Laparoscopic Castration Using Bipolar Forceps vs. Orchiectomy in Dogs: A Comparison of Two Techniques
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures
2.3. Orchiectomy
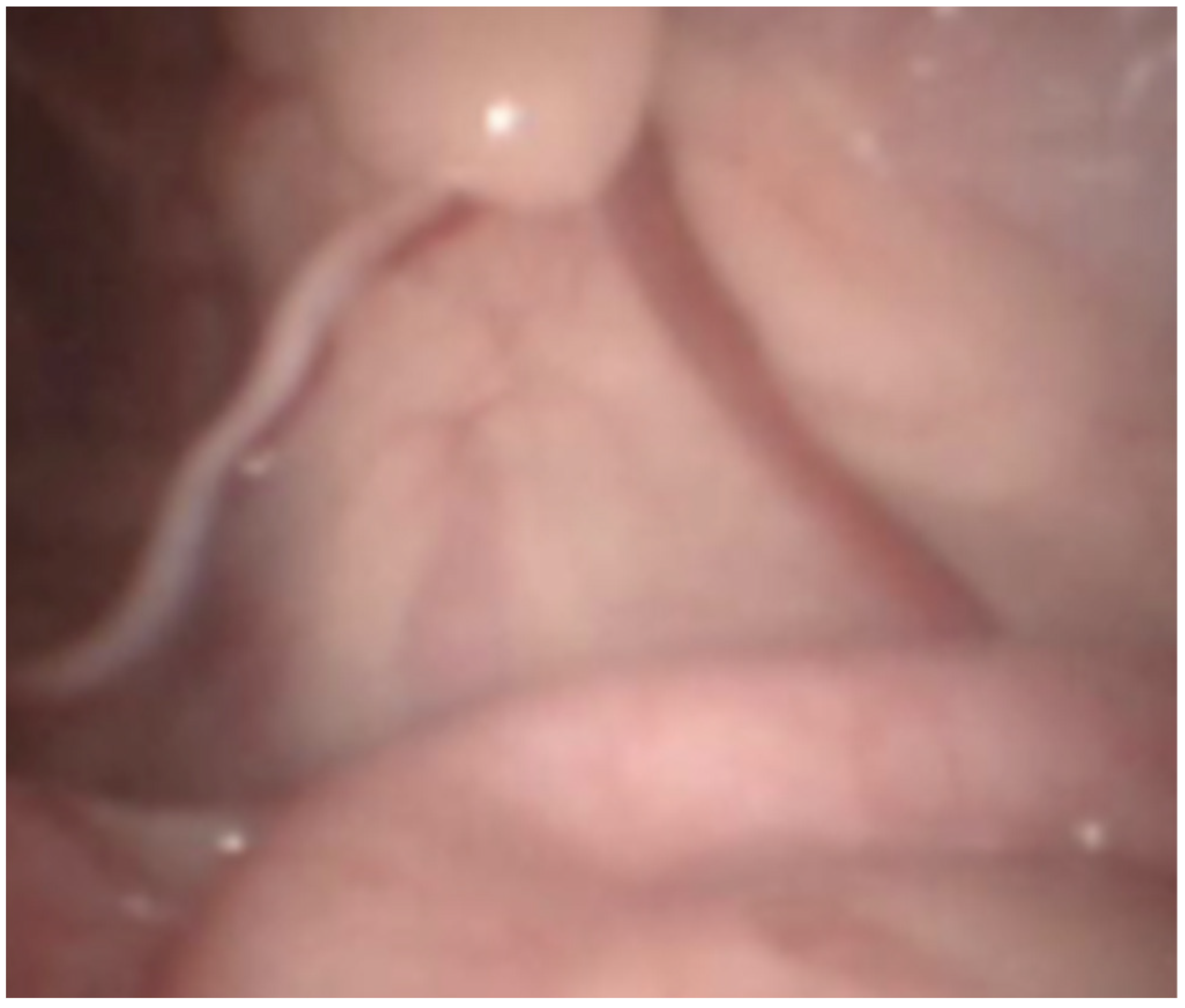
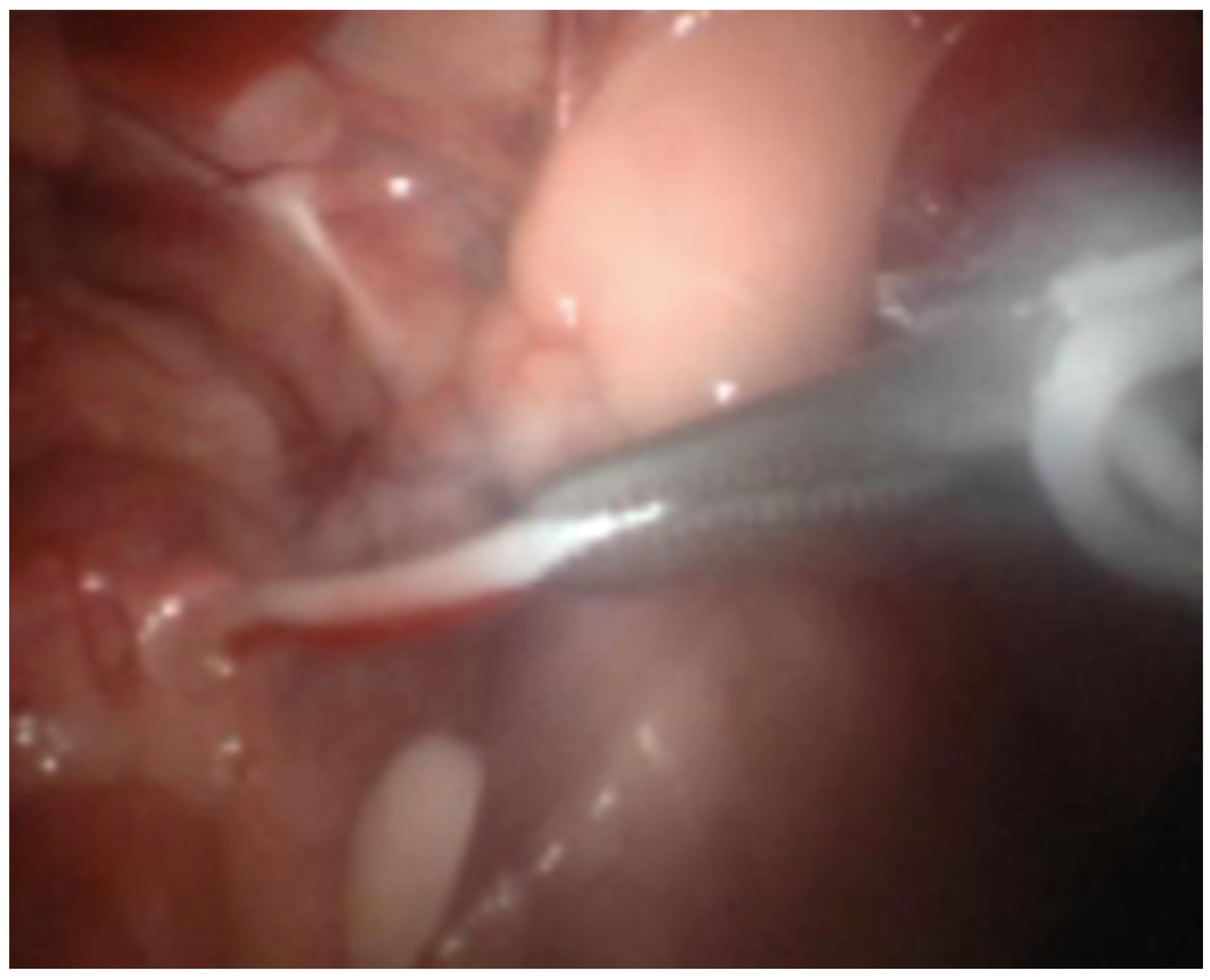
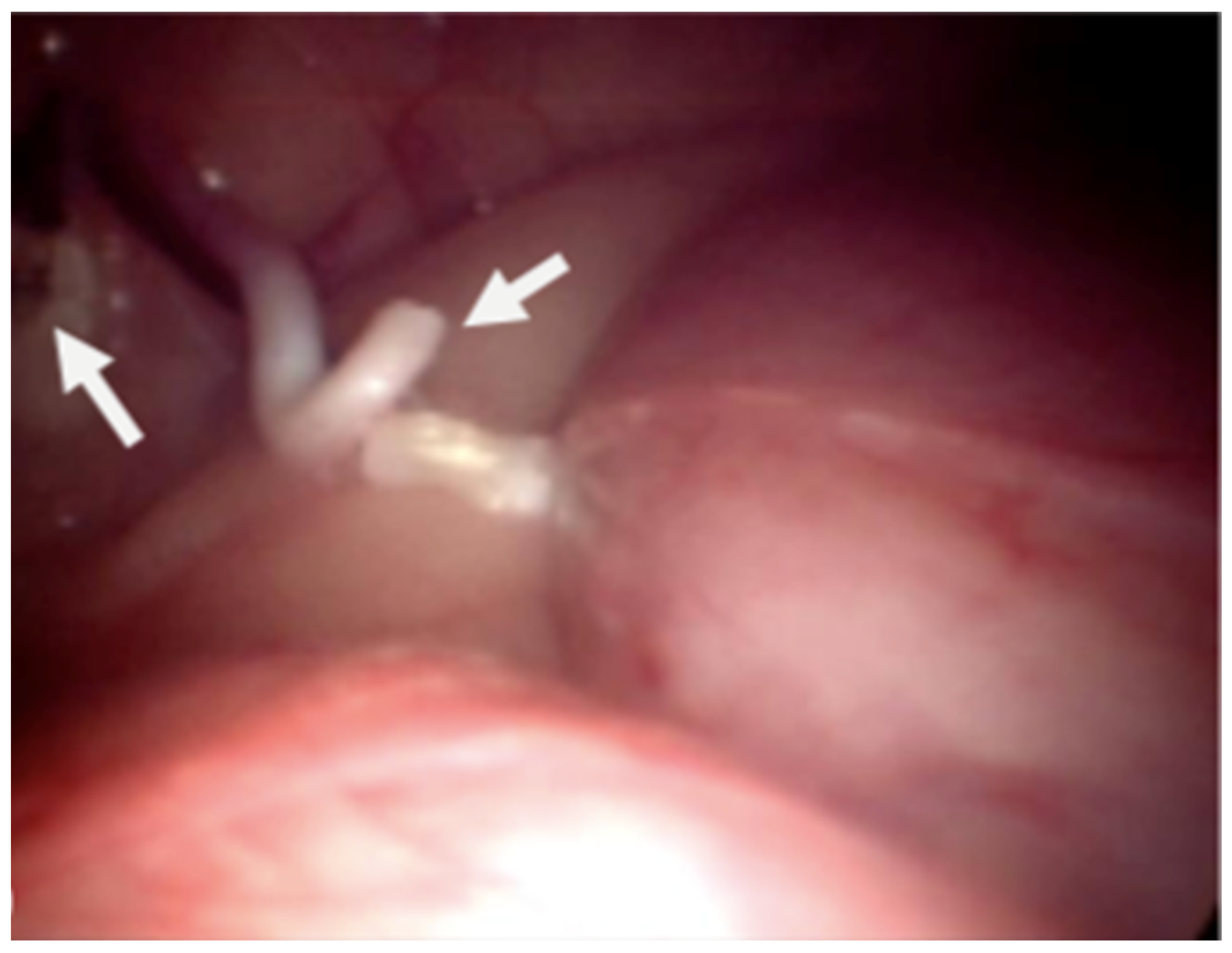
2.4. Laparoscopic Castration
2.5. Recorded Variables and Postoperative Pain Assessment
2.6. Statistical Analysis
3. Results
3.1. Surgical Procedures and Clinical Follow-Up
3.2. UMPS (University of Melbourne Pain Scale)
3.3. Serum Cortisol
3.4. Saliva Cortisol
3.5. C-Reactive Protein (PCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.; Creevy, K.; Promislow, D. Reproductive Capability Is Associated with Lifespan and Cause of Death in Companion Dogs. PLoS ONE 2013, 8, e61082. [Google Scholar] [CrossRef] [Green Version]
- Olson, P.N.; Nett, T.M.; Bowen, R.A.; Amann, R.P.; Sawyer, H.R.; Gorell, T.A.; Niswender, G.D.; Pickett, B.W.; Phemister, R.D. A need for sterilization, contraceptives, and abortifacients: Abandoned and unwanted pets. IV. Potential methods of controlling reproduction. Compend. Contin. Educ. Pract. Vet. 1986, 8, 303–308. [Google Scholar]
- Kustritz, M.V.R. Effects of surgical sterilization on canine and feline health and on society. Reprod. Domest. Anim. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, L.M. Surgical methods of contraception and sterilization. Theriogenology 2006, 66, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Fossum, T. Surgery of the male reproductive tract. In Small Animal Surgery; Mosby Elsevier: Maryland Heights, MI, USA, 2007. [Google Scholar]
- Jana, K.; Samanta, P.K. Sterilization of male stray dogs with a single intratesticular injection of calcium chloride: A dose dependent study. Contraception 2007, 75, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Kumar, N.; Maiti, S.K.; Sharma, A.K.; Dimri, U.; Kataria, M.; Mathew, D.D.; Remya, V.; Mohsina, A. Laparoscopic vasectomy vs laparoscopic sterilization in dogs: A comparison of two techniques. World J. Laparosc. Surg. 2014, 7, 7–15. [Google Scholar] [CrossRef]
