Allonursing in Wild and Farm Animals: Biological and Physiological Foundations and Explanatory Hypotheses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Lactation: An Energetic Costly Period
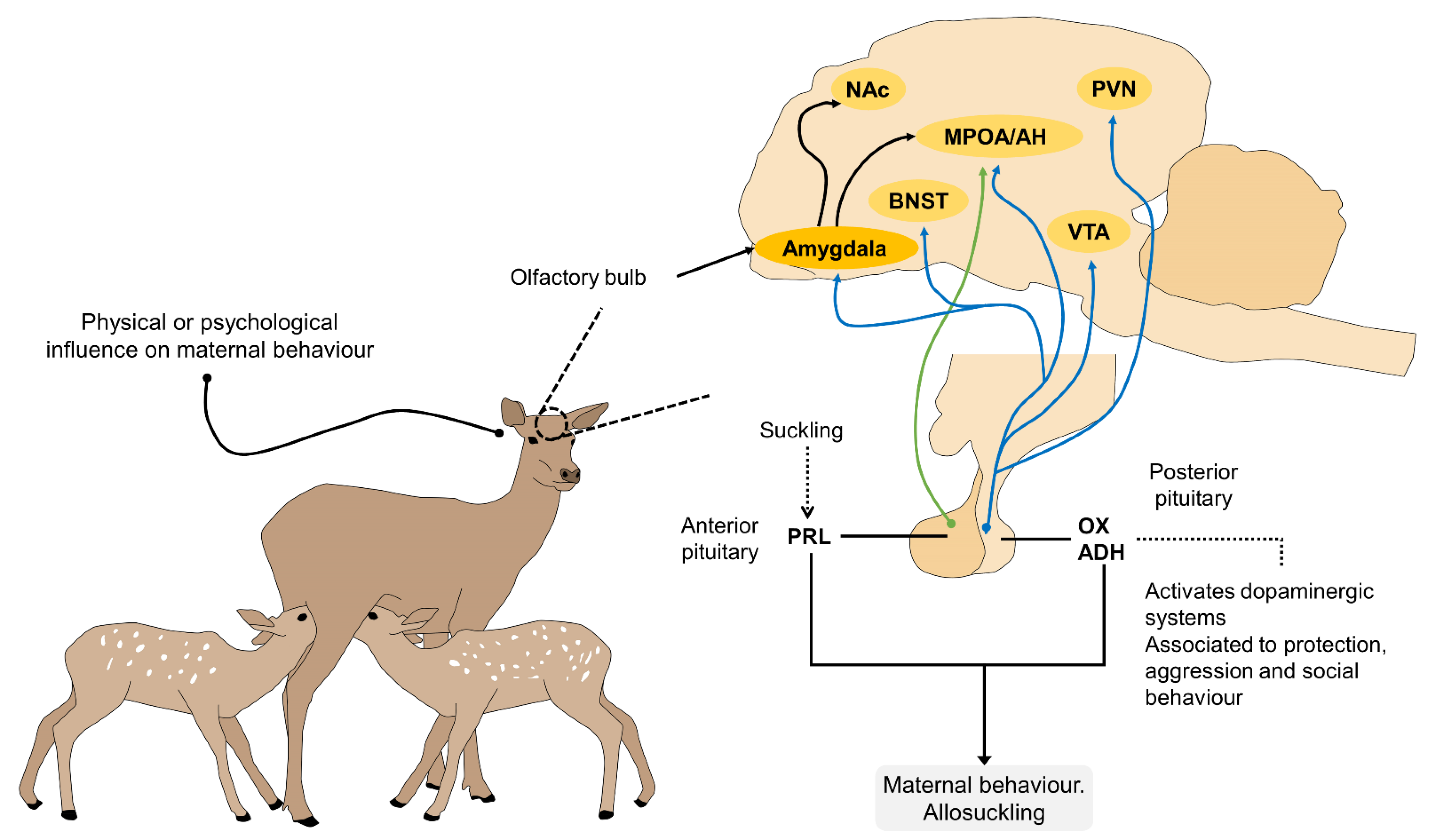
3. The Neurophysiology of Suckling
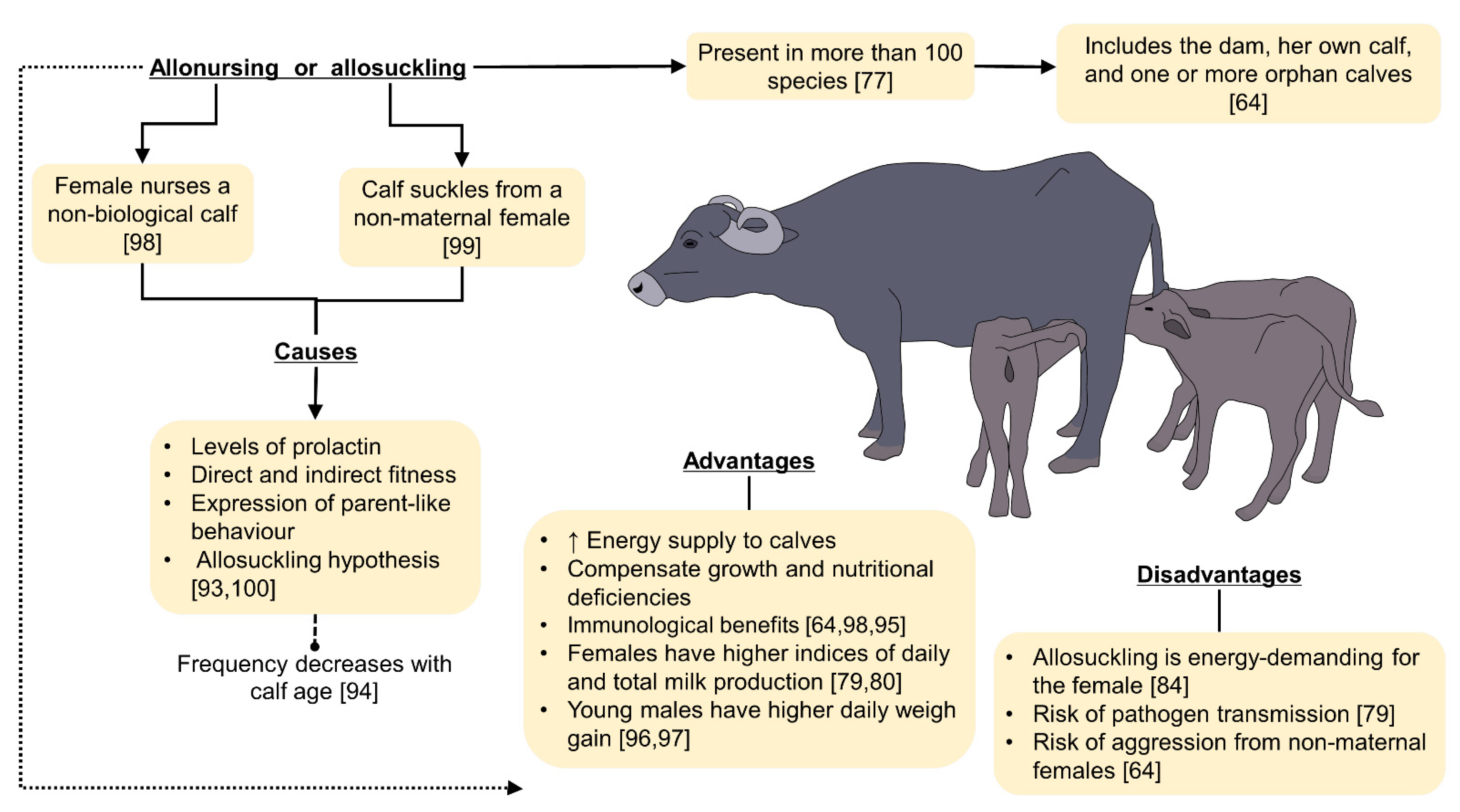
4. The Main Hypotheses Explaining Allonursing and Allosuckling
4.1. Allonursing: A Strategy Adopted by Dams?
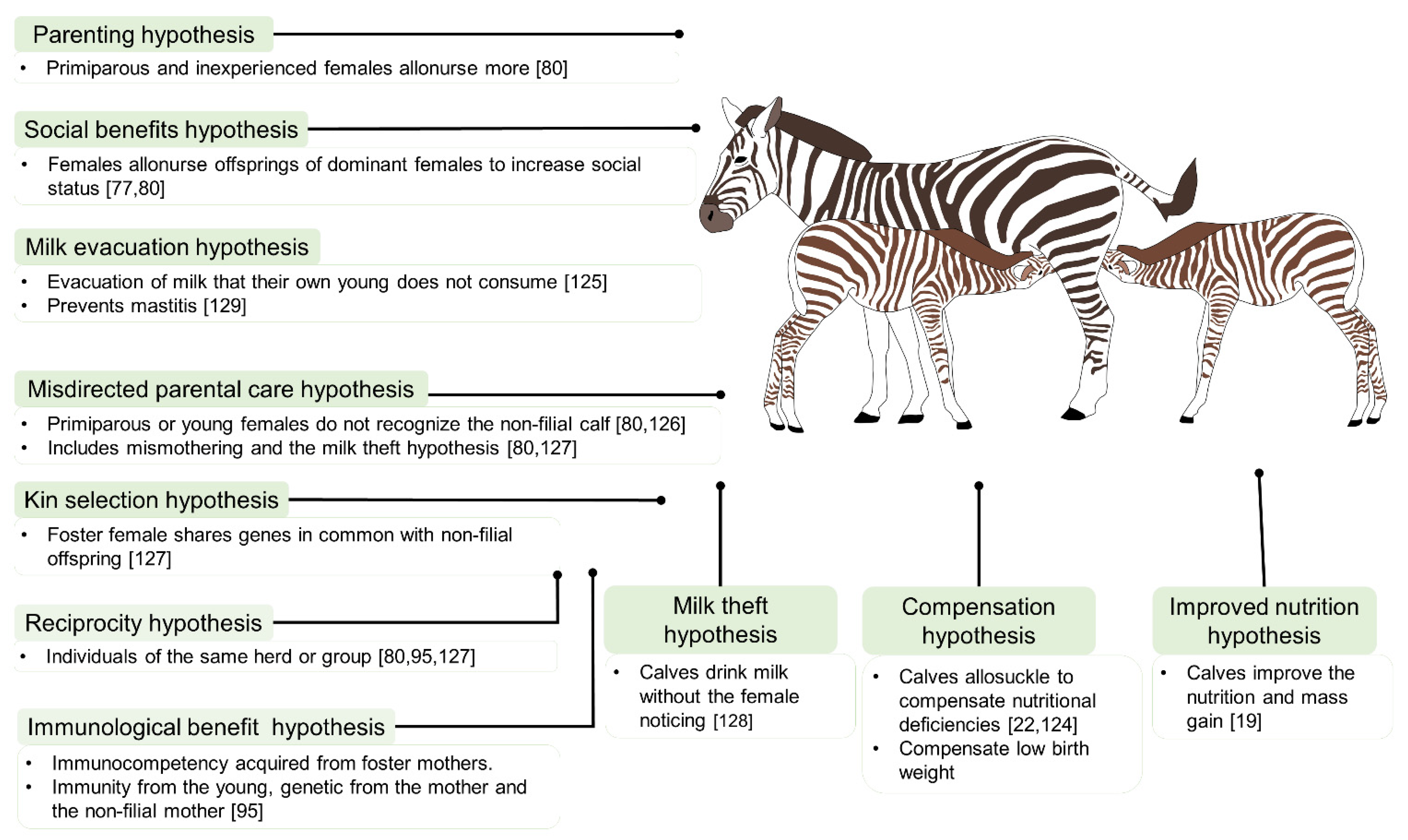
4.1.1. Kin Selection or Selective Parenting
4.1.2. Reciprocity Hypothesis
4.1.3. Parenting Hypothesis
4.1.4. Social Benefits Hypothesis
4.1.5. Milk Evacuation (Milk Dumping)
4.1.6. Misdirected Parental Care
4.2. Allosuckling: A Strategy for Offspring?
4.2.1. Milk Theft
4.2.2. Compensation
4.2.3. Improved Nutrition
Effects of Sex and Age of Offspring on Allonursing
4.2.4. Immunological Function or Benefit
4.3. Altruism in Allomaternal Care?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zilkha, N.; Sofer, Y.; Beny, Y.; Kimchi, T. From classic ethology to modern neuroethology: Overcoming the three biases in social behavior research. Curr. Opin. Neurobiol. 2016, 38, 38–96. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.G. La evolución y la conservación de la biodiversidad. In Evolución: La Base de la Biología; Sur, P., Ed.; Universidad de Extremadura: Extremadura, Spain, 2012; pp. 407–416. [Google Scholar]
- Lévy, F.; Keller, M. Neurobiology of maternal behavior in sheep. Adv. Study Behav. 2008, 38, 399–437. [Google Scholar] [CrossRef]
- Mora-Medina, P.; Orihuela-Trujillo, A.; Santiago, R.; Arch-Tirado, E.; Vázquez-Cruz, C.; Mota-Rojas, D. Metabolic changes during brief periods of ewe-lamb separation at different ages. Anim. Prod. Sci. 2018, 58, 1297–1306. [Google Scholar] [CrossRef]
- Mandujano, C. Ecología y sociobiología de la impronta: Perspectivas para su estudio en los Crocodylia. Cienc. Mar. 2010, 14, 49–54. [Google Scholar]
- Yamaguchi, S.; Aoki, N.; Kitajima, T.; Iikubo, E.; Katagiri, S.; Matsushima, T.; Homma, K.J. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 2012, 3, 1081. [Google Scholar] [CrossRef] [Green Version]
- Poindron, P. Mechanisms of activation of maternal behaviour in mammals. Reprod. Nutr. Dev. 2005, 45, 341–351. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Nowak, R. Socio-spatial criteria are important for the establishment of maternal preference in lambs. Appl. Anim. Behav. Sci. 2006, 96, 269–280. [Google Scholar] [CrossRef]
- Nowak, R.; Keller, M.; Val-Laillet, D.; Lévy, F. Perinatal visceral events and brain mechanisms involved in the development of mother-young bonding in sheep. Horm. Behav. 2007, 52, 92–98. [Google Scholar] [CrossRef]
- Muir, G.D. Early ontogeny of locomotor behaviour: A comparison between altricial and precocial animals. Brain Res. Bull. 2000, 53, 719–726. [Google Scholar] [CrossRef]
- Wöhr, M.; Oddi, D.; D’Amato, F.R. Effect of altricial pup ultrasonic vocalization on maternal behavior. Handb. Behav. Neurosci. 2010, 19, 159–166. [Google Scholar] [CrossRef]
- Broad, K.D.; Curley, J.P.; Keverne, E.B. Mother–infant bonding and the evolution of mammalian social relationships. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, H.; Terrazas, A.; Poindron, P.; Ramírez-Vera, S.; Flores, J.A.; Delgadillo, J.A.; Vielma, J.; Duarte, G.; Fernández, I.G.; Fitz-Rodríguez, G.; et al. Sensorial and physiological control of maternal behavior in small ruminants: Sheep and goats. Trop. Subtrop. Agroecosyst. 2012, 15, S91–S102. [Google Scholar]
- Nowak, R.; Porter, R.H.; Lévy, F.; Orgeur, P.; Schaal, B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev. Reprod. 2000, 5, 153–163. [Google Scholar] [CrossRef]
- Dubey, P.; Singh, R.R.; Choudhary, S.S.; Verma, K.K.; Kumar, A.; Gamit, P.M.; Dubey, S.; Prajapati, K. Post parturient neonatal behaviour and their relationship with maternal behaviour score, parity and sex in surti buffaloes. J. Appl. Anim. Res. 2018, 46, 360–364. [Google Scholar] [CrossRef]
- Glasper, E.R.; Kenkel, W.M.; Bick, J.; Rilling, J.K. More than just mothers: The neurobiological and neuroendocrine underpinnings of allomaternal caregiving. Front. Neuroendocrinol. 2019, 53, 100741. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.D. What drives cooperative breeding? PLoS Biol. 2017, 15, e2002965. [Google Scholar] [CrossRef] [Green Version]
- Lukas, D.; Clutton-Brock, T. Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B Biol. Sci. 2012, 279, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.C.; Weladji, R.B.; Holand, Ø.; Nieminen, M. Allosuckling in reindeer (Rangifer tarandus): A test of the improved nutrition and compensation hypotheses. Mamm. Biol. 2016, 81, 146–152. [Google Scholar] [CrossRef]
