Heterospecific Fear and Avoidance Behaviour in Domestic Horses (Equus caballus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
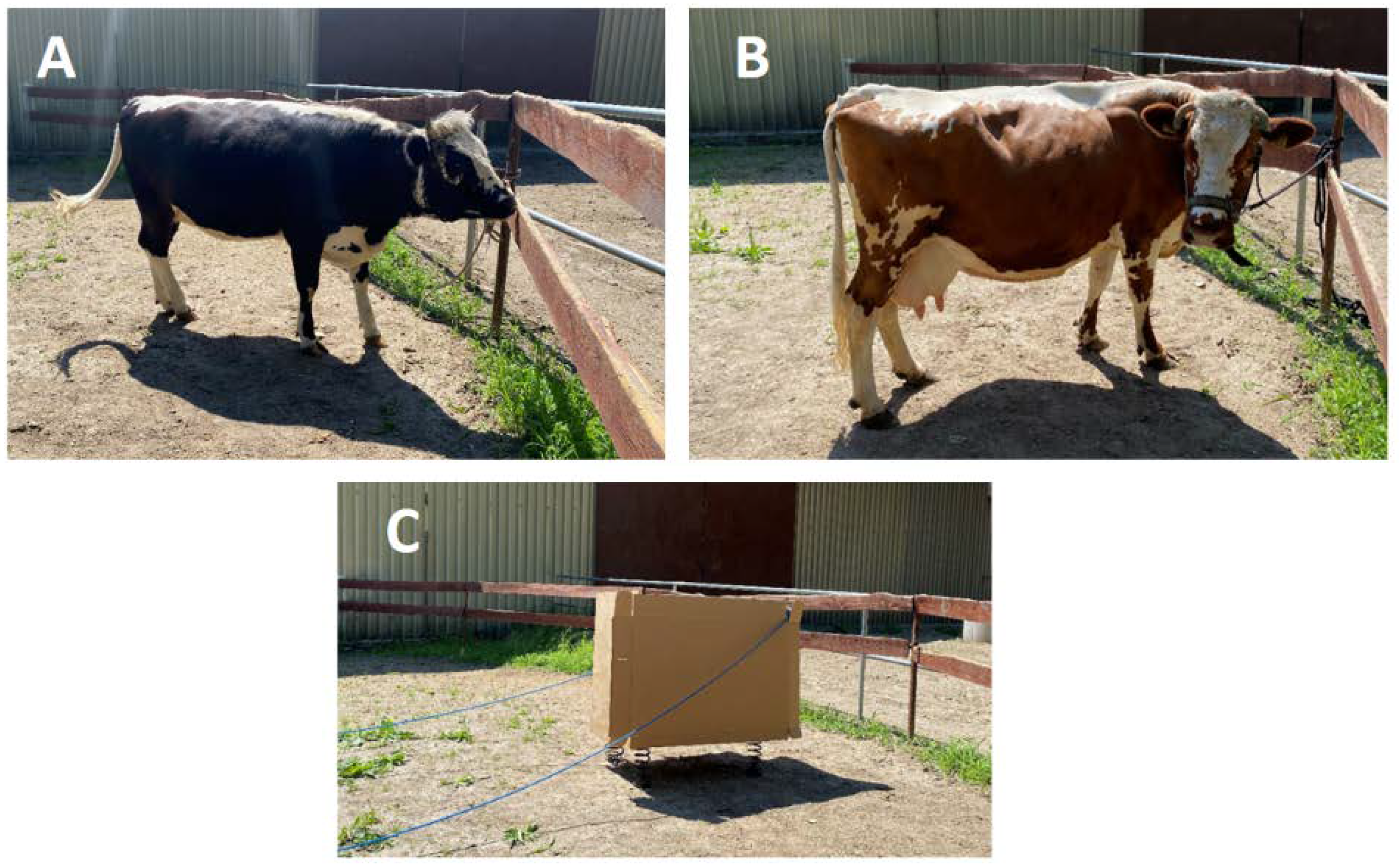
2.2. Animals
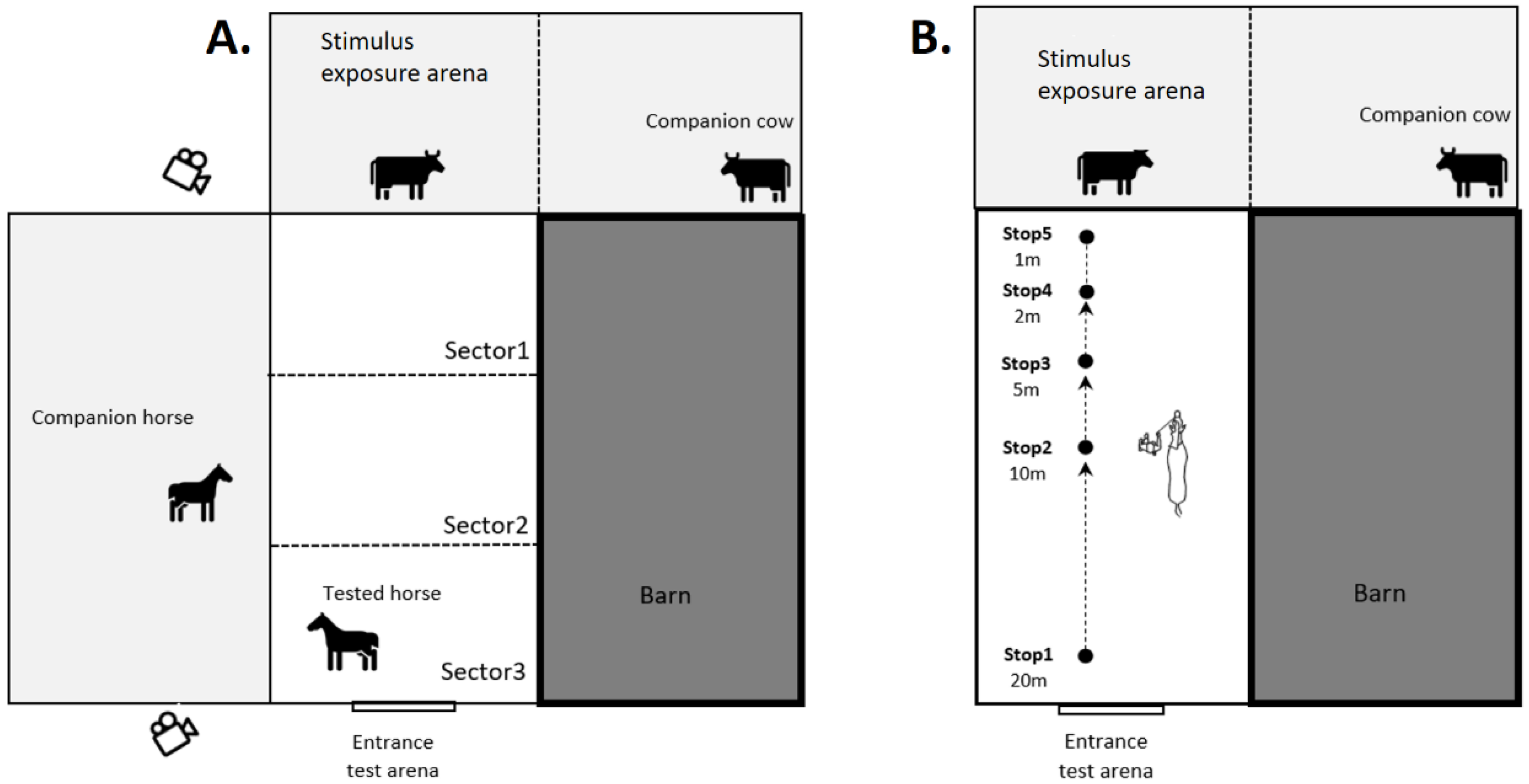
2.3. Arena Test
2.4. Hand-Leading Test
2.5. Statistical Analysis
3. Results
3.1. Arena Test
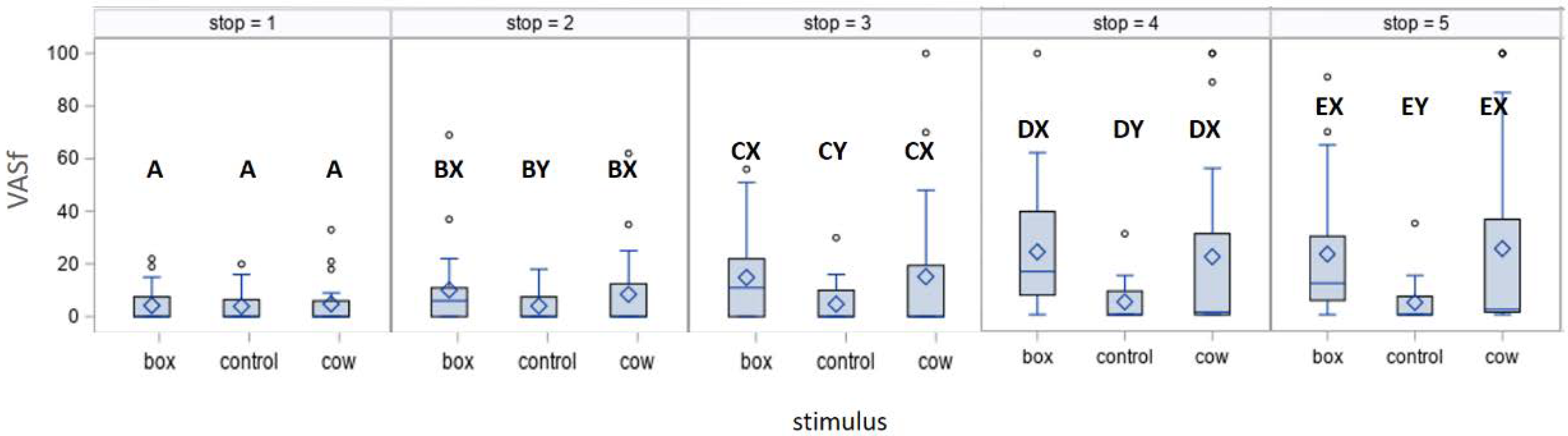
3.2. Hand-Leading Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cromsigt, J.P.; Kemp, Y.J.; Rodriguez, E.; Kivit, H. Rewilding Europe’s large grazer community: How functionally diverse are the diets of European bison, cattle, and horses? Rest. Ecol. 2018, 26, 891–899. [Google Scholar] [CrossRef]
- Boissy, A. Fear and fearfulness in animals. Q. Rev. Biol. 1995, 70, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.W. Fear in Horses. Social Influence, Generalisation and Reactions to Predator Odour. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, University Aarhus, Aarhus, Denmark, 2007; pp. 1–44. [Google Scholar]
- Thomas, K.E.; Annest, J.L.; Gilchrist, J.; Bixby-Hammett, D.M. Non-fatal horse related injuries treated in emergency departments in the United States, 2001–2003. Br. J. Sports Med. 2006, 40, 619–626. [Google Scholar] [CrossRef]
- McGreevy, P.; Christensen, J.W.; König von Borstel, U.; McLean, A.N. Equitation Science, 2nd ed.; Wiley-Blackwell: West Sussex, UK, 2018; pp. 1–393. [Google Scholar]
- Górecka-Bruzda, A.; Jastrzębska, E.; Muszyńska, A.; Jędrzejewska, E.; Jaworski, Z.; Jezierski, T.; Murphy, J. To jump or not to jump? Strategies employed by leisure and sport horses. J. Vet. Behav. 2013, 8, 253–260. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Momozawa, Y.; Ono, T.; Sato, F.; Kikusui, T.; Takeuchi, Y.; Mori, Y.; Kusunose, R. Assessment of equine temperament by a questionnaire survey to caretakers and evaluation of its reliability by simultaneous behavior test. Appl. Anim. Behav. Sci. 2003, 84, 127–138. [Google Scholar] [CrossRef]
- Le Scolan, N.; Hausberger, M.; Wolff, A. Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behav. Process. 1997, 41, 257–266. [Google Scholar] [CrossRef]
- Christensen, J.W.; Zharkikh, T.; Ladewig, J. Do horses generalise between objects during habituation? Appl. Anim. Behav. Sci. 2008, 114, 509–520. [Google Scholar] [CrossRef]
