Dog Receiving Immunosuppressive Therapy. Clin. Case Rep. 2019, 7, 942–948. [Google Scholar] [CrossRef]
- Sakamoto, K.; de Melo, G.; Machado, G. T and B Lymphocytes in the Brains of Dogs with Concomitant Seropositivity to Three Pathogenic Protozoans: Leishmania chagasi, Toxoplasma gondii and Neospora caninum. BMC Res. Notes 2013, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Dogs, Cats, Parasites, and Humans in Brazil: Opening the Black Box. Parasites Vectors 2014, 7, 22. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-Borne Helminths of Dogs and Humans in Europe. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef]
- Saldanha-Elias, A.M.; Silva, M.A.; Silva, V.O.; Amorim, S.L.A.; Coutinho, A.R.; Santos, H.A.; Giunchetti, R.C.; Vitor, R.W.A.; Geiger, S.M. Prevalence of Endoparasites in Urban Stray Dogs from Brazil Diagnosed with Leishmania, with Potential for Human Zoonoses. Acta Parasitol. 2019, 64, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Schnyder, M.; Macchioni, F.; Deplazes, P.; Magi, M. Serological Detection of Circulating Angiostrongylus vasorum Antigen and Specific Antibodies in Dogs from Central and Northern Italy. Vet. Parasitol. 2013, 192, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Tajebe, F.; Getahun, M.; Adem, E.; Hailu, A.; Lemma, M.; Fikre, H.; Raynes, J.; Tamiru, A.; Mulugeta, Z.; Diro, E.; et al. Disease Severity in Patients with Visceral Leishmaniasis Is Not Altered by Co-Infection with Intestinal Parasites. PLoS Negl. Trop. Dis. 2017, 11, e0005727. [Google Scholar] [CrossRef] [PubMed]
- Labarthe, N.V.; Pereira Paiva, J.; Reifur, L.; Mendes-de-Almeida, F.; Merlo, A.; Carvalho Pinto, C.J.; Juliani, P.S.; Ornelas de Almeida, M.A.; Câmara Alves, L. Updated Canine Infection Rates for Dirofilaria immitis in Areas of Brazil Previously Identified as Having a High Incidence of Heartworm-Infected Dogs. Parasites Vectors 2014, 7, 493. [Google Scholar] [CrossRef]
- Self, S.W.; Pulaski, C.N.; McMahan, C.S.; Brown, D.A.; Yabsley, M.J.; Gettings, J.R. Regional and Local Temporal Trends in the Prevalence of Canine Heartworm Infection in the Contiguous United States: 2012–2018. Parasites Vectors 2019, 12, 380. [Google Scholar] [CrossRef]
- Maxwell, E.; Ryan, K.; Reynolds, C.; Pariaut, R. Outcome of a Heartworm Treatment Protocol in Dogs Presenting to Louisiana State University from 2008 to 2011: 50 Cases. Vet. Parasitol. 2014, 206, 71–77. [Google Scholar] [CrossRef]
- Simón, F.; Kramer, L.H.; Román, A.; Blasini, W.; Morchón, R.; Marcos-Atxutegi, C.; Grandi, G.; Genchi, C. Immunopathology of Dirofilaria immitis Infection. Vet. Res. Commun. 2007, 31, 161–171. [Google Scholar] [CrossRef]
- Rodríguez, N.E.; Wilson, M.E. Eosinophils and Mast Cells in Leishmaniasis. Immunol. Res. 2014, 59, 129–141. [Google Scholar] [CrossRef]
- Martinez, R. Epidemiology of Paracoccidioidomycosis. Revista do Instituto de Medicina Tropical de São Paulo 2015, 57, 11–20. [Google Scholar] [CrossRef]
- Ono, M.A.; Bracarense, A.P.F.R.L.; Morais, H.S.A.; Trapp, S.M.; Belitardo, D.R.; Camargo, Z.P. Canine Paracoccidioidomycosis: A Seroepidemiologic Study. Med. Mycol. 2001, 39, 277–282. [Google Scholar] [CrossRef]
