Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
Ethics Statement
2.2. Faecal Microbiota Analysis
2.3. Sequencing of Bacterial 16S rRNA Gene
2.4. Bioinformatics
2.4.1. Sequencing Data Processing
2.4.2. OTU Cluster and Taxonomic Annotation
2.4.3. Alpha and Beta Diversity
3. Results and Discussion
3.1. Demographic Information
3.2. Gut Microbiota Relative Abundance in the Studied Dogs
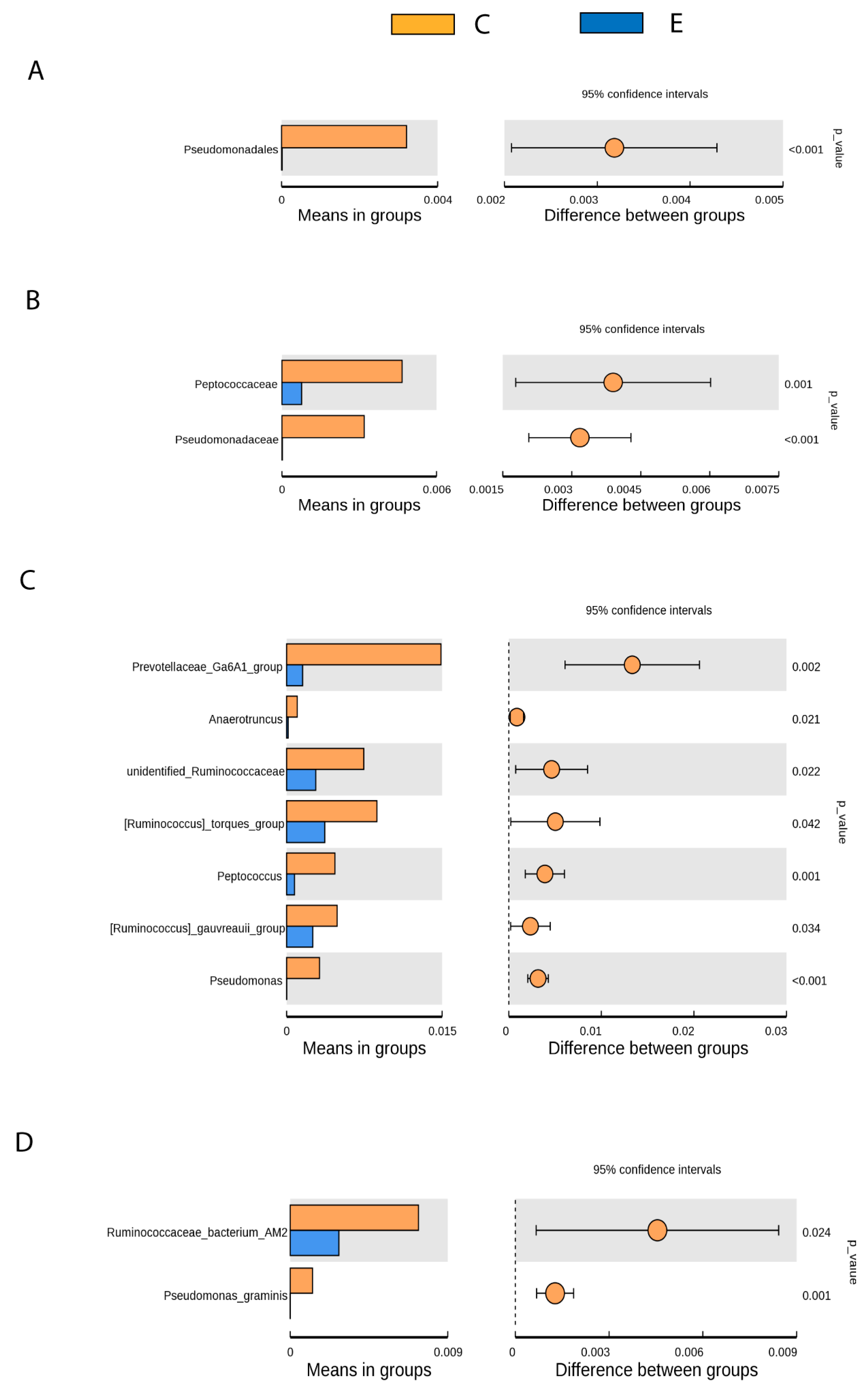
3.3. Gut Microbiota Differences between Healthy and Drug-Naive Epileptic Dogs: Effect of Disease
- Pseudomonadales (Order), Pseudomonadaceae (Family), Pseudomonas (Genus), Pseudomona_graminis (Species) (p < 0.001).
