A Controlled Clinical Study of Intensive Neurorehabilitation in Post-Surgical Dogs with Severe Acute Intervertebral Disc Extrusion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
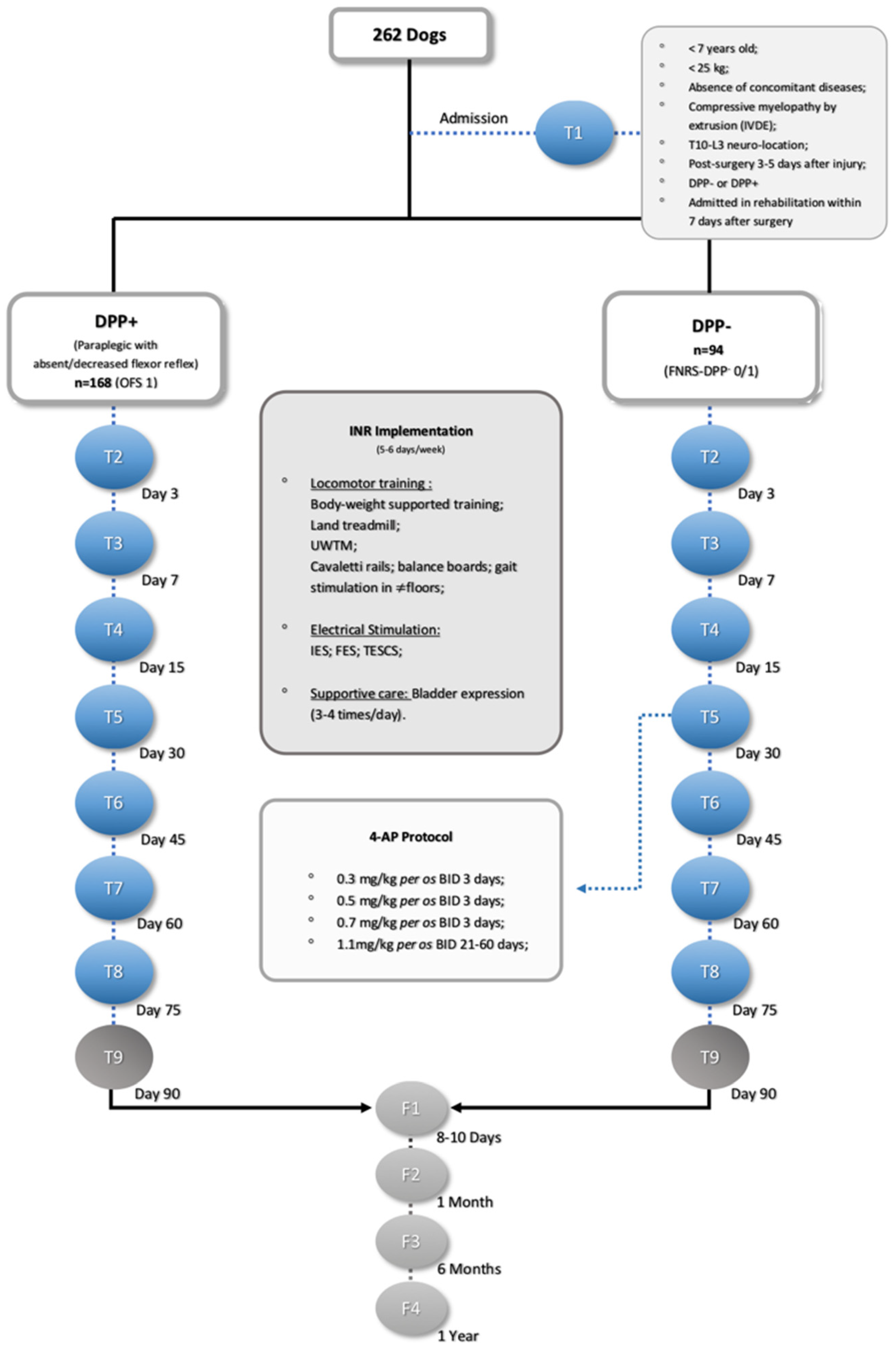
2.1. Participants
2.2. Study Design
2.3. Interventions
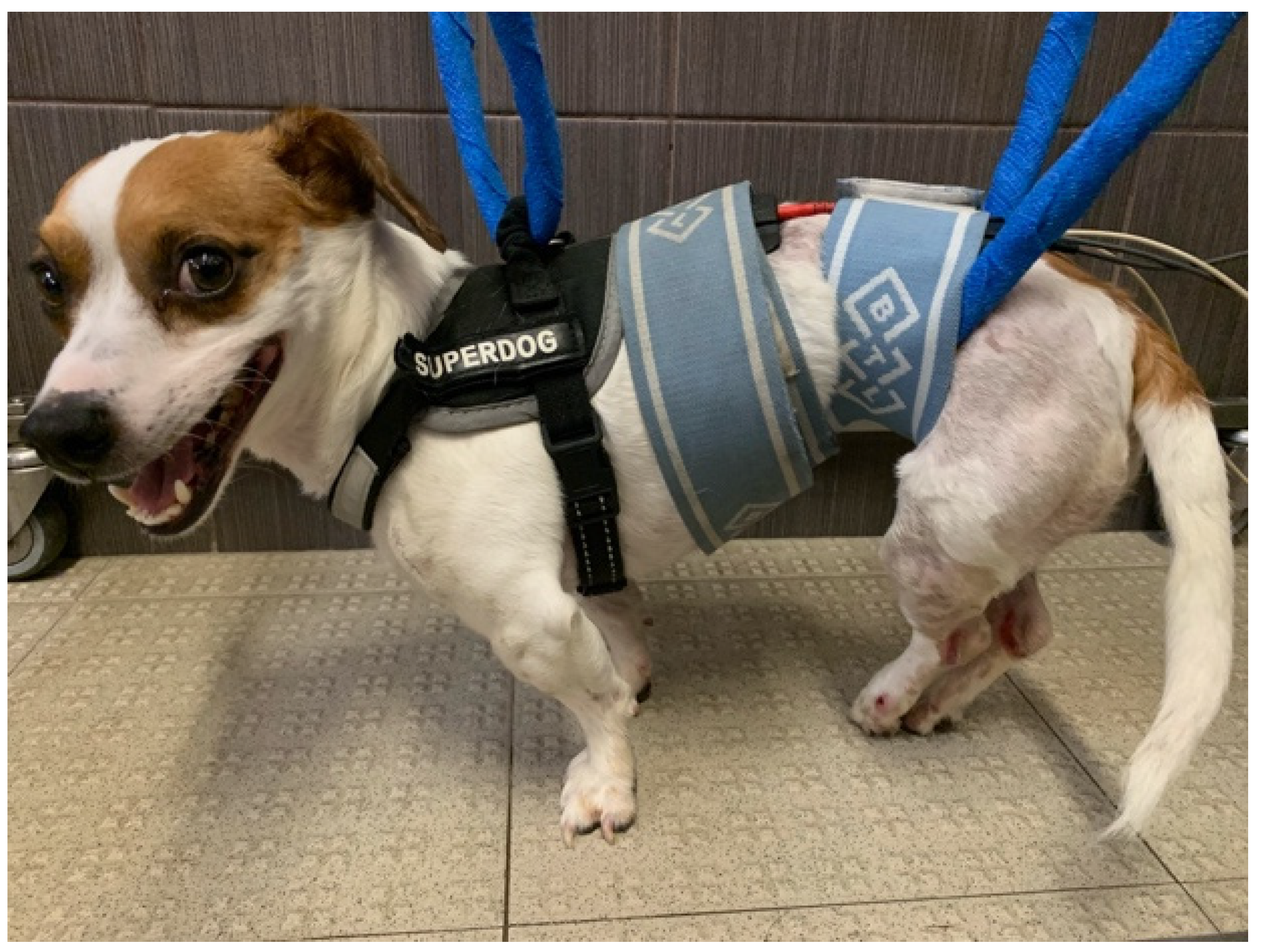
2.3.1. Locomotor Training