- Jennions, M.D.; Macdonald, D.W. Cooperative breeding in mammals. Trends Ecol. Evol. 1994, 9, 89–93. [Google Scholar] [CrossRef]
- Schubert, M.; Pillay, N.; Schradin, C. Parental and alloparental care in a polygynous mammal. J. Mammal. 2009, 90, 724–731. [Google Scholar] [CrossRef]
- Orihuela, A.; Pérez-Torres, L.I.; Ungerfeld, R. Evidence of cooperative calves’ care and providers’ characteristics in zebu cattle (Bos indicus) raised under extensive conditions. Trop. Anim. Health Prod. 2021, 53, 143. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, L.; Orihuela, A.; Corro, M.; Rubio, I.; Cohen, A.; Galina, C.S. Maternal protective behavior of zebu type cattle (Bos indicus) and its association with temperament. J. Anim. Sci. 2014, 92, 4694–4700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriquez, M.F.; Pérez-Torres, L.; Orihuela, A.; Rubio, I.; Corro, M.; Galina, C.S. Relationship between protective maternal behavior and some reproductive variables in zebu-type cows (Bos indicus). J. Anim. Behav. Biometeorol. 2021, 9, 2124. [Google Scholar] [CrossRef]
- Špinka, M.; Illmann, G. Nursing behavior. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 297–318. [Google Scholar]
- Alonso-Spilsbury, M.; Ramirez-Necoechea, R.; Gonzalez-Lozano, M.; Mota-Rojas, D.; Trujillo-Ortega, M. Piglet survival in early lactation: A review. J. Anim. Vet. Adv. 2007, 6, 76–86. [Google Scholar]
- Sánchez-Andrade, G.; James, B.M.; Kendrick, K.M. Neural encoding of olfactory recognition memory. J. Reprod. Dev. 2005, 51, 547558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Medina, P.; Orihuela-Trujillo, A.; Arch-Tirado, E.; Roldan, S.; Terrazas, A.; Mota-Rojas, D. Sensory factors involved in mother-young bonding in sheep: A review. Vet. Med. 2016, 61, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, M.; Soto, R.; Poindron, P.; Alvarez, L.; Valencia, J.; Gonzalez, F.; Terrazas, A. Maternal behaviour around birth and mother-young recognition in Pelibuey sheep. Vet. Mex. 2011, 42, 27–46. [Google Scholar]
- Brus, M.; Meurisse, M.; Keller, M.; Lévy, F. Interactions with the young down-regulate adult olfactory neurogenesis and enhance the maturation of olfactory neuroblasts in sheep mothers. Front. Behav. Neurosci. 2014, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Bădescu, I.; Watts, D.P.; Katzenberg, M.A.; Sellen, D.W. Alloparenting is associated with reduced maternal lactation effort and faster weaning in wild chimpanzees. R. Soc. Open Sci. 2016, 3, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota-Rojas, D.; Orihuela, A.; Napolitano, F.; Mora-Medina, P.; Gregorio, H.; Alonso-Spilsbury, M. Olfaction in animal behaviour and welfare. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Poindron, P.; Lévy, F.; Krehbiel, D. Genital, olfactory, and endocrine interactions in the development of maternal behaviour in the parturient ewe. Psychoneuroendocrinology 1988, 13, 99–125. [Google Scholar] [CrossRef]
- Poindron, P.; Lévy, F. Physiological, sensory, and experiential determinants of maternal behavior in sheep. In Mammalian Parenting: Biochemical; Neurobiological; and Behavioral Determinants; Krasnegor, N.A., Bridges, R.S., Eds.; Oxford University Press: Oxford, UK, 1990; pp. 133–156. [Google Scholar]
- Porter, R.H.; Lévy, F.; Poindron, P.; Litterio, M.; Schaal, B.; Beyer, C. Individual olfactory signatures as major determinants of early maternal discrimination in sheep. Dev. Psychobiol. 1991, 24, 151–158. [Google Scholar] [CrossRef]
- Price, E.O.; Dunn, G.C.; Talbot, J.A.; Dally, M.R. Fostering lambs by odor transfer: The substitution experiment. J. Anim. Sci. 1984, 59, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.; Stevens, D. Odour cues to maternal recognition of lambs: An investigation of some possible sources. Appl. Anim. Ethol. 1982, 9, 165–175. [Google Scholar] [CrossRef]
- Booth, K.; Katz, L.S. Role of the vomeronasal organ in neonatal offspring recognition in sheep. Biol. Reprod. 2000, 63, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.; Stevens, D.; Bradley, L.R. Washing lambs and confinement as aids to fostering. Appl. Anim. Ethol. 1983, 10, 251–261. [Google Scholar] [CrossRef]
- Fraser, E.J.; Shah, N.M. Complex chemosensory control of female reproductive behaviors. PLoS ONE 2014, 9, e90368. [Google Scholar] [CrossRef] [Green Version]
- Kratzing, J. The structure of the vomeronasal organ in the sheep. J. Anat. 1971, 108, 247–260. [Google Scholar]