- Prevotellaceae Ga6A1 group (Genus) (p < 0.05).
- Peptococcaceae (Family), Anaerotruncus, unidentified Ruminococaceae, Ruminococcus torques group, Peptococcus y Ruminococcus gauvreauii group (Genus), Ruminococcaceae bacterium_AM2 (Species) (p < 0.05).
3.4. Gut Microbiota Changes after Introduction of AEDs: Effect of Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzolli, R.; Pessione, E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Eguilaz, M.; Ramón-Trapero, J.L.; Pérez-Marínez, L.; Blanco, J.R. The microbiota-gut-brain axis and its great projections. Rev. Neurol. 2019, 68, 111–117. [Google Scholar] [PubMed]
- Wang, H.X.; Wang, Y.P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome; intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [Green Version]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.F.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Bibbó, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmcol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef]
- Kearsley-Fleet, L.; O’Neill, D.G.; Volk, H.A.; Church, D.B.; Brodbelt, D.C. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec. 2013, 172, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlen, A.; Potschka, H.; Volk, H.A.; Sauter-Louis, C.; O’Neill, D.G. Seizure occurrence in dogs under primary veterinary care in the UK: Prevalence and risk factors. J. Vet. Intern. Med. 2018, 32, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, Y.; Hasegawa, D.; Mizoguchi, S.; Yu, Y.; Wada, M.; Kuwabara, T.; Fujiwara-Igarashi, A.; Fujita, M. Retrospective epidemiological study of canine epilepsy in Japan using the International Veterinary Epilepsy Task Force classification 2015 (2003-2013): Etiological distribution; risk factors; survival time; and lifespan. BMC Vet. Res. 2016, 12, 248. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.; Labruyere, J.; Volk, H.; Cardy, T.J. Estimation of the prevalence of idiopathic epilepsy and structural epilepsy in a general population of 900 dogs undergoing MRI for epileptic seizures. Vet. Rec. 2020, 187, e89. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; French, J.A.; Peruca, E. Challenges in the clinical development of new antiepileptic drugs. Pharmacol. Res. 2016, 103, 95–104. [Google Scholar] [CrossRef]