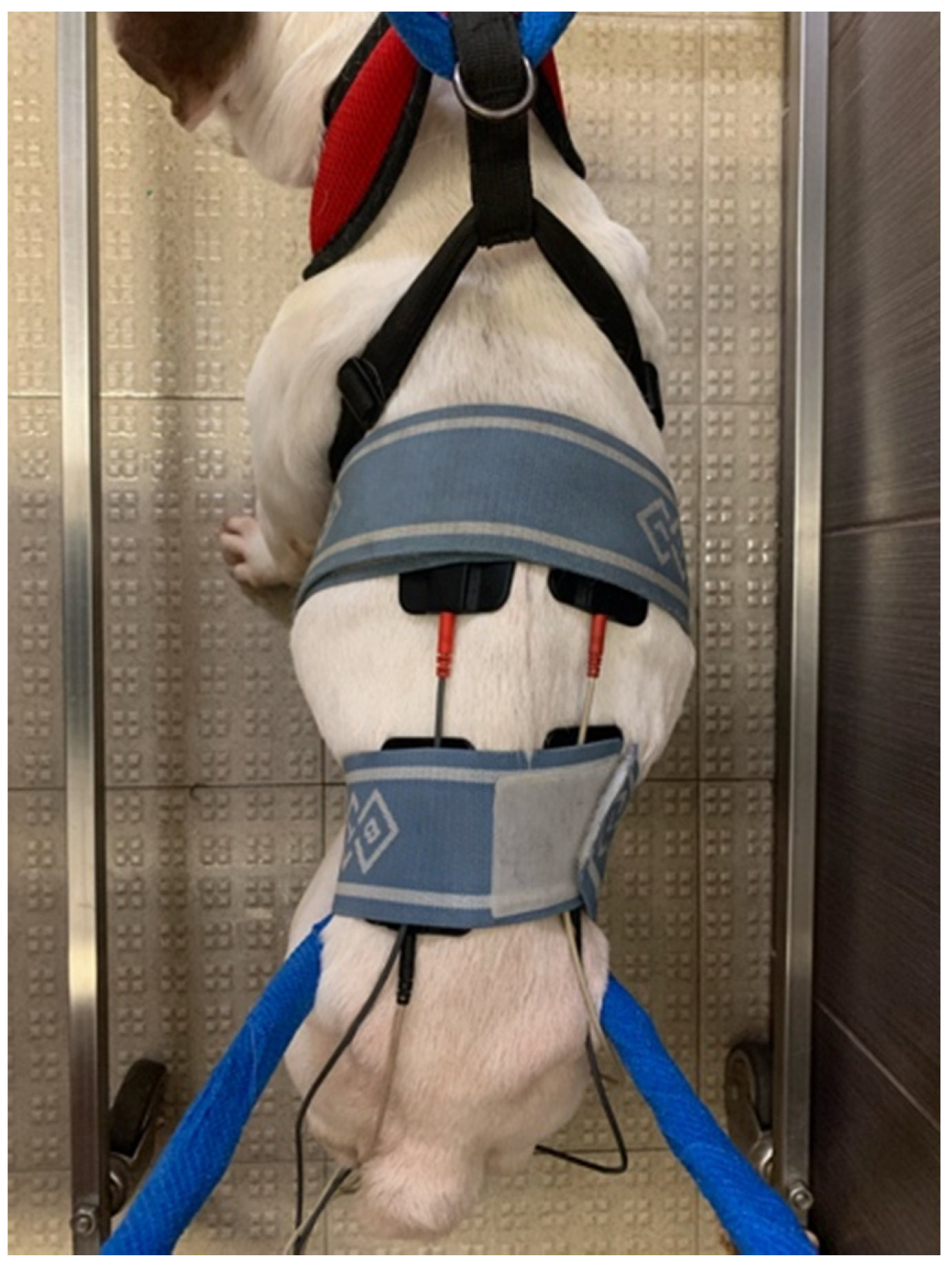
2.3.2. Electrical Stimulation
- Interferential electrical stimulation
- Functional electrical stimulation
- Transcutaneous electrical spinal cord stimulation
