Anti-Obesity Effects of Combined Cornus officinalis and Ribes fasciculatum Extract in High-Fat Diet-Induced Obese Male Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cornus officinali (CO) and Ribes fasciculatum (RF) Extracts and Their Chemical Composition
2.2. Animal Studies
2.3. Histological Analysis and Quantification of Adipose Tissue Area
2.4. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.5. Metabolomic Cage Monitoring System and Analysis of Biochemical Obesity Indicators in Plasma
2.6. Quantiative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results and Discussion
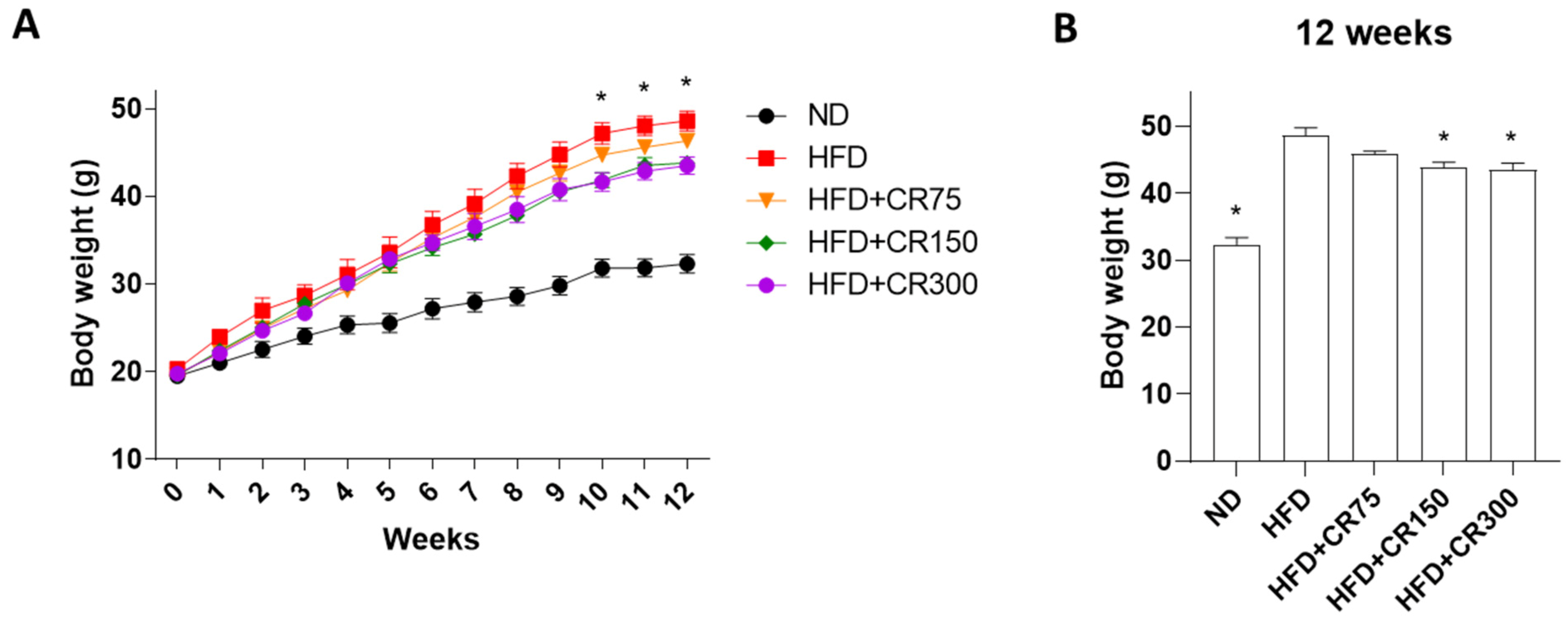
3.1. Anti-Obesity Effects of CR in High-Fat Diet (HFD)-Induced Obese Male Mouse
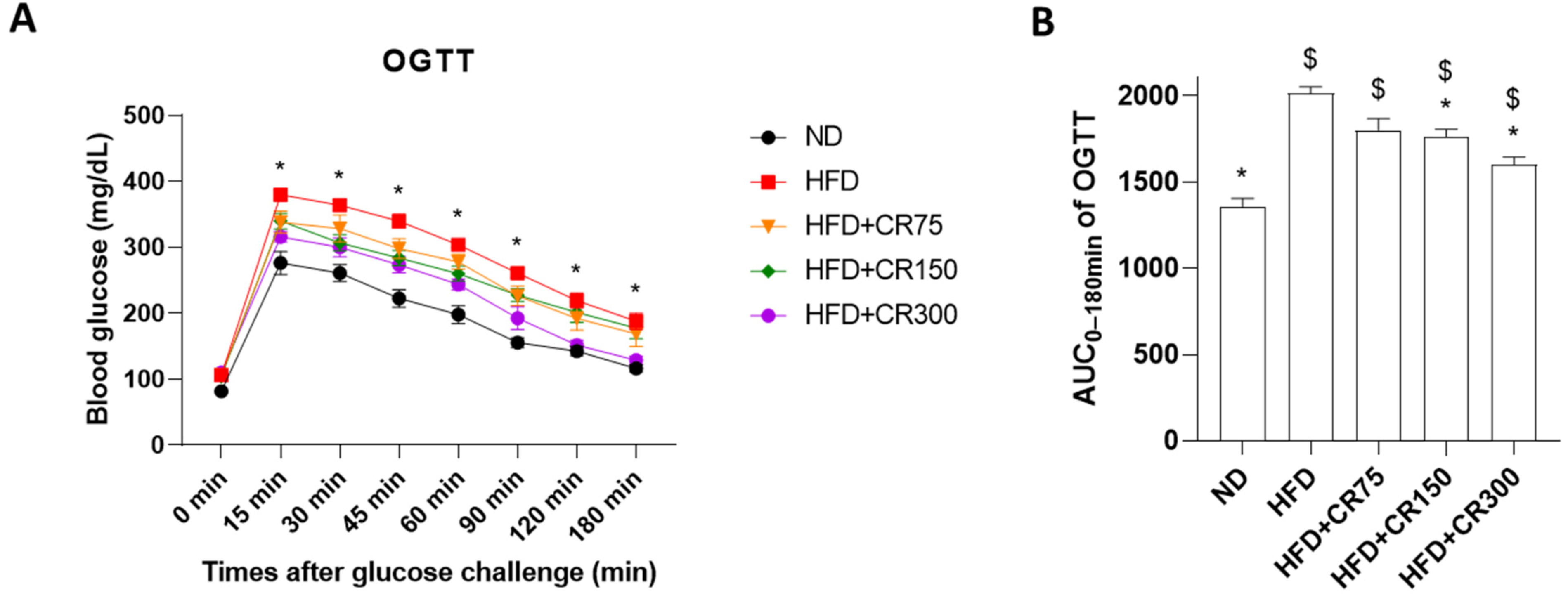
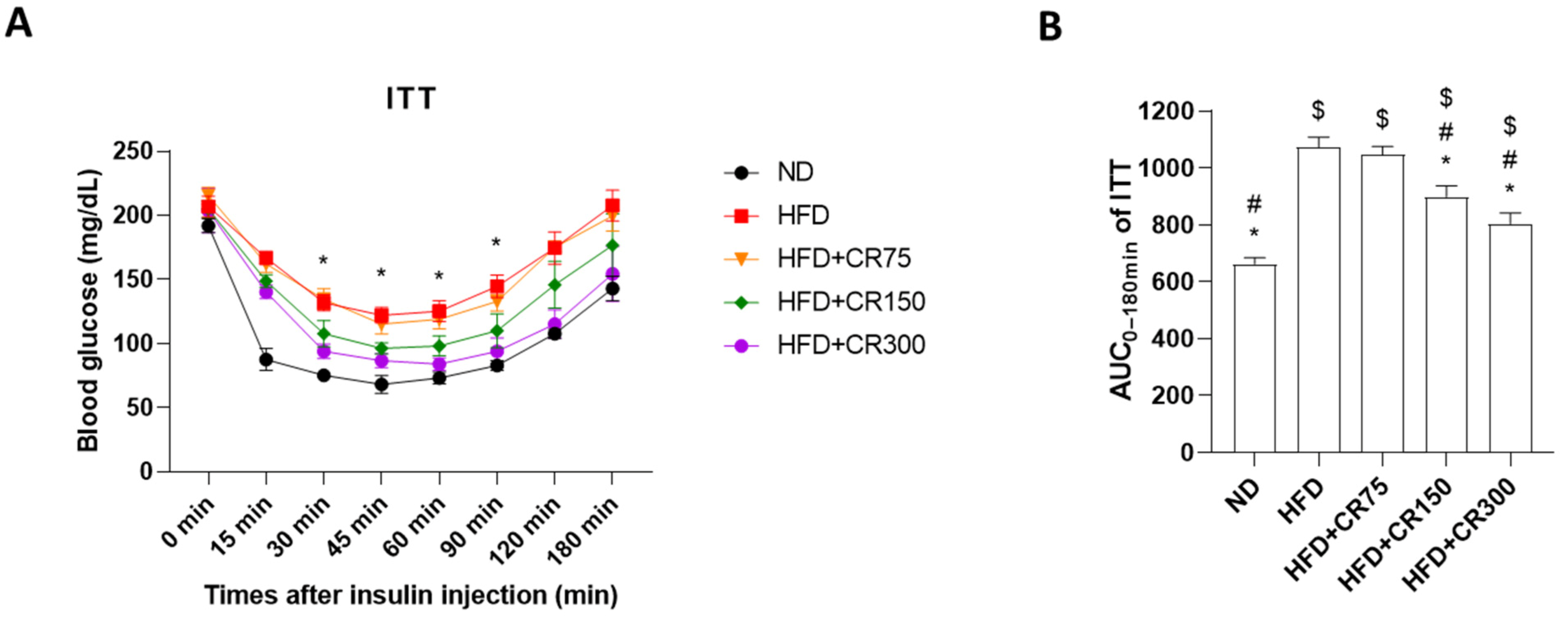
3.2. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT) of High-Fat Diet (HFD)-Induced Obese Male Mice
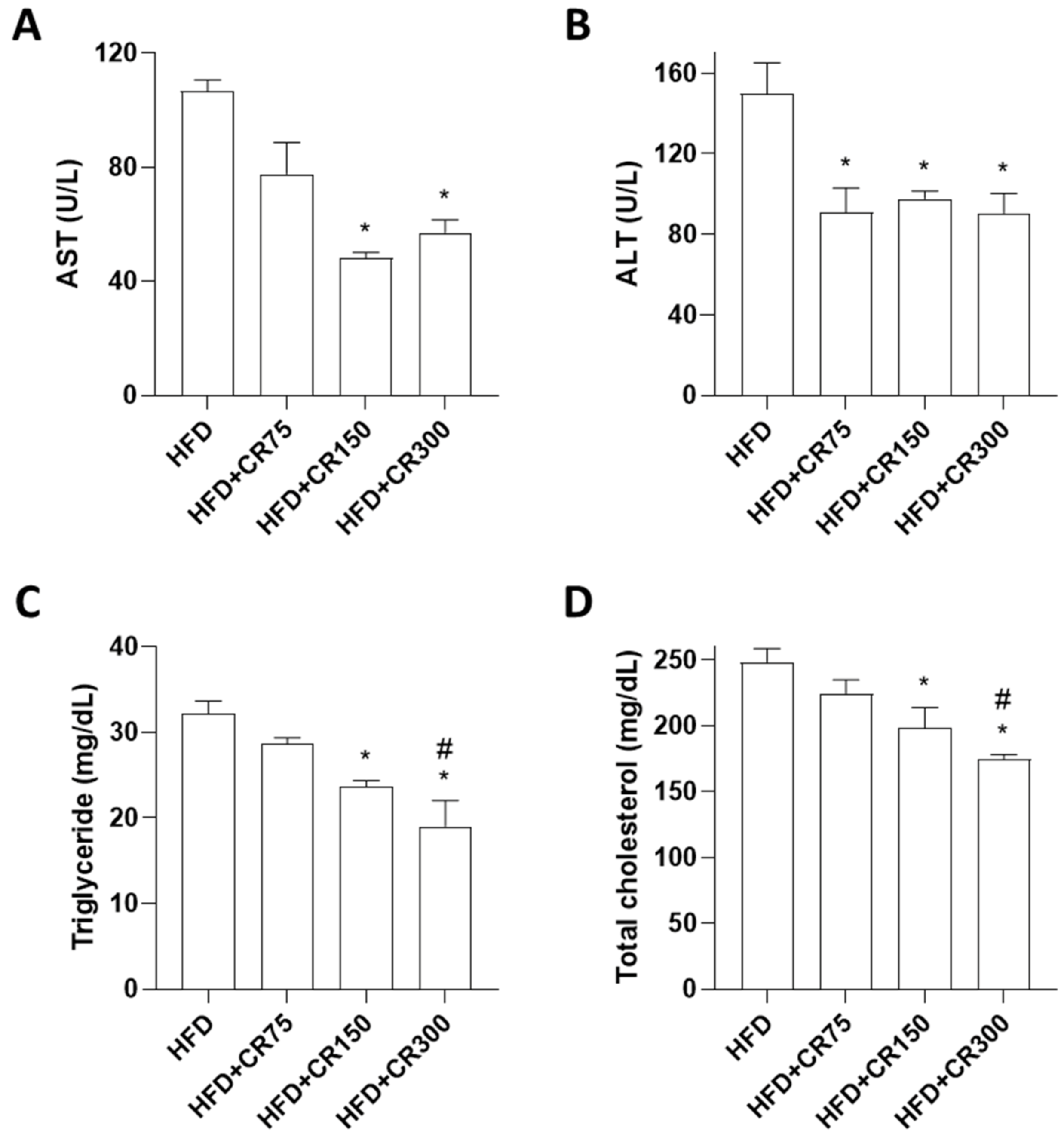
3.3. Effects of CR on Plasma Levels of Biochemical Obesity Indicators in HFD-Induced Obese Male Mice
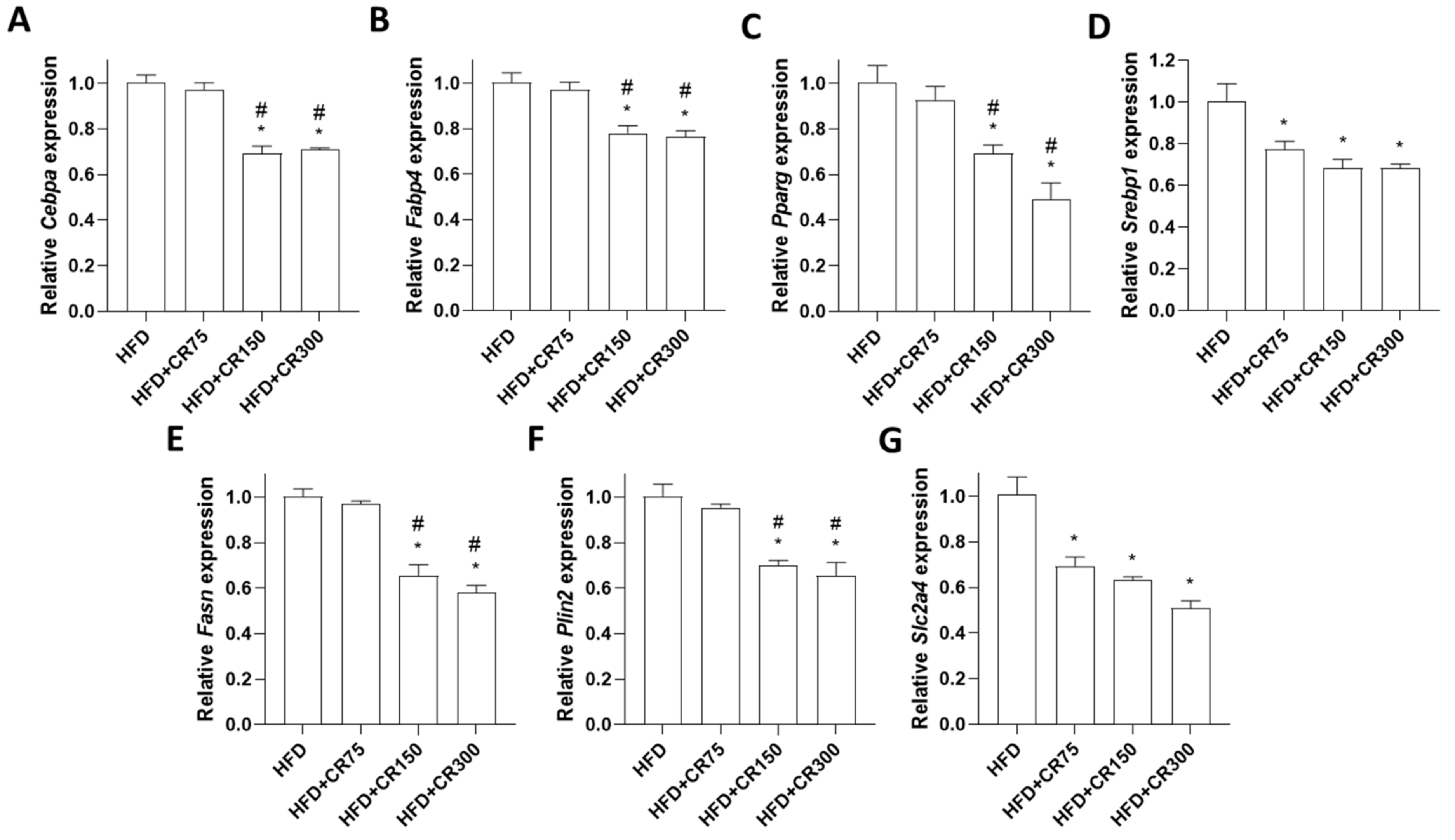
3.4. Effects of CR on Adipogenesis in HFD-Induced Obese Male Mice
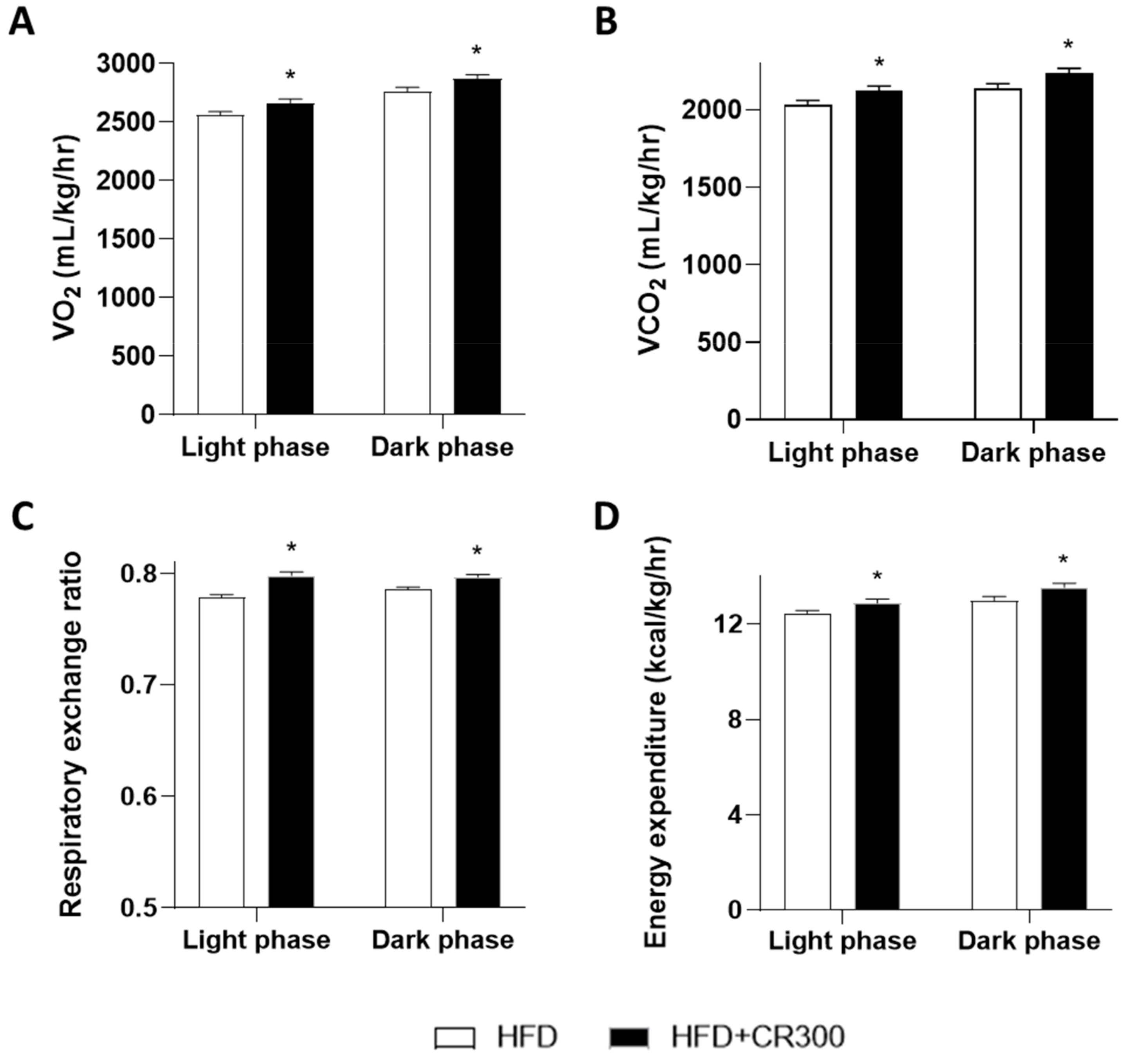
3.5. Effects of CR on Systemic Metabolism in HFD-Induced Male Mice