- Fleming, A.; Vaccarino, F.; Tambosso, L.; Chee, P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science 1979, 203, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J.; Beauchamp, G.K.; Reidinger, R.R.; Wellington, J.L. Access of large and nonvolatile molecules to the vomeronasal organ of mammals during social and feeding behaviors. J. Chem. Ecol. 1985, 11, 1147–1159. [Google Scholar] [CrossRef]
- Bean, N.J. Modulation of agonistic behavior by the dual olfactory system in male mice. Physiol. Behav. 1982, 29, 433–437. [Google Scholar] [CrossRef]
- Bean, N.J. Olfactory and vomeronasal mediation of ultrasonic vocalizations in male mice. Physiol. Behav. 1982, 28, 31–37. [Google Scholar] [CrossRef]
- Winans, S.S.; Powers, J.B. Olfactory and vomeronasal deafferentation of male hamsters: Histological and behavioral analyses. Brain Res. 1977, 126, 325–344. [Google Scholar] [CrossRef] [Green Version]
- Bellringer, J.F.; Hester Pratt, P.M.; Keverne, E.B. Involvement of the vomeronasal organ and prolactin in pheromonal induction of delayed implantation in mice. J. Reprod. Fertil. 1980, 59, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Clancy, A.N.; Macrides, F.; Singer, A.G.; Agosta, W.C. Male hamster copulatory responses to a high molecular weight fraction of vaginal discharge: Effects of vomeronasal organ removal. Physiol. Behav. 1984, 33, 653–660. [Google Scholar] [CrossRef]
- Coquelin, A.; Clancy, A.N.; Macrides, F.; Noble, E.P.; Gorski, R.A. Pheromonally induced release of luteinizing hormone in male mice: Involvement of the vomeronasal system. J. Neurosci. 1984, 4, 2230–2236. [Google Scholar] [CrossRef]
- Johns, M.A.; Feder, H.H.; Komisaruk, B.R.; Mayer, A.D. Urine-induced reflex ovulation in anovulatory rats may be a vomeronasal effect. Nature 1978, 272, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Lehman, M.N.; Winans, S.S.; Powers, J.B. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science 1980, 210, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.B.; Winans, S.S. Vomeronasal organ: Critical role in mediating sexual behavior of the male hamster. Science 1975, 187, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.; Keverne, E.B. The accessory olfactory system and its role in the pheromonally mediated suppression of oestrus in grouped mice. J. Reprod. Fertil. 1979, 57, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Wysocki, C.J.; Katz, Y.; Bernhard, R. Male vomeronasal organ mediates female-induced testosterone surges in mice. Biol. Reprod. 1983, 28, 917–922. [Google Scholar] [CrossRef]
- Wysocki, C.J.; Nyby, J.; Whitney, G.; Beauchamp, G.K.; Katz, Y. The vomeronasal organ: Primary role in mouse chemosensory gender recognition. Physiol. Behav. 1982, 29, 315–327. [Google Scholar] [CrossRef]
- Estes, R.D. Mammalia: The role of the vomeronasal organ in mammalian reproduction. Mammalia 1972, 36, 315–341. [Google Scholar] [CrossRef]
- McCotter, R.E. The connection of the vomeronasal nerves with the accessory olfactory bulb in the opossum and other mammals. Anat. Rec. 1912, 6, 299–318. [Google Scholar] [CrossRef] [Green Version]
- Karimi, H.; Mansoori Ale Hashem, R.; Ardalani, G.; Sadrkhanloo, R.; Hayatgheibi, H. Structure of vomeronasal organ (jacobson organ) in male camelus domesticus var. dromedaris persica. Anat. Histol. Embryol. 2014, 43, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Hovis, K.R.; Ramnath, R.; Dahlen, J.E.; Romanova, A.L.; LaRocca, G.; Bier, M.E.; Urban, N.N. Activity regulates functional connectivity from the vomeronasal organ to the accessory olfactory bulb. J. Neurosci. 2012, 32, 7907–7916. [Google Scholar] [CrossRef]
- Bergan, J.F.; Ben-Shaul, Y.; Dulac, C. Sex-specific processing of social cues in the medial amygdala. eLife 2014, 3, e02743. [Google Scholar] [CrossRef]
- Charra, R.; Datiche, F.; Casthano, A.; Gigot, V.; Schaal, B.; Coureaud, G. Brain processing of the mammary pheromone in newborn rabbits. Behav. Brain Res. 2012, 226, 179–188. [Google Scholar] [CrossRef]
- Poindron, P.; Otal, J.; Ferreira, G.; Keller, M.; Guesdon, V.; Nowak, R.; Lévy, F. Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal 2010, 4, 2057–2064. [Google Scholar] [CrossRef]
- Poindron, P.; Lévy, F.; Keller, M. Maternal responsiveness and maternal selectivity in domestic sheep and goats: The two facets of maternal attachment. Dev. Psychobiol. 2007, 49, 54–70. [Google Scholar] [CrossRef]