- Górecka-Bruzda, A.; Jaworski, Z.; Suwała, M.; Boroń, M.; Ogłuszka, M.; Earley, B.; Sobczyńska, M. Longitudinal study on human-related behaviour in horses—Can horses (Equus caballus) be de-domesticated? Appl. Anim. Behav. Sci. 2017, 195, 50–59. [Google Scholar] [CrossRef]
- Corgan, M.E.; Grandin, T.; Matlock, S. Evaluating the Reaction to a Complex Rotated Object in the American Quarter Horse (Equus caballus). Animals 2021, 11, 1383. [Google Scholar] [CrossRef]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 104–109. [Google Scholar] [CrossRef]
- Visser, E.K.; Van Reenen, C.G.; Hopster, H.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Quantifying aspects of young horses’ temperament: Consistency of behavioural variables. Appl. Anim. Behav. Sci. 2001, 74, 241–258. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; van der Werf, J.T.N.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Phys. Behav. 2002, 76, 289–296. [Google Scholar] [CrossRef]
- Lee, K.E.; Kim, J.G.; Lee, H.; Kim, B.S. Behavioral and cardiac responses in mature horses exposed to a novel object. J. Anim. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Lloyd, A.S.; Martin, J.E.; Bornet-Gauci, H.L.I.; Wilkinson, R.G. Evaluation of a novel method of horse personality assessment: Rater-agreement and links to behavior. Appl. Anim. Behav. Sci. 2007, 105, 205–222. [Google Scholar] [CrossRef]
- Hornblow, A.R.; Kidson, M.A. The visual analogue scale for anxiety: A validation study. Aust. N. Z. J. Psychiatry 1976, 10, 339–341. [Google Scholar] [CrossRef]
- van Duinen, M.; Rickelt, J.; Griez, E. Validation of the electronic visual analogue scale of anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1045–1047. [Google Scholar] [CrossRef]
- Sutton, G.A.; Dahan, R.; Turner, D.; Paltiel, O. A behaviour-based pain scale for horses with acute colic: Scale construction. Vet. J. 2013, 196, 394–401. [Google Scholar] [CrossRef] [PubMed]
- De Grauw, J.C.; Van Loon, J.P.A.M. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Letterier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann. Noninvasive Electrocardiol. 1996, 1, 151–181. [Google Scholar]
- Lansade, L.; Bouissou, M.F.; Erhard, H.W. Fearfulness in horses: A temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 2008, 115, 182–200. [Google Scholar] [CrossRef]