- Nikpasand, A.; Behfar, M.; Hashemi, M.; Tehrani, A.-A.; Mohammadi, V. Evaluation of bilateral vasocystostomy for canine sterilization. Theriogenology 2020, 156. [Google Scholar] [CrossRef] [PubMed]
- Boothe, H. Testes and epididymides. In Textbook of Small Animal Surgery; Slatter, D., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 1527–1529. [Google Scholar]
- Mahalingam, A.; Kumar, N.; Maiti, S.K.; Sharma, A.K.; Dimri, U.; Kataria, M. Laparoscopic sterilization vs. open method sterilization in dogs: A comparison of two techniques. Turk. J. Vet. Anim. Sci. 2009, 33, 427–436. [Google Scholar] [CrossRef]
- Jana, K.; Samanta, P.K.; Ghosh, D. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization of male Black Bengal goats (Capra hircus): A dose-dependent study. Anim. Reprod. Sci. 2005, 86, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Brückner, M. Laparoscopic-assisted cryptorchidectomy in a cat. Tierärztliche Prax. Ausg. K Kleintiere Heimtiere 2015, 43, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Lanz, O.; Hamilton, S. Laparoscopic ovariohysterectomy in nine dogs. J. Am. Anim. Hosp. Assoc. 2003, 39, 391–396. [Google Scholar]
- Davidson, E.B.; Moll, H.D.; Payton, M.E. Comparison of laparoscopic ovariohysterectomy and ovariohysterectomy in dogs. Vet. Surg. 2004, 33, 62–69. [Google Scholar] [CrossRef]
- Devitt, C.M.; Cox, R.E.; Hailey, J.J. Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 921–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gower, S.; Mayhew, P. Canine laparoscopic and laparo-scopic assisted ovariohysterectomy and ovariectomy. Compend. Contin. Educ. Vet. 2008, 30, 430–440. [Google Scholar]