- Bankstahl, M.; Bankstahl, J.P. Recent Advances in Radiotracer Imaging Hold Potential for Future Refined Evaluation of Epilepsy in Veterinary Neurology. Front. Vet. Sci. 2017, 4, 218. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, M.; Prast-Nielsen, S. The gut microbiome and epilepsy. EBioMedicine 2019, 44, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef] [Green Version]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Lazarow, A.; Nissim, I. Ketogenic diet; amino acid metabolism; and seizure control. J. Neurosci. Res. 2001, 66, 931–940. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, Y.; Yang, H.; Rao, Y.; Miao, J.; Lu, X. Intestinal microbiota as an alternative therapeutic target for epilepsy. Can. J. Infect. Dis. Med. Microbiol. 2016, 9032809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Eguilaz, M.; Ramón-Trapero, J.L.; Pérez-Marínez, L.; Blanco, J.R. The beneficial effect of probiotics as a supplementary treatment in drug-resistent epilepsy: A pilot study. Benef. Microbes 2018, 9, 875–881. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [Green Version]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, N.D.; Barker, A.K.; Alcott, C.J.; Levine, J.M.; Meren, I.; Wengert, J.; Jergens, A.E.; Suchodolski, J.S. The association of specific constituents of the fecal microbiota with immune-mediated brain disease in dogs. PLoS ONE 2017, 12, e0170589. [Google Scholar] [CrossRef] [Green Version]
- Kirchoff, N.S.; Udell, M.A.R.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef] [Green Version]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P.A. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef] [PubMed]
- Muñana, K.R.; Jacob, M.E.; Callahan, B.J. Evaluation of fecal Lactobacillus populations in dogs with idiopathic epilepsy: A pilot study. Anim. Microbiome 2020, 2, 19. [Google Scholar] [CrossRef]
- Pilla, R.; Law, T.H.; Pan, Y.; Zanghi, B.M.; Li, Q.; Want, E.J.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S.; Volk, H.A. The effects of a ketogenic medium-chain triglyceride diet on the feces in dogs with idiopathic epilepsy. Front. Vet. Sci. 2020, 7, 541547. [Google Scholar] [CrossRef] [PubMed]
- Law, T.H.; Davies, E.S.; Pan, Y.; Zanghi, B.; Want, E.; Volk, H.A. A randomised trial of a medium-chain TAG diet as treatment for dogs with idiopathic epilepsy. Br. J. Nutr. 2015, 114, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berk, B.J.; Packer, R.M.A.; Law, T.H.; Wessmann, A.; Bathen-Nöthen, A.; Jokinen, T.S.; Knebel, A.; Tipold, A.; Pelligand, L.; Volk, H.A. A double-blinded randomised dietary supplement crossover trial design to investigate the short-term influence of medium chain fatty acid (MCT) supplement on canine idiopathic epilepsy: Study protocol. BMC Vet. Res. 2019, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Jean-Philippe, C.; Conboy, L.; Añor, S.; de la Fuente, C.; Wrzosek, M.A.; Spycher, A.; Luchsinger, E.; Wenger-Riggenbach, B.; Montoliu, P.; et al. Efficacy of medium chain triglyceride oil dietary supplementation in reducing seizure frequency in dogs with idiopathic epilepsy without cluster seizures: A non-blinded; prospective clinical trial. Vet. Rec. 2020, 187, 356. [Google Scholar] [CrossRef]