- Bulens, A.; Sterken, H.; Van Beirendonck, S.; Van Thielen, J.; Driessen, B. The use of different objects during a novel object test in stabled horses. J. Vet. Behav. 2015, 10, 54–58. [Google Scholar] [CrossRef]
- Christensen, J.W.; Beblein, C.; Malmkvist, J. Development and consistency of fearfulness in horses from foal to adult. Appl. Anim. Behav. Sci. 2020, 232, 105106. [Google Scholar] [CrossRef]
- Grandin, T.; Shivley, C. How farm animals react and perceive stressful situations such as handling, restraint, and transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Keeling, L.J.; Jonare, L.; Lanneborn, L. Investigating horse–human interactions: The effect of a nervous human. Vet. J. 2009, 181, 70–71. [Google Scholar] [CrossRef]
- Scopa, C.; Contalbrigo, L.; Greco, A.; Lanatà, A.; Scilingo, E.P.; Baragli, P. Emotional transfer in human–horse interaction: New perspectives on equine assisted interventions. Animals 2019, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, A.; Single, M.S.; Nawroth, C. Social Referencing in the Domestic Horse. Animals 2020, 10, 164. [Google Scholar] [CrossRef]
- Christensen, J.W. Object habituation in horses: The effect of voluntary versus negatively reinforced approach to frightening stimuli. Equine Vet. J. 2013, 45, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Topál, J.; Miklósi, Á.; Csányi, V. Dog-human relationship affects problem solving behavior in the dog. Anthrozoös 1997, 10, 214–224. [Google Scholar] [CrossRef]
- Nawroth, C.; Brett, J.M.; McElligott, A.G. Goats display audience-dependent human-directed gazing behaviour in a problem-solving task. Biol. Lett. 2016, 12, 20160283. [Google Scholar] [CrossRef] [PubMed]
- Lesimple, C.; Sankey, C.; Richard, M.A.; Hausberger, M. Do horses expect humans to solve their problems? Front. Psychol. 2012, 3, 306. [Google Scholar] [CrossRef] [PubMed]
- Górecka, A.; Bakuniak, M.; Chruszczewski, M.H.; Jezierski, T.A. A note on the habituation to novelty in horses: Handler effect. Anim. Sci. Pap. Rep. 2007, 25, 143–152. [Google Scholar]
- Proops, L.; McComb, K.; Reby, D. Cross-modal individual recognition in domestic horses (Equus caballus). Proc. Natl. Acad. Sci. USA 2009, 106, 947–951. [Google Scholar] [CrossRef]