- Eisele, R.C.; Juliani, L.C.; Belitardo, D.R.; Itano, E.N.; Estevão, D.; Bracarense, A.P.F.R.L.; Camargo, Z.P.; Ono, M.A. Immune Response in Dogs Experimentally Infected with Paracoccidioides brasiliensis. Med. Mycol. 2004, 42, 549–553. [Google Scholar] [CrossRef]
- Headley, S.A.; Pretto-Giordano, L.G.; Di Santis, G.W.; Gomes, L.A.; Macagnan, R.; da Nóbrega, D.F.; Leite, K.M.; de Alcântara, B.K.; Itano, E.N.; Alfieri, A.A.; et al. Paracoccidioides brasiliensis-Associated Dermatitis and Lymphadenitis in a Dog. Mycopathologia 2017, 182, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Farias, M.; Anuska Zeni Condas, L.; Garcia Ribeiro, M.; de Moraes Gimenes Bosco, S.; Dominguez Muro, M.; Werner, J.; Cordeiro Theodoro, R.; Bagagli, E.; Alencar Marques, S.; Franco, M. Paracoccidioidomycosis in a Dog: Case Report of Generalized Lymphadenomegaly. Mycopathologia 2011, 172, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Petroni, T.F.; Bonfietti, L.X.; Zaninelli, T.H.; Itano, E.N.; Ono, M.A. Serological Evidence of Infection by Paracoccidioides brasiliensis in Dogs with Leishmaniasis. Mycopathologia 2017, 182, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.H.; Domingos, I.H.; Kouchi, K.; Itano, E.N.; Silva, E.A.; Landgraf, V.O.; Werneck, S.M.; Camargo, Z.P.; Ono, M.A. Serological Detection of Antibodies against Paracoccidioides brasiliensis in Dogs with Leishmaniasis. Mycopathologia 2006, 162, 325–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brummer, E.; Hanson, L.H.; Stevens, D.A. Gamma-Interferon Activation of Macrophages for Killing of Paracoccidioides brasiliensis and Evidence for Nonoxidative Mechanisms. Int. J. Immunopharmacol. 1988, 10, 945–952. [Google Scholar] [CrossRef]
- Soares, A.M.V.C.; Calvi, S.A.; Peraçoli, M.T.S.; Fernandez, A.C.; Dias, L.A.; dos Anjos, A.R. Modulatory Effect of Prostaglandins on Human Monocyte Activation for Killing of High- and Low-Virulence Strains of Paracoccidioides brasiliensis. Immunology 2001, 102, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.B.; Araújo-Santos, T.; Lázaro-Souza, M.; Carneiro, A.B.; Ibraim, I.C.; Jesus-Santos, F.H.; Luz, N.F.; de Moura Pontes, S.; Entringer, P.F.; Descoteaux, A.; et al. Leishmania infantum Lipophosphoglycan Induced-Prostaglandin E2 Production in Association with PPAR-γ Expression via Activation of Toll like Receptors-1 and 2. Sci. Rep. 2017, 7, 14321. [Google Scholar] [CrossRef] [PubMed]
- Burger, E. Paracoccidioidomycosis Protective Immunity. J. Fungi 2021, 7, 137. [Google Scholar] [CrossRef]
- Bozzi, A.; Pereira, P.P.N.; Reis, B.S.; Goulart, M.I.; Pereira, M.C.N.; Pedroso, E.P.; Leite, M.F.; Goes, A.M. Interleukin-10 and Tumor Necrosis Factor–α Single Nucleotide Gene Polymorphism Frequency in Paracoccidioidomycosis. Hum. Immunol. 2006, 67, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Parise-Fortes, M.R.; Marques, S.A.; Soares, A.M.V.C.; Kurokawa, C.S.; Marques, M.E.A.; Peracoli, M.T.S. Cytokines Released from Blood Monocytes and Expressed in Mucocutaneous Lesions of Patients with Paracoccidioidomycosis Evaluated before and during Trimethoprim–Sulfamethoxazole Treatment. Br. J. Dermatol. 