- Ambrosini, Y.M.; Borcherding, D.; Kanthasamy, A.; Kim, H.J.; Willette, A.A.; Jergens, A.; Allenspach, K.; Mochel, J.P. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: A review. Font. Aging Neurosci. 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Overall, K. Natural animal models of human psychiatric conditions: Assessment of mechanism and validity. Prog. Neuropsychopharmacol. Biol. Psychiat. 2000, 24, 727–776. [Google Scholar] [CrossRef]
- Sarasa, M.; Pesini, P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potschka, H.; Fischer, A.; von Rüden, E.L.; Hülsmeyer, V.; Baumgärtner, W. Canine epilepsy as a translational model? Epilepsia 2013, 54, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef]
- De Risio, L.; Bhatti, S.; Muñana, K.; Penderis, J.; Stein, V.; Tipold, A.; Berendt, M.; Farqhuar, R.; Fischer, A.; Long, S.; et al. International veterinary epilepsy task force consensus proposal: Diagnostic approach to epilepsy in dogs. Vet. Res. 2015, 11, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum; jejunum; ileum and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Middelbos, I.S.; Vester Boler, B.M.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey, G.C., Jr. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 545 pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandri, G.; Milani, C.; Mancabelli, L.; Mangifesta, M.; Lugli, G.A.; Viappini, A.; Duranti, S.; Turroni, F.; Ossiprandi, M.C.; van Sinderen, D.; et al. Metagenomic dissection of the canine gut microbiota: Insights into taxonomic, metabolic and nutritional features. Environ. Microbiol. 2019, 21, 1331–1343. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans; dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel16S rRNAgene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthydogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- You, I.; Kim, M.J. Comparison of gut microbiota of 96 healthy dogs by individual traits: Breed, age, and body condition score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Reddy, K.E.; Kim, H.R.; Jeong, J.Y.; So, K.M.; Lee, S.; Ji, S.Y.; Kim, M.; Lee, H.J.; Lee, S.; Kim, K.H.; et al. Impact of breed on the fecal microbiome of dogs under the same dietary condition. J. Microbiol. Biotechnol. 2019, 29, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Scarsella, E.; Stefanon, B.; Cintio, M.; Licastro, D.; Sgorlon, S.; Dal Monego, S.; Sandri, M. Learning machine approach reveals microbial signatures of diet and sex in dog. PLoS ONE 2020, 15, e0237874. [Google Scholar] [CrossRef]