- Mora-Medina, P.; Napolitano, F.; Mota-Rojas, D.; Berdugo-Gutiérrez, J.; Ruiz-Buitrago, J.; Guerrero-Legarreta, I. Imprinting, sucking and allosucking behaviors in buffalo calves. J. Buffalo Sci. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- Olléová, M.; Pluháček, J.; King, S.R.B. Effect of social system on allosuckling and adoption in zebras. J. Zool. 2012, 288, 127–134. [Google Scholar] [CrossRef]
- Quigley, J.D.; Strohbehn, R.E.; Kost, C.J.; O’Brien, M.M. Formulation of colostrum supplements, colostrum replacers and acquisition of passive immunity in neonatal calves. J. Dairy Sci. 2001, 84, 2059–2065. [Google Scholar] [CrossRef]
- Silva, F.L.M.; Miqueo, E.; da Silva, M.D.; Torrezan, T.M.; Rocha, N.B.; Salles, M.S.V.; Bittar, C.M.M. Thermoregulatory responses and performance of dairy calves fed different amounts of colostrum. Animals 2021, 11, 703. [Google Scholar] [CrossRef]
- Solomon, N.G.; French, J.A. The study of mammalian cooperative breeding. In Cooperative Breeding in Mammals; Solomon, N., French, J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 1–10. [Google Scholar]
- Riedman, M.L. The evolution of alloparental care and adoption in mammals and birds. Q. Rev. Biol. 1982, 57, 405–435. [Google Scholar] [CrossRef]
- Komdeur, J. The effect of kinship on helping in the cooperative breeding Seychelles warbler (Acrocephalus sechellensis). Proc. R. Soc. B Biol. Sci. 1994, 256, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Bon, R.; Campan, R. Unexplained sexual segregation in polygamous ungulates: A defense of an ontogenetic approach. Behav. Processes 1996, 38, 131–154. [Google Scholar] [CrossRef]
- Doolan, S.P.; Macdonald, D.W. Co-operative rearing by slender-tailed meerkats (Suricata suricatta) in the southern Kalahari. Ethology 2001, 105, 851–866. [Google Scholar] [CrossRef]
- Miková, K.; Sovják, R. Agricultura tropica et subtropica a review: Possibilities of allosuckling occurrence in camels (Camelus bactrianus). Agric. Trop. Subtrop. 2005, 38, 91–94. [Google Scholar]
- Lukas, D.; Clutton-Brock, T. Monotocy and the evolution of plural breeding in mammals. Behav. Ecol. 2020, 31, 943. [Google Scholar] [CrossRef]
- Patel, E. Non-maternal infant care in wild silky sifakas non-maternal infant care in wild silky sifakas (Propithecus candidus). Lemur News 2007, 12, 39–42. [Google Scholar]
- Dušek, A. Maternal Investment Strategy in Model Monotocous and Polytocous Mammals: A Life-History Perspective. Ph.D. Thesis, Charles University, Prague, Czech Republic, 12 May 2011. [Google Scholar]
- Packer, C.; Lewis, S.; Pusey, A. A comparative analysis of non-offspring nursing. Anim. Behav. 1992, 43, 265–281. [Google Scholar] [CrossRef]
- MacLeod, K.J.; Lukas, D. Revisiting non-offspring nursing: Allonursing evolves when the costs are low. Biol. Lett. 2014, 10, 20140378. [Google Scholar] [CrossRef]
- Murphey, R.M.; Paranhos da Costa, M.J.R.; Da Silva, R.G.; De Souza, R.C. Allonursing in river buffalo, Bubalus bubalis: Nepotism, incompetence, or thievery? Anim. Behav. 1995, 49, 1611–1616. [Google Scholar] [CrossRef]
- Roulin, A. Why do lactating females nurse alien offspring? A review of hypotheses and empirical evidence. Anim. Behav. 2002, 63, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Royle, N.J.; Smiseth, P.T.; Kölliker, M. The Evolution of Parental Care; Oxford University Press: Oxford, UK, 2013; pp. 1–377. [Google Scholar]
- Davyt, P.D.; Talmón, T.D.; Villanueva, C.Á. Efecto de la Estrategia de Alimentación sobre el Gasto Energético en Vacas Lecheras. Bachelor’s Thesis, Universidad de la República, Montevideo, Uruguay, 4 September 2017. [Google Scholar]
- Gallina, S.; Bello, J. El gasto energético del venado cola blanca (Odocoileus virginianus texanus) en relación a la precipitación en una zona semiárida de México. Therya 2010, 1, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Gittleman, J.L.; Thompson, S.D. Energy allocation in mammalian reproduction. Integr. Comp. Biol. 1988, 28, 863–875. [Google Scholar] [CrossRef]
- Freer, M.; Dove, H.; Nolan, J. Nutrient Requirements of Domesticated Ruminants; Csiro Publishing: Collingwood, ON, Canada, 2007; pp. 50–61. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Hambly, C.; Shi, L.L.; Bi, Z.Q.; Cao, J.; Speakman, J.R. Late lactation in small mammals is a critically sensitive window of vulnerability to elevated ambient temperature. Proc. Natl. Acad. Sci. USA 2020, 117, 24352–24358. [Google Scholar] [CrossRef] [PubMed]
- Marotta, E. Determinación del requerimiento energético de la cerda reproductora mantenida a campo en base al clima y la etología. Analecta Vet. 2003, 23, 28–35. [Google Scholar]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Parental investment in male and female offspring in polygynous mammals. Nature 1981, 289, 487–489. [Google Scholar] [CrossRef]