3.6. Gender-Specific Differences in the Effects of CR in HFD-Induced Obese Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thaker, V.V. Genetic and Epigenetic Causes of Obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar]
- Aleksandar, K. Social causes of obesity. Med. Pregl. 1986, 39, 591–592. [Google Scholar]
- Kotsis, V.; Stabouli, S.; Papakatsika, S.; Rizos, Z.; Parati, G. Mechanisms of obesity-induced hypertension. Hypertens. Res. 2010, 33, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Ezquerra, E.A.; Vazquez, J.M.C.; Barrero, A.A. Obesity, metabolic syndrome and diabetes: Cardiovascular implications and therapy. Rev. Esp. Cardiol. 2008, 61, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.; McLachlan, A.J.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 1. Australia and New Zealand. Expert Rev. Clin. Pharmacol. 2016, 9, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sammons, H.M.; Gubarev, M.I.; Krepkova, L.V.; Bortnikova, V.V.; Corrick, F.; Job, K.M.; Sherwin, C.M.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part 2. European Union and Russia. Expert Rev. Clin. Pharmacol. 2016, 9, 1117–1127. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jin, B.; Lee, S.; Oh, J.-Y.; Song, J.; Lee, D.; Kim, Y.-S.; Kim, H. A herbal formula HT051, a combination of Pueraria lobata and Rehmannia glutinosa, Prevents postmenopausal obesity in ovariectomized rats. Evid. Based Complement. Altern. Med. 2017, 2017, 8641535. [Google Scholar] [CrossRef]