- Bresciani, F.; Minamoto, Y.; Suchodolski, J.S.; Galiazzo, G.; Vecchiato, C.G.; Pinna, C.; Biagi, G.; Pietra, M. Effect of an extruded animal protein-free diet on fecal microbiota of dogs with food-responsive enteropathy. J. Vet. Intern. Med. 2018, 32, 1903–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial Diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity; stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koponen, K.K.; Salosensaari, A.; Ruuskanen, M.O.; Havulinna, A.S.; Männistö, S.; Jousilahti, P.; Palmu, J.; Salido, R.; Sanders, K.; Brennan, C.; et al. Associations of healthy food choices with gut microbiota profiles. Am. J. Clin. Nutr. 2021, 21, nqab077. [Google Scholar] [CrossRef]
- Schmitz, S.; Suchodolski, J.S. Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics—What is the evidence? Vet. Med. Sci. 2016, 2, 71–94. [Google Scholar] [CrossRef]
- Gong, X.; Cai, Q.; Liu, X.; An, D.; Zhou, D.; Luo, R.; Peng, R.; Hong, Z. Gut flora and metabolism are altered in epilepsy and partially restored after ketogenic diets. Microb. Pathog. 2021, 155, 104899. [Google Scholar] [CrossRef] [PubMed]
- Gadea, A.; López-Colomé, A.M. Glial transporters for glutamate; glycine; and GABA: II. GABA transporters. J. Neurosci. Res. 2001, 63, 461–468. [Google Scholar] [CrossRef]
- Reyes-Darias, J.A.; García, V.; Rico-Jiménez, M.; Corral-Lugo, A.; Lesouhaitier, O.; Juárez-Hernández, D.; Yang, Y.; Bi, S.; Feuilloley, M.; Muñoz-Rojas, J.; et al. Specific gamma-aminobutyrate chemotaxis in pseudomonads with different lifestyle. Mol. Microbiol. 2015, 97, 488–501. [Google Scholar] [CrossRef]
- Dagorn, A.; Hillion, M.; Chapalain, A.; Lesouhaitier, O.; Duclairoir Poc, C.; Vieillard, J.; Chevalier, S.; Taupin, L.; Le Derf, F.; Feuilloley, M.G.J. Gamma-aminobutyric acid acts as a specific virulence regulator in Pseudomonas aeruginosa. Microbiology 2013, 159, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Abadias, M.; Anguera, M.; Viñas, I. Efficacy of Pseudomonas graminis CPA-7 against Salmonella spp. and Listeria monocytogenes on fresh-cut pear and setting up of the conditions for its commercial application. Food Microbiol. 2018, 70, 103–112. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Feng, X.; Li, D.; Li, X.; Ouyang, Q.; Dai, W.; Wu, G.; Zhou, Q.; Wang, P.; et al. Distinct gut microbiota composition and functional category in children with cerebral palsy and epilepsy. Front. Pediatr. 2019, 7, 394. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary probiotic treatment and vitamin B7 in depression-a randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Albhaisi, S.; Shamsaddini, A.; Fagan, A.; McGeorge, S.; Sikaroodi, M.; Gavis, E.; Patel, S.; Davis, B.C.; Acharya, C.; Sterling, R.K.; et al. Gut microbial signature of hepatocellular cancer in men with cirrhosis. Liver Transpl. 2021, 27, 629–640. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Singh, A.K.; Lee, C.N.; Jha, R.; Mishra, B. Dietary supplementation of dried plum: A novel strategy to mitigate heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 58. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo, M.G.; San Juan, C.; Larrosa, M. Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
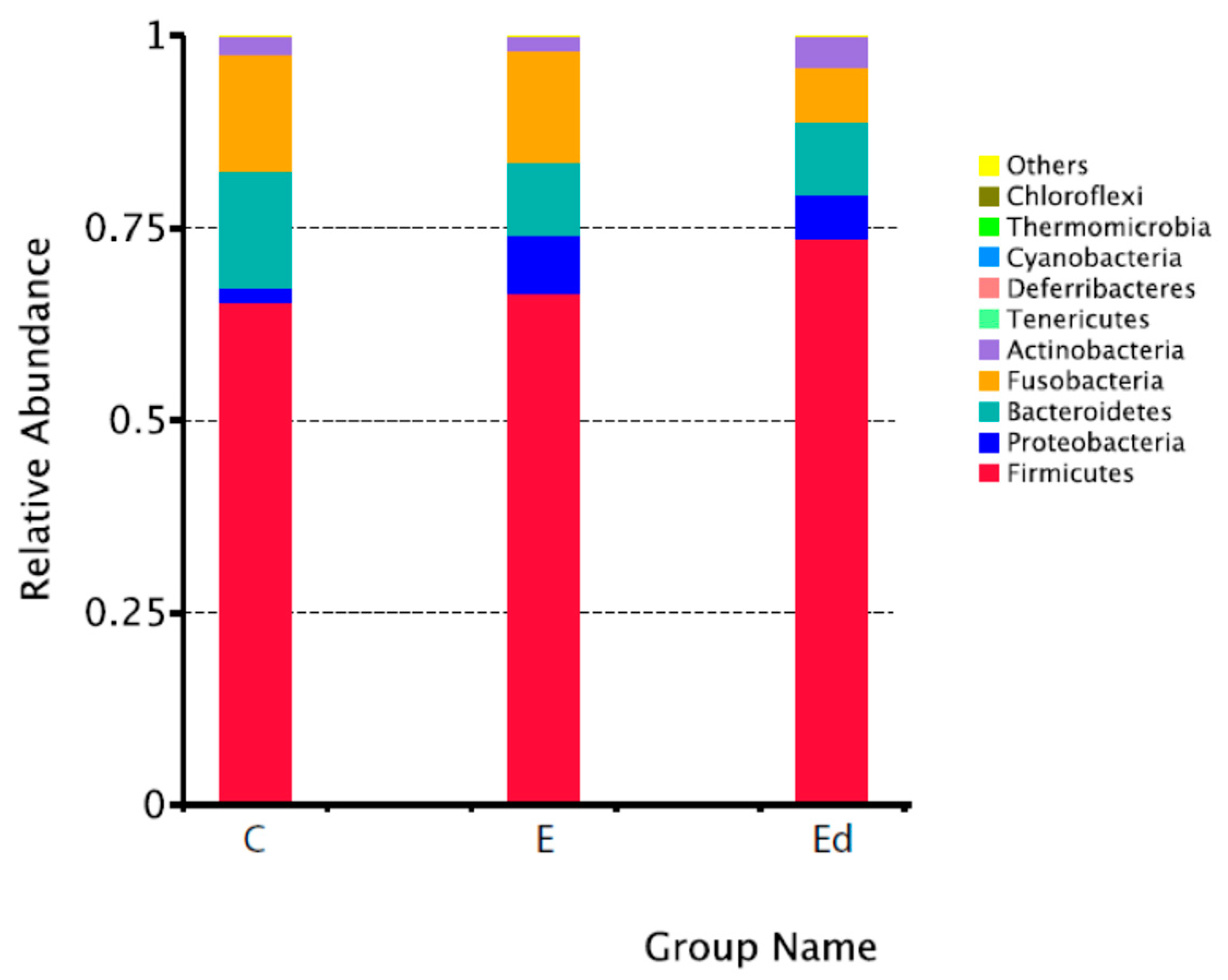


| Philum | Class | Order | Family | Genus |
|---|---|---|---|---|
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Peptoclostridium |
| C: 65.4 ± 16.7% | C: 54.5 ± 15.4% | C: 54.5 ± 15.4% | C: 26.5 ± 10.4% | C: 21.8 ± 7.9% |
| E: 66.6 ± 16.1% | E: 50.0 ± 13.6% | E: 50.0 ± 13.6% | E: 26.6 ± 11.6% | E: 17.9 ± 8.0% |
| Ed: 73.7 ± 14.3% | Ed: 54.0 ± 11.3% | Ed: 54.0 ± 11.3% | Ed: 29.0 ± 8.0% | Ed: 19.3 ± 9.6% |
| Bacteroidetes | Bacteroidia | Bacteroidales | Peptostreptococcaceae | Fusobacterium |
| C: 15.3 ± 9.5% | C: 15.3 ± 9.5% | C: 15.3 ± 9.5% | C: 22.5 ± 7.9% | C: 12.8 ± 8.2% |
| E: 9.5 ± 7.3% | E: 9.5 ± 7.3% | E: 9.5 ± 7.3% | E: 19.5 ± 7.6% | E: 12.5 ± 8.4% |
| Ed: 9.5 ± 8.2% | Ed: 9.5 ± 8.2% | Ed: 9.5 ± 8.2% | Ed: 20.6 ± 9.2% | Ed: 5.7 ± 5.5% |
| Fusobacteria | Fusobacteriia | Fusobacteriales | Fusobacteriaceae | Blautia |
| C: 15.2 ± 9.1% | C: 15.2 ± 9.2% | C: 15.2 ± 9.2% | C: 12.8 ± 8.2% | C: 12.2 ± 5.5% |
| E: 14.5 ± 10.0% | E: 14.5 ± 10.0% | E: 14.5 ± 10.0% | E: 12.5 ± 8.4% | E: 12.5 ± 7.1% |
| Ed: 7.0 ± 7.1% | Ed: 7.0 ± 7.1% | Ed: 7.0 ± 7.1% | Ed: 5.7 ± 5.5% | Ed: 13.8 ± 4.3% |
| Proteobacteria | Erysipelotrichia | Erysipelotrichiales | Bacteroidaceae | Bacteroides |
| C: 1.8 ± 0.8% | C: 6.6 ± 3.3% | C: 6.6 ± 3.3% | C: 8.0 ± 5.8% | C: 8.0 ± 5.8% |
| E: 7.4 ± 15.3% | E: 4.8 ± 2.8% | E: 4.8 ± 2.8% | E: 6.1 ± 6.0% | E: 6.1 ± 6.0% |
| Ed: 5.7 ± 11.0% | Ed: 7.2 ± 5.9% | Ed: 7.2 ± 5.9% | Ed: 4.8 ± 4.8% | Ed: 4.8 ± 4.8% |
| Actinobacteria | Negativicutes | Selenomonadales | Erysipelotrichaceae | Ruminococcus |
| _gnavus_group | ||||
| C: 2.3 ± 1.6% | C: 3.6 ± 3.0% | C: 3.6 ± 3.0% | C: 6.6 ± 3.3% | C: 5.0 ± 6.3% |
| E: 1.9 ± 1.8% | E: 9.8 ± 15.7% | E: 9.8 ± 15.7% | E: 4.8 ± 2.8% | E: 4.1 ± 2.3% |
| Ed: 4.0 ± 5.1% | Ed: 7.1 ± 6.8% | Ed: 7.1 ± 6.8% | Ed: 7.2 ± 5.9% | Ed: 4.9 ± 3.5% |