- Bérubé, C.H.; Festa-Bianchet, M.; Jorgenson, J.T. Reproductive costs of sons and daughters in Rocky Mountain bighorn sheep. Behav. Ecol. 1996, 7, 60–68. [Google Scholar] [CrossRef]
- Moen, A.N. Seasonal changes in heart rates, activity, metabolism, and forage intake of white-tailed deer. J. Wildl. Manag. 1978, 42, 715–738. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Albon, S.D.; Guinness, F.E. Fitness costs of gestation and lactation in wild mammals. Nature 1989, 337, 260–262. [Google Scholar] [CrossRef]
- Stábile, F. Aloamamantamiento en Arctocephalus australis en Isla de Lobos (Uruguay): Estrategia individual de las crías? Bachelor’s Thesis, Universidad de la República de Uruguay, Montevideo, Uruguay, February 2013. [Google Scholar]
- Andriolo, A.; Paranhos, M.J.R.; Costa, D.A.; Schmidek, W.R.; Costa, M.J.R.P.; De Estudos, E.-G. Suckling behaviour in water buffalo suckling behaviour in water buffalo (Bubalus bubalis): Development and individual differences. Rev. Etol. 2001, 3, 129–136. [Google Scholar]
- Víchová, J.; Bartoš, L. Allosuckling in cattle: Gain or compensation? Appl. Anim. Behav. Sci. 2005, 94, 223–235. [Google Scholar] [CrossRef]
- Roulin, A.; Heeb, P. The immunological function of allosuckling. Ecol. Lett. 1999, 2, 319–324. [Google Scholar] [CrossRef]
- Paranhos Da Costa, M.J.R.; Andriolo, A.; Simplício De Oliveira, J.F.; Schmidek, W.R. Suckling and allosuckling in river buffalo calves and its relation with weight gain. Appl. Anim. Behav. Sci. 2000, 66, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.D.F.M.; Quirino, C.R.; Bastos, R. Effect of nursing behaviour, sex of the calf, and parity order on milk production of buffaloes. Rev. Colomb. De Cienc. Pecu. 2017, 30, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Bartoš, L.; Vaňková, D.; Šiler, J.; Illmann, G. Adoption, allonursing and allosucking in farmed red deer (Cervus elaphus). Anim. Sci. 2001, 72, 483–492. [Google Scholar] [CrossRef]
- Zapata, B.; Gaete, G.; Correa, L.A.; González, B.A.; Ebensperger, L.A. A case of allosuckling in wild guanacos (Lama guanicoe). J. Ethol. 2009, 27, 295–297. [Google Scholar] [CrossRef]
- Mumme, R.L. A bird’s-eye view of mammalian cooperative breeding. In Cooperative Breeding in Mammals; Solomon, N., French, J., Eds.; Cambridge University Press: Omaha, NE, USA, 2009; pp. 364–388. [Google Scholar]
- Fuquay, J.W. Heat stress as it affects animal production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef]
- Black, J.L.; Mullan, B.P.; Lorschy, M.L.; Giles, L.R. Lactation in the sow during heat stress. Livest. Prod. Sci. 1993, 35, 153–170. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Herrenkohl, L.R.; Whitney, J.B. Effects of prepartal stress on postpartal nursing behavior, litter development and adult sexual behavior. Physiol. Behav. 1976, 17, 1019–1021. [Google Scholar] [CrossRef]
- Lay, D.C.; Randel, R.D.; Friend, T.H.; Jenkins, O.C.; Neuendorff, D.A.; Bushong, D.M.; Lanier, E.K.; Bjorge, M.K. Effects of prenatal stress on suckling calves. J. Anim. Sci. 1997, 75, 3143–3151. [Google Scholar] [CrossRef]
- Benson, G.K.; Fitzpatrick, R.J. The Neurohypophysis and the Mammary Gland. In Pars Intermedia and Neurohypophysis; Butterworthpp & Co.: London, UK, 2020; pp. 414–443. [Google Scholar]
- Bisset, G.W. The Milk-Ejection Reflex and the Actions of Oxytocin, Vasopressin and Synthetic Analogues on the Mammary Gland. In Neurohypophysial Hormones and Similar Polypeptides; Berde, B., Ed.; Springer: Berlin, Germany, 1968. [Google Scholar]
- Ni, Y.; Chen, Q.; Cai, J.; Xiao, L.; Zhang, J. Three lactation-related hormones: Regulation of hypothalamus-pituitary axis and function on lactation. Mol. Cell. Endocrinol. 2021, 520, 111084. [Google Scholar] [CrossRef]
- Arizala, J.A.; Olivera, M. Fisiología de la Producción Láctea en Bovinos: Involución de la Glándula Mamaria, Lactogénesis, Galactopoyesis, y Eyección de la Leche. In Buenas Prácticas de Producción Primaria de Leche; Olivera, M.A., Ed.; Fondo Editorial Biogénesis: Medellín, Colombia, 2007; pp. 143–151. [Google Scholar]
- Svennersten-Sjaunja, K.; Olsson, K. Endocrinology of milk production. Domest. Anim. Endocrinol. 2005, 29, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Perez-Marquez, H.J.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Infrared thermography and behavioral biometrics associated with estrus indicators and ovulation in estrus-synchronized dairy cows housed in tiestalls. J. Dairy Sci. 2019, 102, 4427–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, C.; Robert, S. Hormonal, behavioural and performance characteristics of Meishan sows during pregnancy and lactation. Can. J. Anim. Sci. 2003, 83, 1–12. [Google Scholar] [CrossRef]