- Lansdowne, J.L.; Mehler, S.J.; Bouré, L.P. Minimally invasive abdominal and Thoracic Surgery: Principles and Instrumentation. Compend. Contin. Educ. Vet. 2012, 34, E1. [Google Scholar] [PubMed]
- Rawlings, C.A.; Howerth, E.W.; Mahaffey, M.B.; Foutz, T.L.; Bement, S.; Canalis, C. Laparoscopic-assisted cystopexy in dogs. Am. J. Vet. Res. 2002, 63, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.F.; Cotard, J.P.; Viguier, E. Management of urethral sphincter mechanism incompetence in a male dog with laparoscopic-guided deferentopexy. J. Small Anim. Pract. 2002, 43, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Mathon, D.H.; Palierne, S.; Meynaud-Collard, P.; Layssol-Lamour, C.; Dulaurent-Ferrieres, A.; Colson, A.; Lacroix, M.; Bousquet-Melou, A.; Delverdier, M.; Autefage, A. Laparoscopic-Assisted Colopexy and Sterilization in Male Dogs: Short-Term Results and Physiologic Consequences. Vet. Surg. 2011, 40, 500–508. [Google Scholar] [CrossRef]
- Rivier, P.; Furneaux, R.; Viguier, E. Combined laparoscopic ovariectomy and laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatation-volvulus. Can. Vet. J. 2011, 52, 62–66. [Google Scholar] [PubMed]
- Lew, M.; Jałyński, M.; Kasprowicz, A.; Brzeski, W. Laparoscopic cryptorchidectomy in dogs-report of 15 cases. Pol. J. Vet. Sci. 2005, 8, 251–254. [Google Scholar]
- Mayhew, P.D.; Brown, D.C. Comparison of three techniques for ovarian pedicle hemostasis during laparoscopic-assisted ovariohysterectomy. Vet. Surg. 2007, 36, 541–547. [Google Scholar] [CrossRef]
- Case, J.; Marvel, S.; Boscan, P.; Monnet, E. Surgical time and severity of postoperative pain in dogs undergoing laparoscopic ovariectomy with one, two, or three instrument cannulas. J. Am. Vet. Med. Assoc. 2011, 239, 203–208. [Google Scholar] [CrossRef]
- McClaran, J.; Buote, N. Complications and Need for Conversion to Laparotomy in Small Animals. Vet. Clin. North Am. Small Anim. Pract. 2009, 39, 941–951. [Google Scholar] [CrossRef]