- Yinghong, L.; Ming, K.J.; Sai, C.L.; Khang, G.N. Synergistic Effect of Traditional Chinese Medicine. Asian J. Chem. 2007, 19, 867. [Google Scholar]
- Park, E.; Ryu, M.J.; Kim, N.K.; Bae, M.H.; Seo, Y.; Kim, J.; Yeo, S.; Kanwal, M.; Choi, C.W.; Heo, J.Y.; et al. Synergistic Neuroprotective Effect of Schisandra chinensis and Ribes fasciculatum on Neuronal Cell Death and Scopolamine-Induced Cognitive Impairment in Rats. Int. J. Mol. Sci. 2019, 20, 4517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechesso, A.F.; Lee, S.J.; Park, N.H.; Kim, J.Y.; Im, Z.E.; Suh, J.W.; Park, S.C. Preventive effects of a novel herbal mixture on atopic dermatitis-like skin lesions in BALB/C mice. BMC Complement. Altern. Med. 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Quah, Y.; Lee, S.J.; Lee, E.B.; Birhanu, B.T.; Ali, M.S.; Abbas, M.A.; Boby, N.; Im, Z.E.; Park, S.C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients 2020, 12, 3317. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Ranjbar, A.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effect of (Cornus mas L.) fruit extract on liver function among patients with nonalcoholic fatty liver: A double-blind randomized clinical trial. Phytother. Res. PTR 2021, 35, 5259–5268. [Google Scholar] [CrossRef]