- Brogan, R.S.; Mitchell, S.E.; Trayhurn, P.; Smith, M.N. Suppression of leptin during lactation: Contribution of the suckling stimulus versus milk production. Endocrinology 1999, 140, 2621–2627. [Google Scholar] [CrossRef]
- Bridges, R.S. The behavioral neuroendocrinology of maternal behavior: Past accomplishments and future directions. Horm. Behav. 2020, 120, 104662. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.A.; Spoon, R.A.; Ryan, D.P.; Williams, G.L. Evidence for maternal behavior as a requisite link in suckling-mediated anovulation in cows. Biol. Reprod. 1993, 49, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D. Some factors influencing the availability of colostrum to piglets. Anim. Sci. 1984, 39, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Muns, V. Welfare and Management Strategies to Reduce Pre-Weaning Mortality in Piglets. Ph.D. Thesis, Universitat Autonoma de Barcelona, Barcelona, Spain, 20 June 2013. [Google Scholar]
- Muns, V.R.; Tummaruk, P. Management strategies in farrowing house to improve piglet pre-weaning survival and growth. Thai J. Vet. Med. 2016, 46, 347–354. [Google Scholar]
- Algers, B. Nursing in pigs: Communicating needs and distributing resources. J. Anim. Sci. 1993, 71, 2826–2831. [Google Scholar] [CrossRef]
- Brachet, M.A.A.; Vullioud, P.; Ganswindt, A.; Manser, M.B.; Keller, M.; Clutton-Brock, T.H. Parity predicts allonursing in a cooperative breeder. J. Mammal. 2021, 1, gyab084. [Google Scholar] [CrossRef]
- Monticelli, P.F.; Tokumaru, R.S.; Ades, C. Allosuckling in a captive group of wild cavies Cavia aperea. Mammalia 2018, 82, 355–359. [Google Scholar] [CrossRef]
- Gloneková, M.; Brandlová, K.; Pluháček, J. Stealing milk by young and reciprocal mothers: High incidence of allonursing in giraffes, Giraffa camelopardalis. Anim. Behav. 2016, 113, 113–123. [Google Scholar] [CrossRef]
- Zapata, B.; Correa, L.; Soto-Gamboa, M.; Latorre, E.; González, B.A.; Ebensperger, L.A. Allosuckling allows growing offspring to compensate for insufficient maternal milk in farmed guanacos (Lama guanicoe). Appl. Anim. Behav. Sci. 2010, 122, 119–126. [Google Scholar] [CrossRef]
- Wilkinson, G.S. Communal nursing in the evening bat, Nycticeius humeralis. Behav. Ecol. Sociobiol. 1992, 31, 225–235. [Google Scholar] [CrossRef]
- Maniscalco, J.M.; Harris, K.R.; Atkinson, S.; Parker, P. Alloparenting in Steller sea lions (Eumetopias jubatus): Correlations with misdirected care and other observations. J. Ethol. 2006, 25, 125–131. [Google Scholar] [CrossRef]
- Engelhardt, S.C.; Weladji, R.B.; Holand, Ø.; Røed, K.H.; Nieminen, M. Evidence of reciprocal allonursing in reindeer, Rangifer tarandus. Ethology 2015, 121, 245–259. [Google Scholar] [CrossRef]
- Zapata, B.; González, B.A.; Ebensperger, L.A. Allonursing in captive guanacos, lama guanicoe: Milk theft or misdirected parental care? Ethology 2009, 115, 731–737. [Google Scholar] [CrossRef]
- Lee, P.C. Allomothering among African elephants. Anim. Behav. 1987, 35, 278–291. [Google Scholar] [CrossRef]
- Vitikainen, E.I.K.; Marshall, H.H.; Thompson, F.J.; Sanderson, J.L.; Bell, M.B.V.; Gilchrist, J.S.; Hodge, S.J.; Nichols, H.J.; Cant, M.A. Biased escorts: Offspring sex, not relatedness explains alloparental care patterns in a cooperative breeder. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162384. [Google Scholar] [CrossRef]
- Lukas, D.; Huchard, E. The evolution of infanticide by females in mammals. Philos. Trans. R. Soc. B 2019, 374, 20180075. [Google Scholar] [CrossRef] [Green Version]
- Tučková, V.; Šumbera, R.; Čížková, B. Alloparental behaviour in Sinai spiny mice Acomys dimidiatus: A case of misdirected parental care? Behav. Ecol. Sociobiol. 2016, 70, 437–447. [Google Scholar] [CrossRef]
- Engelhardt, S.C.; Weladji, R.B.; Holand, Ø.; De Rioja, C.M.; Ehmann, R.K.; Nieminen, M. Allosuckling in reindeer (Rangifer tarandus): Milk-theft, mismothering or kin selection? Behav. Process. 2014, 107, 133–141. [Google Scholar] [CrossRef]
- Queller, D.C. Kinship, reciprocity and synergism in the evolution of social behaviour. Nature 1985, 318, 366–367. [Google Scholar] [CrossRef]
- Taborsky, M. Cichlid Fishes: A Model for the Integrative Study of Social Behavior. In Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution, and Behavior; Koenig, W.D., Dickinson, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 272–292. [Google Scholar]