- Lansade, L.; Colson, V.; Parias, C.; Trösch, M.; Reigner, F.; Calandreau, L. Female horses spontaneously identify a photograph of their keeper, last seen six months previously. Sci. Rep. 2020, 10, 6302. [Google Scholar] [CrossRef]
- Millman, S.T.; Duncan, I.J.H. Social Cognition of Farm Animals. In Social Behaviour in Farm Animals; Keeling, L.J., Gonyou, H.W., Eds.; CABI Publishing, CAB International: Oxon, UK, 2005; pp. 373–399. [Google Scholar]
- Saslow, C.A. Factors affecting stimulus visibility for horses. Appl. Anim. Behav. Sci. 1999, 61, 273–284. [Google Scholar] [CrossRef]
- McGreevy, P. Equine Behavior. Perception. In A Guide for Veterinarians and Equine Scientists; McGreevy, P., Ed.; Saunders: Philadephia, PA, USA, 2004; pp. 37–54. [Google Scholar]
- Vaina, L.M.; Solomon, J.; Chowdhury, S.; Sinha, P.; Belliveau, J.W. Functional neuroanatomy of biological perception in humans. Proc. Natl. Acad. Sci. USA 2001, 98, 11656–11661. [Google Scholar] [CrossRef]
- Regolin, L.; Tommasi, L.; Vallortigara, G. Visual perception of biological motion in newly hatched chicks as revealed by an imprinting procedure. Anim. Cognit. 2000, 3, 53–60. [Google Scholar] [CrossRef]
- Witzany, G. Why Biocommunication of Animals? In Biocommunication of Animals; Witzany, G., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Meise, K.; Franks, D.W.; Bro-Jørgensen, J. Alarm communication networks as a driver of community structure in African savannah herbivores. Ecol. Lett. 2020, 23, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Sablik, P.; Kobak, P.; Biała, M.; Matkowski, D. Porównanie behawioryzmu udomowionych zwierząt roślinożernych (bydła mięsnego i koni) w naturalnych warunkach bytowania w otulinie przyrodniczego Parku Narodowego “Ujście Warty”. Acta Sci. Pol. Zootech. 2010, 9, 207–214. [Google Scholar]
- Fenner, K.; Caspar, G.; Hyde, M.; Henshall, C.; Dhand, N.; Probyn-Rapsey, F.; Dashper, K.; McLean, A.; McGreevy, P. It’s all about the sex, or is it? Humans, horses and temperament. PLoS ONE 2019, 14, e0216699. [Google Scholar] [CrossRef] [PubMed]