- Tapia-Araya, A.E.; Martin-Portugués, I.D.G.; Bermejo, L.F.; Sánchez-Margallo, F.M. Laparoscopic ovariectomy in dogs: Comparison between laparoendoscopic single-site and three-portal access. J. Vet. Sci. 2015, 16, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados, J.-R.; Usón, J.; Martínez, J.-M.; Sánchez Margallo, F.; Perez, E. Canine laparoscopic ovariectomy using two 3- and 5-mm portal sites: A prospective randomized clinical trial. Can. Vet. J. 2017, 58, 565–570. [Google Scholar]
- Wildt, D.E.; Seager, S.W.; Bridges, C.H. Sterilization of the male dog and cat by laparoscopic occlusion of the ductus deferens. Am. J. Vet. Res. 1981, 42, 1888–1897. [Google Scholar] [PubMed]
- Mathews, K. A Pain assessment and general approach to management. Vet. Clin. North Am. Small Anim. Pract. 2000, 30, 729–755. [Google Scholar]
- Mich, P.M.; Hellyer, P.W. Objective, Categoric Methods for Assessing Pain and Analgesia. In Handbook of Veterinary Pain Management; Elsevier: Amsterdam, The Netherlands, 2009; pp. 78–109. ISBN 9780323046794. [Google Scholar]
- Hancock, R.B.; Lanz, O.I.; Waldron, D.R.; Duncan, R.B.; Broadstone, R.V.; Hendrix, P.K. Comparison of postoperative pain after ovariohysterectomy by harmonic scalpel-assisted laparoscopy compared with median celiotomy and ligation in dogs. Vet. Surg. 2005, 34, 273–282. [Google Scholar] [CrossRef]
- Hansen, B.D.; Hardie, E.M.; Carroll, G.S. Physiological measurements after ovariohysterectomy in dogs: What’s normal? Appl. Anim. Behav. Sci. 1997, 51, 101–109. [Google Scholar] [CrossRef]
- Fox, S.M.; Mellor, D.J.; Lawoko, C.R.O.; Hodge, H.; Firth, E.C. Changes in plasma cortisol concentrations in bitches in response to different combinations of halothane and butorphanol, with or without ovariohysterectomy. Res. Vet. Sci. 1998, 65, 125–133. [Google Scholar] [CrossRef]