- Magrath, R.D.; Whittingham, L.A. Subordinate males are more likely to help if unrelated to the breeding female in cooperatively breeding white-browed scrubwrens. Behav. Ecol. Sociobiol. 1997, 41, 185–192. [Google Scholar] [CrossRef]
- Riehl, C. Living with strangers: Direct benefits favour non-kin cooperation in a communally nesting bird. Proc. R. Soc. B Biol. Sci. 2011, 278, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- Kingma, S.A. Direct benefits explain interspecific variation in helping behaviour among cooperatively breeding birds. Nat. Commun. 2017, 8, 1094. [Google Scholar] [CrossRef]
- Griffin, A.S.; West, S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 2003, 302, 634–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clutton-Brock, T. Breeding together: Kin selection and mutualism in cooperative vertebrates. Science 2002, 296, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Trivers, R.L. The Evolution of reciprocal altruism. Q. Rev. Biol. 1971, 46, 35–57. [Google Scholar] [CrossRef]
- Jones, J.D.; Treanor, J.J. Allonursing and cooperative birthing behavior in Yellowstone Bison, Bison bison. Can. Field-Nat. 2008, 122, 2008. [Google Scholar] [CrossRef] [Green Version]
- Taborsky, M.; Frommen, J.G.; Riehl, C. Correlated pay-offs are key to cooperation. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150084. [Google Scholar] [CrossRef] [Green Version]
- Creel, S.R.; Monfort, S.L.; Wildt, D.E.; Waser, P.M. Spontaneous lactation is an adaptive result of pseudopregnancy. Nature 1991, 351, 660–662. [Google Scholar] [CrossRef]
- Montgomery, T.M.; Pendleton, E.L.; Smith, J.E. Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 2018, 193, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.R.; Clutton-Brock, T.H. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc. Biol. Sci. 2011, 278, 1189–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebensperger, L.A.; Ramírez-Estrada, J.; León, C.; Castro, R.A.; Tolhuysen, L.O.; Sobrero, R.; Quirici, V.; Burger, J.R.; Soto-Gamboa, M.; Hayes, L.D. Sociality, glucocorticoids and direct fitness in the communally rearing rodent, Octodon degus. Horm. Behav. 2011, 60, 346–352. [Google Scholar] [CrossRef]
- Pluháček, J.; Bartošová, J. A case of suckling and allosuckling behaviour in captive common hippopotamus. Mamm. Biol. 2011, 76, 380–383. [Google Scholar] [CrossRef]
- MacLeod, K.J.; Clutton-Brock, T.H. Low costs of allonursing in meerkats: Mitigation by behavioral change? Behav. Ecol. 2015, 26, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Ogino, M.; Maldonado-Chaparro, A.A.; Farine, D.R. Drivers of alloparental provisioning of fledglings in a colonially breeding bird. Behav. Ecol. 2021, 32, 316–326. [Google Scholar] [CrossRef]
- Kingma, S.A.; Santema, P.; Taborsky, M.; Komdeur, J. Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 2014, 29, 476–484. [Google Scholar] [CrossRef]
- Kokko, H.; Johnstone, R.A.; Clutton-Brock, T.H. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. B Biol. Sci. 2001, 268, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teunissen, N.; Kingma, S.A.; Fan, M.; Roast, M.J.; Peters, A. Context-dependent social benefits drive cooperative predator defense in a bird. Curr. Biol. 2021, 31, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Gloneková, M.; Brandlová, K.; Pluháček, J. Further behavioural parameters support reciprocity and milk theft as explanations for giraffe allonursing. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Engelhardt, S.C.; Weladji, R.B.; Holand, Ø.; Røed, K.H.; Nieminen, M. Allonursing in reindeer, Rangifer tarandus: A test of the kin-selection hypothesis. J. Mammal. 2016, 97, 689–700. [Google Scholar] [CrossRef] [Green Version]
- König, B. Cooperative care of young in mammals. Naturwissenschaften 1997, 84, 95–104. [Google Scholar] [CrossRef] [PubMed]
- König, B. Non-Offspring Nursing in Mammals: General Implications from a Case Study on House Mice. In Cooperation in Primates and Humans. Mechanism and Evolution; Kappeler, P.P., Van Schaik, C.P., Eds.; Springer: Berlin, Germany, 2009; pp. 191–205. [Google Scholar] [CrossRef] [Green Version]
- Drake, A.; Fraser, D.; Weary, D.M. Parent–offspring resource allocation in domestic pigs. Behav. Ecol. Sociobiol. 2007, 62, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, M.; McManus, C.M.; De Paula Neto, J.B.; Da Costa, M.J.R.P.; Mendes, F.D.C.; Sereno, J.R.B.; Bértoli, C.D.; Fioravanti, M.C.S. Maternal offspring behaviour in curraleiro pé duro naturalized cattle in brazil. Rev. Bras. Zootec. 2013, 42, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Villagrán, B.M. Evolución del Comportamiento Alimenticio del Venado de Campo (Ozotoceros bezoarticus, Linnaeus 1758) en Semicautiverio Durante las 12 Primeras Semanas de Vida. Ph.D. Thesis, Universidad de la República, Montevideo, Uruguay, 17 April 2009. [Google Scholar]