- Aune, A.; Fenner, K.; Wilson, B.; Cameron, E.; McLean, A.; McGreevy, P. Reported behavioural differences between geldings and mares challenge sex-driven stereotypes in ridden equine behaviour. Animals 2020, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Aurich, J.; Möstl, E.; Müller, J.; Aurich, C. Changes in cortisol release and heart rate and heart rate variability during the initial training of 3-year-old sport horses. Horm. Behav. 2010, 58, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Vandenheede, M.; Bouissou, M.F. Sex differences in fear reactions in sheep. Appl. Anim. Behav. Sci. 1993, 37, 39–55. [Google Scholar] [CrossRef]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Brit. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
| Measurement | Variable | Definition | Unit |
|---|---|---|---|
| Arena test | TimeSector | The total percentage of time the horse spent in Sector1–3 during the Control Stay or in the presence of Cow1, Cow2 or the Box | % |
| Alert_occurr | The total number of occurrences of the following alert behaviours: vocalisations (whinny, neigh), snorts (loud, forceful burst of the air through the nostrils), defecations (eliminating faeces) during exposure to Control Stay, Cow1, Cow2 or Box | count | |
| Alert_dur | The total durations of the following alert behaviours: holding the tail high (above the horizontal line relative to its base) and the head and neck high (above the horizontal line relative to its withers) during exposure to Control Stay, Cow1, Cow2 or Box | seconds | |
| Hand-leading test | HR | Mean heart rate during the 10 s stay at each stop | beats per minute (bpm) |
| RMSSD | Mean RMSSD (Root mean square of successive differences between heartbeats) during the 10 s stay at each stop | milliseconds | |
| VASf | The researcher’s score of the perception of the fearfulness of the horse at stops 1–5. This was scored using a continuous scale from 0 (not at all afraid) to 100 (extremely fearful) by the researcher marking a point on a 100 mm length bar. | mm |
| Variable | Sector | Stimulus | Sector * Stimulus | Sex | Age |
|---|---|---|---|---|---|
| F; p | |||||
| TimeSector (%) | 16,714.1; <0.01 | 5.18; <0.01 | 504.73; <0.01 | 0.18; 0.67 | 0.38; 0.53 |
| Stimulus Variable | Control Stay | Cow1 | Cow2 | Box |
|---|---|---|---|---|
| Mean, Standard Error | ||||
| TimeSector1 (%) | 30.4, 0.24 AX | 17.8, 0.20 BX | 30.6, 0.24 AX | 25.3, 0.22 CX |
| TimeSector2 (%) | 20.7, 0.21 AY | 24.6, 0.23 BY | 19.8, 0.21 AY | 22.7, 0.22 CY |