- Smith, J.; Allen, S.; Quandt, J. Indicators of post-operative pain in cats and correlation with clinical criteria. Am. J. Vet. Res. 1996, 57, 1674–1678. [Google Scholar] [PubMed]
- Smith, J.D.; Allen, S.W.; Quandt, J.E. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables. Am. J. Vet. Res. 1999, 60, 432–436. [Google Scholar]
- Conzemius, M.G.; Hill, C.M.; Sammarco, J.L.; Perkowski, S.Z. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J. Am. Vet. Med. Assoc. 1997, 210, 1619–1622. [Google Scholar] [PubMed]
- Benson, G.J.; Wheaton, L.G.; Thurmon, J.C.; Tranquilli, W.J.; Olson, W.A.; Davis, C.A. Postoperative Catecholamine Response to Onychectomy in Isoflurane-anesthetized Cats Effect of Analgesics. Vet. Surg. 1991, 20, 222–225. [Google Scholar] [CrossRef]
- Popilskis, S.; Kohn, D.; Laurent, L. Efficacy of epidural morphine versus intravenous morphine for post-thoracotomy pain in dogs. Vet. Anaesth. Analg. 1993, 20, 21–25. [Google Scholar]
- Lin, H.C.; Benson, G.J.; Thurmon, J.C.; Tranquilli, W.J.; Olson, W.A.; Bevill, R.F. Influence of anesthetic regimens on the perioperative catecholamine response associated with onychectomy in cats. Am. J. Vet. Res. 1993, 54, 1721–1724. [Google Scholar]
- Beerda, B.; Schilder, M.B.H.; Janssen, N.S.C.R.M.; Mol, J.A. The use of saliva cortisol, urinary cortisol, and cateoholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 1996, 30, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Vincent, I.C.; Michell, A.R. Comparison of cortisol concentrations in saliva and plasma of dogs. Res. Vet. Sci. 1992, 53, 342–345. [Google Scholar] [CrossRef]
- Ko, J.C.; Mandsager, R.E.; Lange, D.N.; Fox, S.M. Cardiorespiratory responses and plasma cortisol concentrations in dogs treated with medetomidine before undergoing ovariohysterectomy. J. Am. Vet. Med. Assoc. 2000, 217, 509–514. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shida, T.; Miyaji, S.; Santsuka, H.; Fujise, H.; Mukawa, K.; Furukawa, E.; Nagae, T.; Naiki, M. Changes in serum C-reactive protein levels in dogs with various disorders and surgical traumas. Vet. Res. Commun. 1993, 17, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Subiela, S. Acute phase proteins in the Dog: Analytical and Clinical validation Studies. Ph.D. Thesis, University of Murcia, Murcia, Spain, 2003. [Google Scholar]
- Kjelgaard-Hansen, M.; Strom, H.; Mikkelsen, L.F.; Eriksen, T.; Jensen, A.L.; Luntang-Jensen, M. Canine serum C-reactive protein as a quantitative marker of the inflammatory stimulus of aseptic elective soft tissue surgery. Vet. Clin. Pathol. 2013, 42, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. Iron metabolism and its disorders. In Clinical Bioquemistry of Domestic Animals; Academic Press: New York, NY, USA, 1997; pp. 223–240. [Google Scholar]
- Conner, J.G.; Eckersall, P.D.; Ferguson, J.; Douglas, T.A. Acute phase response in the dog following surgical trauma. Res. Vet. Sci. 2018, 45, 107–110. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Zhang, N.; Zhang, J.-T.; Wang, H.-B.; Pan, L. Laparoscopic Colopexy in Dogs. J. Vet. Med. Sci. 2013, 75. [Google Scholar] [CrossRef] [Green Version]
- Stedile, R.; Beck, C.; Schiochet, F.; Ferreira, M.; Oliveira, S.; Martens, F.; Tessari, J.; Bernades, S.; Oliveira, C.; Dos Santos, A.; et al. Laparoscopic versus open splenectomy in dogs. Pesqui. Veterinária Bras. 2009, 29, 653–660. [Google Scholar] [CrossRef] [Green Version]



| Number of Animals by Breed | ||||
|---|---|---|---|---|
| Mixed-Breed | Miniature Poodle | Labrador | Yorkshire Terrier | |
| OR | 6 | 2 | 1 | 1 |
| LS | 10 | - | - | - |
| Patient Characteristics and Intraoperative Variables | OR | LS |
|---|---|---|
| Age (years) | 3.3 (0.6–7) | 1.04 (0.4–2) |
| Weight (kg) | 14 (4.5–27) | 14.1 (5.7–24.4) |
| Duration of surgery (hours) | 41.4 (36–49.8) | 42 (30–75) |