- Bertoni, A.; Napolitano, F.; Mota-Rojas, D.; Sabia, E.; Álvarez-Macías, A.; Mora-Medina, P.; Morales-Canela, A.; Berdugo-Gutiérrez, J.; Guerrero-Legarreta, I. Similarities and differences between river buffaloes and cattle: Health, physiological, behavioral and productivity aspects. J. Buffalo Sci. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Pélabon, C.; Yoccoz, N.G.; Ropert-Coudert, Y.; Caron, M.; Peirera, V. Suckling and allosuckling in captive fallow deer (Dama dama, Cervidae). Ethology 1998, 104, 75–86. [Google Scholar] [CrossRef]
- Réale, D.; Boussès, P.; Chapuis, J.L. Nursing behaviour and mother–lamb relationships in mouflon under fluctuating population densities. Behav. Process. 1999, 47, 81–94. [Google Scholar] [CrossRef]
- Waltl, B.; Appleby, M.C.; Sölkner, J. Effects of relatedness on the suckling behaviour of calves in a herd of beef cattle rearing twins. Appl. Anim. Behav. Sci. 1995, 45, 1–9. [Google Scholar] [CrossRef]
- Drábková, J.; Bartošová, J.; Bartoš, L.; Kotrba, R.; Pluháček, J.; Švecová, L.; Dušek, A.; Kott, T. Sucking and allosucking duration in farmed red deer (Cervus elaphus). Appl. Anim. Behav. Sci. 2008, 113, 215–223. [Google Scholar] [CrossRef]
- Loberg, J. Behaviour of Foster Cows and Calves in Dairy Production Acceptaof Calves, Cow-Calf Interactions and Weaning. Ph.D. Thesis, Universitatis Agriculturae Sueciae, Uppsala, Sweden, 23 November 2007. [Google Scholar]
- Ebensperger, L.A.; León, C.; Ramírez-Estrada, J.; Abades, S.; Hayes, L.D.; Nova, E.; Salazar, F.; Bhattacharjee, J.; Becker, M.I. Immunocompetence of breeding females is sensitive to cortisol levels but not to communal rearing in the degu (Octodon degus). Physiol. Behav. 2015, 140, 61–70. [Google Scholar] [CrossRef]
- Becker, M.I.; De Ioannes, A.E.; León, C.; Ebensperger, L.A. Females of the communally breeding rodent, Octodon degus, transfer antibodies to their offspring during pregnancy and lactation. J. Reprod. Immunol. 2007, 74, 68–77. [Google Scholar] [CrossRef]
- Dalto, A.C.; Bandarra, P.M.; Pavarini, S.P.; Boabaid, F.M.; De Bitencourt, A.P.G.; Gomes, M.P.; Chies, J.; Driemeier, D.; Da Cruz, C.E.F. Clinical and pathological insights into Johne’s disease in buffaloes. Trop. Anim. Health Prod. 2012, 44, 1899–1904. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; De Rosa, G.; Mora-Medina, P.; Braghieri, A.; Guerrero-Legarreta, I.; Napolitano, F. Dairy buffalo behaviour and welfare from calving to milking. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Brandlová, K.; Bartoš, L.; Haberová, T. Camel calves as opportunistic milk thefts? the first description of allosuckling in domestic bactrian camel (Camelus bactrianus). PLoS ONE 2013, 8, e53052. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Altemus, M. Integrative functions of lactational hormones in social behavior and stress management. Ann. N. Y. Acad. Sci. 1977, 807, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L. Alterations in hypothalamic-pituitary responsiveness during lactation. Ann. N. Y. Acad. Sci. 1992, 652, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Schlein, P.A.; Zarrow, M.X.; Denenberg, V.H. The role of prolactin in the depressed or ‘buffered’ adrenocorticosteroid response of the rat. J. Endocrinol. 1974, 62, 93–99. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Marcet-Rius, M.; Freitas-de-Melo, A.; Muns, R.; Mora-Medina, P.; Domínguez-Oliva, A.; Orihuela, A. Allonursing in Wild and Farm Animals: Biological and Physiological Foundations and Explanatory Hypotheses. Animals 2021, 11, 3092. https://doi.org/10.3390/ani11113092
Mota-Rojas D, Marcet-Rius M, Freitas-de-Melo A, Muns R, Mora-Medina P, Domínguez-Oliva A, Orihuela A. Allonursing in Wild and Farm Animals: Biological and Physiological Foundations and Explanatory Hypotheses. Animals. 2021; 11(11):3092. https://doi.org/10.3390/ani11113092
Chicago/Turabian StyleMota-Rojas, Daniel, Míriam Marcet-Rius, Aline Freitas-de-Melo, Ramon Muns, Patricia Mora-Medina, Adriana Domínguez-Oliva, and Agustín Orihuela. 2021. "Allonursing in Wild and Farm Animals: Biological and Physiological Foundations and Explanatory Hypotheses" Animals 11, no. 11: 3092. https://doi.org/10.3390/ani11113092