- Jeon, H.; Cha, D.S. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chin. J. Nat. Med. 2016, 14, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, S.J.; Ahn, E.M.; Oh, S.R.; Lee, H.J.; Jeong, J.A.; Lee, J.Y. Ribes fasciculatum var. chinense Attenuated Allergic Inflammation In Vivo and In Vitro. Biomol. Ther. 2014, 22, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Lim, E.; Yeo, S.; Yong, Y.; Yang, J.; Jeong, S.Y. Anti-Menopausal Effects of Cornus officinalis and Ribes fasciculatum Extract In Vitro and In Vivo. Nutrients 2020, 12, 369. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Lee, C.G.; Jeong, H.; Yeo, S.; Kim, J.A.; Jeong, S.Y. Antiadipogenic Effects of Mixtures of Cornus officinalis and Ribes fasciculatum Extracts on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Mice. Molecules 2020, 25, 2350. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.G.; Kim, J.; Kang, J.H.; Cho, Y.G.; Jeong, S.Y. Efficacy and Safety of Combined Extracts of Cornus officinalis and Ribes fasciculatum for Body Fat Reduction in Overweight Women. J. Clin. Med. 2020, 9, 3629. [Google Scholar] [CrossRef]
- Lin, I.T.; Lee, M.Y.; Wang, C.W.; Wu, D.W.; Chen, S.C. Gender Differences in the Relationships among Metabolic Syndrome and Various Obesity-Related Indices with Nonalcoholic Fatty Liver Disease in a Taiwanese Population. Int. J. Environ. Res. Public Health 2021, 18, 857. [Google Scholar] [CrossRef] [PubMed]
- Kroll, D.S.; Feldman, D.E.; Biesecker, C.L.; McPherson, K.L.; Manza, P.; Joseph, P.V.; Volkow, N.D.; Wang, G.J. Neuroimaging of Sex/Gender Differences in Obesity: A Review of Structure, Function, and Neurotransmission. Nutrients 2020, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Golay, A.; Bobbioni, E. The role of dietary fat in obesity. Int. J. Obes. Relat. Metab. Disord. 1997, 21 (Suppl. S3), S2–S11. [Google Scholar]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, e56672. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Rutter, G.A. Insulin secretion: Fatty acid signalling via serpentine receptors. Curr. Biol. 2003, 13, R403–R405. [Google Scholar] [CrossRef] [Green Version]
- Pedro, P.F.; Tsakmaki, A.; Bewick, G.A. The Glucose Tolerance Test in Mice. Methods Mol. Biol. 2020, 2128, 207–216. [Google Scholar] [CrossRef]
- Cozar-Castellano, I.; Perdomo, G. Assessment of Insulin Tolerance In Vivo in Mice. Methods Mol. Biol. 2020, 2128, 217–224. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef] [Green Version]