| Variable | Group | Time after Extubation | ||
|---|---|---|---|---|
| 1 h | 12 h | 24 h | ||
| UMPS Score | OR | 3.56 ± 2.4 | 3.8 ± 2.68 | 2.55 ± 1.8 |
| LS | 2 ± 1.12 | 1.44 ± 1.5 * | 0.44 ± 0.73 * | |
| Variable | Group | Time after Extubation | |||
|---|---|---|---|---|---|
| 0 h | 1 h | 12 h | 24 h | ||
| Serum cortisol | OR | 182 ± 133.67 | 234 ± 81.17 | 113.9 ± 51.99 | 110 ± 83.66 |
| LS | 92.5 ± 36.02 | 119 ± 62.47 * | 88.7 ± 36.18 | 95.1 ± 93.30 | |
| Salivary Cortisol | OR | 8 ± 7.6 | 15 ± 4.1 | 5.6 ± 1.9 | 4.5 ± 1.77 |
| LS | 4.9 ± 2.8 | 11.1 ± 3.8 * | 5.2 ± 2.7 | 4.75 ± 2.71 | |
| Variable | Group | Time after Extubation | ||
|---|---|---|---|---|
| 0 h | 24 h | 168 h | ||
| C-reactive Protein | OR | 17.8 ± 15.8 | 22.9 ± 11.2 | 20.8 ± 16.9 |
| LS | 7.1 ± 4.1 | 18.7 ± 8.2 | 5.8 ± 3.3 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, I.T.; Barreno, R.R.; Sales-Luís, J.P.; Vaudano, C.G.; Jaber, J.R. Laparoscopic Castration Using Bipolar Forceps vs. Orchiectomy in Dogs: A Comparison of Two Techniques. Animals 2021, 11, 3041. https://doi.org/10.3390/ani11113041
Tavares IT, Barreno RR, Sales-Luís JP, Vaudano CG, Jaber JR. Laparoscopic Castration Using Bipolar Forceps vs. Orchiectomy in Dogs: A Comparison of Two Techniques. Animals. 2021; 11(11):3041. https://doi.org/10.3390/ani11113041
Chicago/Turabian StyleTavares, Inês Tenreiro, Ramón R. Barreno, José P. Sales-Luís, Carlo G. Vaudano, and José Raduan Jaber. 2021. "Laparoscopic Castration Using Bipolar Forceps vs. Orchiectomy in Dogs: A Comparison of Two Techniques" Animals 11, no. 11: 3041. https://doi.org/10.3390/ani11113041
APA StyleTavares, I. T., Barreno, R. R., Sales-Luís, J. P., Vaudano, C. G., & Jaber, J. R. (2021). Laparoscopic Castration Using Bipolar Forceps vs. Orchiectomy in Dogs: A Comparison of Two Techniques. Animals, 11(11), 3041. https://doi.org/10.3390/ani11113041