2006, 154, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Bastos, K.R.; Russo, M.; Almeida, S.R. Interaction between Paracoccidioides brasiliensis and Pulmonary Dendritic Cells Induces Interleukin-10 Production and Toll-Like Receptor–2 Expression: Possible Mechanisms of Susceptibility. J. Infect. Dis. 2007, 196, 1108–1115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreira, M.C.; de Oliveira, R.T.D.; da Silva, R.M.; Blotta, M.H.S.L.; Mamoni, R.L. Involvement of Regulatory T Cells in the Immunosuppression Characteristic of Patients with Paracoccidioidomycosis. Infect. Immun. 2010, 78, 4392–4401. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A Moving Target in Immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- DE F Michelin, A.; Perri, S.H.V.; De Lima, V.M.F. Evaluation of TNF-α, IL-4, and IL-10 and Parasite Density in Spleen and Liver of L. (L.) chagasi Naturally Infected Dogs. Ann. Trop. Med. Parasitol. 2011, 105, 373–383. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing Canine Vector-Borne Diseases of Zoonotic Concern: Part Two. Trends Parasitol. 2009, 25, 228–235. [Google Scholar] [CrossRef]
- Oliva, G.; Scalone, A.; Foglia Manzillo, V.; Gramiccia, M.; Pagano, A.; Di Muccio, T.; Gradoni, L. Incidence and Time Course of Leishmania infantum Infections Examined by Parasitological, Serologic, and Nested-PCR Techniques in a Cohort of Naive Dogs Exposed to Three Consecutive Transmission Seasons. J. Clin. Microbiol. 2006, 44, 1318–1322. [Google Scholar] [CrossRef]
- Savani, E.S.M.M.; Nunes, V.L.B.; Galati, E.A.B.; Castilho, T.M.; de Araujo, F.S.; Ilha, I.M.N.; de Oliveira Camargo, M.C.G.; D’Auria, S.R.N.; Floeter-Winter, L.M. Ocurrence of Co-Infection by Leishmania (Leishmania) chagasi and Trypanosoma (Trypanozoon) evansi in a Dog in the State of Mato Grosso Do Sul, Brazil. Memorias do Instituto Oswaldo Cruz 2005, 100, 739–741. [Google Scholar] [CrossRef]
- Viol, M.A.; Lima, V.M.F.; Aquino, M.C.C.; Gallo, G.; Alves, I.P.; Generoso, D.; Perri, S.H.V.; Lucheis, S.B.; Langoni, H.; Nunes, C.M.; et al. Detection of Cross Infections by Leishmania spp. and Trypanosoma spp. in Dogs Using Indirect Immunoenzyme Assay, Indirect Fluorescent Antibody Test and Polymerase Chain Reaction. Parasitol. Res. 2012, 111, 1607–1613. [Google Scholar] [CrossRef]
- Guillén Llera, J.L.; López García, M.L.; Martín Reinoso, E.; De Vivar González, R. Differential Serological Testing by Simultaneous Indirect Immunofluorescent Antibody Test in Canine Leishmaniosis and Ehrlichiosis. Vet. Parasitol. 2002, 109, 185–190. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the Diagnosis, Clinical Staging, Treatment and Prevention of Canine Leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Miró, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-Gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel Areas for Prevention and Control of Canine Leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Napoli, E.; Arfuso, F.; Gaglio, G.; Giannetto, S.; Halos, L.; Beugnet, F.; Brianti, E. Six-Month Field Efficacy and Safety of the Combined Treatment of Dogs with Frontline Tri-Act® and NexGard Spectra®. Parasites Vectors 2018, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Liu, Y.; Li, T.; Chen, Y.; Wang, S.; Han, X.; Wang, Q. Seroepidemiological Study of Canine Leishmania infantum and Toxoplasma gondii Infections in Shanghai, China, and Analysis of Risk Factors. Ann. Agric. Environ. Med. 2016, 23, 420–424. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Brandão-Filho, S.P. Visceral Leishmaniasis in Brazil: Revisiting Paradigms of Epidemiology and Control. Revista do Instituto de Medicina Tropical de São Paulo 2006, 48, 151–156. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Type of Pathogen | Main Vector(s) | Region(s) Primarily Found | Reference(s) |