- Gerber, L.; Otgonsuren, M.; Mishra, A.; Escheik, C.; Birerdinc, A.; Stepanova, M.; Younossi, Z.M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment. Pharmacol. Ther. 2012, 36, 772–781. [Google Scholar] [CrossRef]
- Salmela, P.I.; Sotaniemi, E.A.; Niemi, M.; Maentausta, O. Liver function tests in diabetic patients. Diabetes Care 1984, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Saligram, S.; Williams, E.J.; Masding, M.G. Raised liver enzymes in newly diagnosed Type 2 diabetes are associated with weight and lipids, but not glycaemic control. Indian J. Endocrinol. Metab. 2012, 16, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Al-Jameil, N.; Khan, F.A.; Arjumand, S.; Khan, M.F.; Tabassum, H. Associated liver enzymes with hyperlipidemic profile in type 2 diabetes patients. Int. J. Clin. Exp. Pathol. 2014, 7, 4345–4349. [Google Scholar]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Kloting, N.; Fasshauer, M.; Schon, M.R.; Korner, A.; Stumvoll, M.; Bluher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- McManaman, J.L.; Bales, E.S.; Orlicky, D.J.; Jackman, M.; MacLean, P.S.; Cain, S.; Crunk, A.E.; Mansur, A.; Graham, C.E.; Bowman, T.A.; et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 2013, 54, 1346–1359. [Google Scholar] [CrossRef] [Green Version]
- Heyde, I.; Begemann, K.; Oster, H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021, 162, bqab009. [Google Scholar] [CrossRef]