| Tenericutes | Coriobacteriia | Coriobacteriales | Prevotellaceae | Alloprevotella |
| C: 0.001 ± 0.0003% | C: 2.3 ± 1.6% | C: 2.3 ± 1.6% | C: 7.2 ± 5.9% | C: 2.9 ± 3.7% |
| E: 0.001 ± 0.0003% | E: 1.9 ± 1.8% | E: 1.9 ± 1.8% | E: 3.4 ± 4.4% | E: 2.7 ± 4.0% |
| Ed: 0.003 ± 0.005% | Ed: 3.9 ± 5.1% | Ed: 3.9 ± 5.1% | Ed: 4.6 ± 5.0% | Ed: 2.0 ± 2.1% |
| Deferribacteres | Gammaproteobacteria | Lactobacillales | Veillonellaceae | Prevotella_9 |
| C: 0.003 ± 0.0004% | C: 1.1 ± 0.6% | C: 0.7 ± 1.7% | C: 2.5 ± 3.0% | C: 2.8 ± 3.7% |
| E: 0.0001 ± 0.0002% | E: 6.5 ± 14.3% | E: 2.0 ± 3.9% | E: 9.1 ± 15.8% | E: 0.5 ± 0.6% |
| Ed: 0.001 ± 0.003% | Ed: 4.1 ± 8.1% | Ed: 5.2 ± 7.6% | Ed: 6.6 ± 6.7% | Ed: 2.5 ± 3.1% |
| Cyanobacteria | Bacilli | Enterobacteriales | Streptococcaceae | Megamonas |
| C: 0.001 ± 0.004% | C: 0.8 ± 1.7% | C: 0.3 ± 0.2% | C: 0.7 ± 1.7% | C: 2.5 ± 3.0% |
| E: 0.0 ± 0.0% | E: 2.0 ± 3.9% | E: 6.0 ± 14.4% | E: 1.3 ± 3.4% | E: 9.1 ± 15.7% |
| Ed: 0.0 ± 0.0% | Ed: 5.2 ± 7.6% | Ed: 3.9 ± 8.2% | Ed: 3.8 ± 7.6% | Ed: 5.5 ± 6.9% |
| Thermomicrobia | Betaproteobacteria | Burkholderiales | Enterobacteriaceae | Streptococcus |
| C: 0.0 ± 0.0% | C: 0.6 ± 0.6% | C: 0.6 ± 0.6% | C: 0.3 ± 0.2% | C: 0.7 ± 1.7% |
| E: 0.0001 ± 0.003% | E: 0.8 ± 10.1% | E: 0.8 ± 10.1% | E: 6.0 ± 14.4% | E: 1.3 ± 3.4% |
| Ed: 0.0007 ± 0.0002% | Ed: 1.5 ± 3.0% | Ed: 1.5 ± 3.0% | Ed: 3.9 ± 8.2% | Ed: 3.8 ± 7.6% |
| Chloroflexi | Unidentified | Aeromonadales | Lactobacillaceae | Lactobacillus |
| C: 0.0 ± 0.0% | Actinobacteria | C: 0.5 ± 0.4% | C: 0.05 ± 0.1% | C: 0.05 ± 1.2% |
| E: 0.0 ± 0.0% | C: 0.01 ± 0.01% | E: 0.5 ± 0.5% | E: 0.5 ± 1.0% | E: 0.5 ± 1.0% |
| Ed: 0.001 ± 0.003% | E: 0.02 ± 0.02% | Ed: 0.2 ± 0.2% | Ed: 0.1 ± 0.2% | Ed: 0.1 ± 0.2% |
| Ed: 0.07 ± 0.09% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Belenguer, S.; Grasa, L.; Valero, O.; Palacio, J.; Luño, I.; Rosado, B. Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment. Animals 2021, 11, 3121. https://doi.org/10.3390/ani11113121
García-Belenguer S, Grasa L, Valero O, Palacio J, Luño I, Rosado B. Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment. Animals. 2021; 11(11):3121. https://doi.org/10.3390/ani11113121
Chicago/Turabian StyleGarcía-Belenguer, Sylvia, Laura Grasa, Olga Valero, Jorge Palacio, Isabel Luño, and Belén Rosado. 2021. "Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment" Animals 11, no. 11: 3121. https://doi.org/10.3390/ani11113121
APA StyleGarcía-Belenguer, S., Grasa, L., Valero, O., Palacio, J., Luño, I., & Rosado, B. (2021). Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment. Animals, 11(11), 3121. https://doi.org/10.3390/ani11113121