|---|---|---|---|---|
| Leishmania infantum | Protozoa | Phlebotomus spp. | Mediterranean basin Southern Europe Northern Africa | [1,2] |
| Lutzomyia longipalpis | South America | [2,3] | ||
| None | North America (enzootic) | [6,7] | ||
| Ehrlichia canis | Bacteria | Rhipicephalus sanguineus | North America South America Mediterranean basin | [13,14,15,16] |
| Ehrlichia ewingii | Bacteria | Amblyomma americanum | North America | [13,14,15,17] |
| Ehrlichia chaffeensis | Bacteria | Amblyomma americanum | North America | [13,14,15] |
| Anaplasma phagocytophilum | Bacteria | Ixodes scapularis | North America | [18] |
| Ixodes pacificus | Western U.S. | [18] | ||
| Ixodes ricinus | Europe | [18,19] | ||
| Anaplasma platys | Bacteria | Rhipicephalus sanguineus | Brazil Europe | [9,16,20] |
| Borrelia burgdorferi | Bacteria | Ixodes scapularis | North America | [21,22,23] |
| Ixodes pacificus | Western U.S. | [21] | ||
| Borrelia garinii | Bacteria | Ixodes ricinus | Europe | [19,22,24] |
| Borrelia afzelii | Bacteria | Ixodes ricinus | Europe | [22,24] |
| Babesia canis | Protozoa | Dermacentor reticulatus | Europe | [25,26,27] |
| Rhipicephalus sanguineus | Brazil | [25,28] | ||
| Babesia vogeli | Protozoa | Rhipicephalus sanguineus | Brazil | [28] |
| Babesia gibsoni | Protozoa | Haemaphysalis bispinosa | Asia | [29] |
| Haemaphysalis longicornis | Asia | [27] | ||
| Trypanosoma cruzi | Protozoa | Triatoma gerstaeckeri, T. sanguisuga | North America | [30,31,32,33,34,35,36] |
| T. dimidiata | Central America | [33,37] | ||
| T. infestans | South America | [33,38,39] | ||
| Toxoplasma gondii | Protozoa | None | South America North America Europe Asia | [40,41] |
| Dirofilaria immitis | Helminth | Aedes, Anopheles, Culex | North America, South America, Europe | [42,43,44,45,46,47] |
| Paracoccidiodes brasiliensis | Fungi | None | South America Central America | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beasley, E.A.; Pessôa-Pereira, D.; Scorza, B.M.; Petersen, C.A. Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis. Animals 2021, 11, 3206. https://doi.org/10.3390/ani11113206
Beasley EA, Pessôa-Pereira D, Scorza BM, Petersen CA. Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis. Animals. 2021; 11(11):3206. https://doi.org/10.3390/ani11113206
Chicago/Turabian StyleBeasley, Erin A., Danielle Pessôa-Pereira, Breanna M. Scorza, and Christine A. Petersen. 2021. "Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis" Animals 11, no. 11: 3206. https://doi.org/10.3390/ani11113206
APA StyleBeasley, E. A., Pessôa-Pereira, D., Scorza, B. M., & Petersen, C. A. (2021). Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis. Animals, 11(11), 3206. https://doi.org/10.3390/ani11113206