- Moreno-Fernandez, S.; Garces-Rimon, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, G.I.; Henstridge, D.C. Body Composition and Metabolic Caging Analysis in High Fat Fed Mice. J. Vis. Exp. 2018, 135, 57280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, P. Simple equations for complex physiology: Can we use VCO2 for calculating energy expenditure? Crit. Care 2016, 20, 72. [Google Scholar] [CrossRef] [Green Version]
- Marvyn, P.M.; Bradley, R.M.; Mardian, E.B.; Marks, K.A.; Duncan, R.E. Data on oxygen consumption rate, respiratory exchange ratio, and movement in C57BL/6J female mice on the third day of consuming a high-fat diet. Data Brief 2016, 7, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Hua, Y.; Lapchak, P.A.; Topchiy, E.; Lehmann, T.E.; Ren, J.; Nair, S. Novel curcumin derivative CNB-001 mitigates obesity-associated insulin resistance. J. Pharmacol. Exp. Ther. 2014, 349, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Kendig, S. Implications for policy to support healthy weight for women. J. Obstet. Gynecol. Neonatal Nurs. 2015, 44, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nut. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- White, M.C.; Miller, A.J.; Loloi, J.; Bingaman, S.S.; Shen, B.; Wang, M.; Silberman, Y.; Lindsey, S.H.; Arnold, A.C. Sex differences in metabolic effects of angiotensin-(1–7) treatment in obese mice. Biol. Sex Differ. 2019, 10, 36. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, C.-G.; Jeon, H.; Jeong, H.; Yeo, S.; Yong, Y.; Jeong, S.-Y. Anti-Obesity Effects of Combined Cornus officinalis and Ribes fasciculatum Extract in High-Fat Diet-Induced Obese Male Mice. Animals 2021, 11, 3187. https://doi.org/10.3390/ani11113187
Park E, Lee C-G, Jeon H, Jeong H, Yeo S, Yong Y, Jeong S-Y. Anti-Obesity Effects of Combined Cornus officinalis and Ribes fasciculatum Extract in High-Fat Diet-Induced Obese Male Mice. Animals. 2021; 11(11):3187. https://doi.org/10.3390/ani11113187
Chicago/Turabian StylePark, Eunkuk, Chang-Gun Lee, Hyoju Jeon, Hyesoo Jeong, Subin Yeo, Yoonjoong Yong, and Seon-Yong Jeong. 2021. "Anti-Obesity Effects of Combined Cornus officinalis and Ribes fasciculatum Extract in High-Fat Diet-Induced Obese Male Mice" Animals 11, no. 11: 3187. https://doi.org/10.3390/ani11113187
APA StylePark, E., Lee, C.-G., Jeon, H., Jeong, H., Yeo, S., Yong, Y., & Jeong, S.-Y. (2021). Anti-Obesity Effects of Combined Cornus officinalis and Ribes fasciculatum Extract in High-Fat Diet-Induced Obese Male Mice. Animals, 11(11), 3187. https://doi.org/10.3390/ani11113187