| TimeSector3 (%) | 48.8, 0.26 AZ | 58.2, 0.26 BZ | 49.6, 0.26 AZ | 52.1, 0.26 CZ |
| Variable | Stop | Stimulus | Stop * Stimulus | Sex | Age |
|---|---|---|---|---|---|
| F; p | |||||
| HR (bpm) | 0.29; 0.89 | 133.0; <0.01 | 0.61; 0.77 | 5.27; 0.02 | 2.42; 0.12 |
| RMSSD (ms) | 1.44; 0.22 | 25.24; <0.01 | 0.90; 0.52 | 6.48; 0.01 | 0.52; 0.47 |
| VASf (mm) | 4.13; 0.01 | 7.78; <0.01 | 1.47; 0.17 | 0.07; 0.78 | 1.34; 0.25 |
| Variable | Stimulus | Sex | |||
|---|---|---|---|---|---|
| Control | Cow | Box | Geldings | Mares | |
| Mean 95% CI (Lower; Upper) | |||||
| HR | 50.8 (45.2; 56.7) A | 64.7 (67.7; 72.2) B | 49.8 (44.4; 55.6) A | 51.4 (43.3; 58.1) a | 58.1 (43.0; 68.7) b |
| RMSSD | 71.7 (52.3; 97.1) A | 43.3 (31.8; 58.7) B | 69.9 (51.3; 94.7) A | 76.1(53.8; 107.0) A | 47.4 (29.6; 75.6) B |
| HR | RMSSD | ||||
|---|---|---|---|---|---|
| Stimulus | rs | p | rs | p | |
| Control | 0.31 | 0.17 | −0.51 | 0.02 | |
| VASf, | Cow | 0.48 | 0.03 | −0.50 | 0.02 |
| Stop1 | Box | 0.59 | 0.01 | −0.41 | 0.07 |
| Control | 0.04 | 0.85 | −0.22 | 0.34 | |
| VASf, | Cow | 0.30 | 0.20 | −0.07 | 0.74 |
| Stop5 | Box | 0.39 | 0.09 | −0.44 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, A.; Janczarek, I.; Wilk, I.; Tkaczyk, E.; Mierzicka, M.; Stanley, C.R.; Górecka-Bruzda, A. Heterospecific Fear and Avoidance Behaviour in Domestic Horses (Equus caballus). Animals 2021, 11, 3081. https://doi.org/10.3390/ani11113081
Wiśniewska A, Janczarek I, Wilk I, Tkaczyk E, Mierzicka M, Stanley CR, Górecka-Bruzda A. Heterospecific Fear and Avoidance Behaviour in Domestic Horses (Equus caballus). Animals. 2021; 11(11):3081. https://doi.org/10.3390/ani11113081
Chicago/Turabian StyleWiśniewska, Anna, Iwona Janczarek, Izabela Wilk, Ewelina Tkaczyk, Martyna Mierzicka, Christina R. Stanley, and Aleksandra Górecka-Bruzda. 2021. "Heterospecific Fear and Avoidance Behaviour in Domestic Horses (Equus caballus)" Animals 11, no. 11: 3081. https://doi.org/10.3390/ani11113081
APA StyleWiśniewska, A., Janczarek, I., Wilk, I., Tkaczyk, E., Mierzicka, M., Stanley, C. R., & Górecka-Bruzda, A. (2021). Heterospecific Fear and Avoidance Behaviour in Domestic Horses (Equus caballus). Animals, 11(11), 3081. https://doi.org/10.3390/ani11113081