2.3.3. Pharmacological Management
2.3.4. Supportive Measures
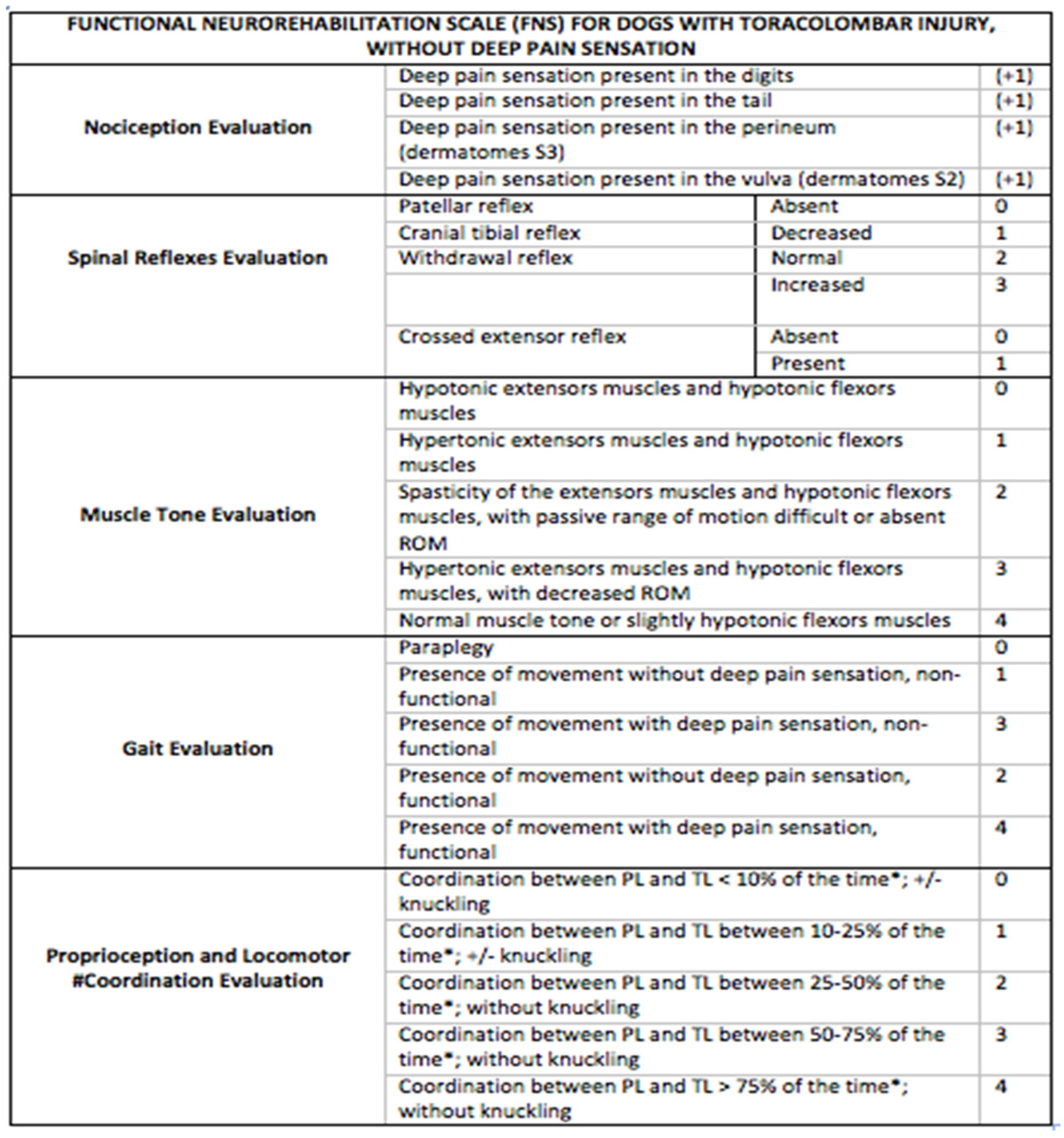
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
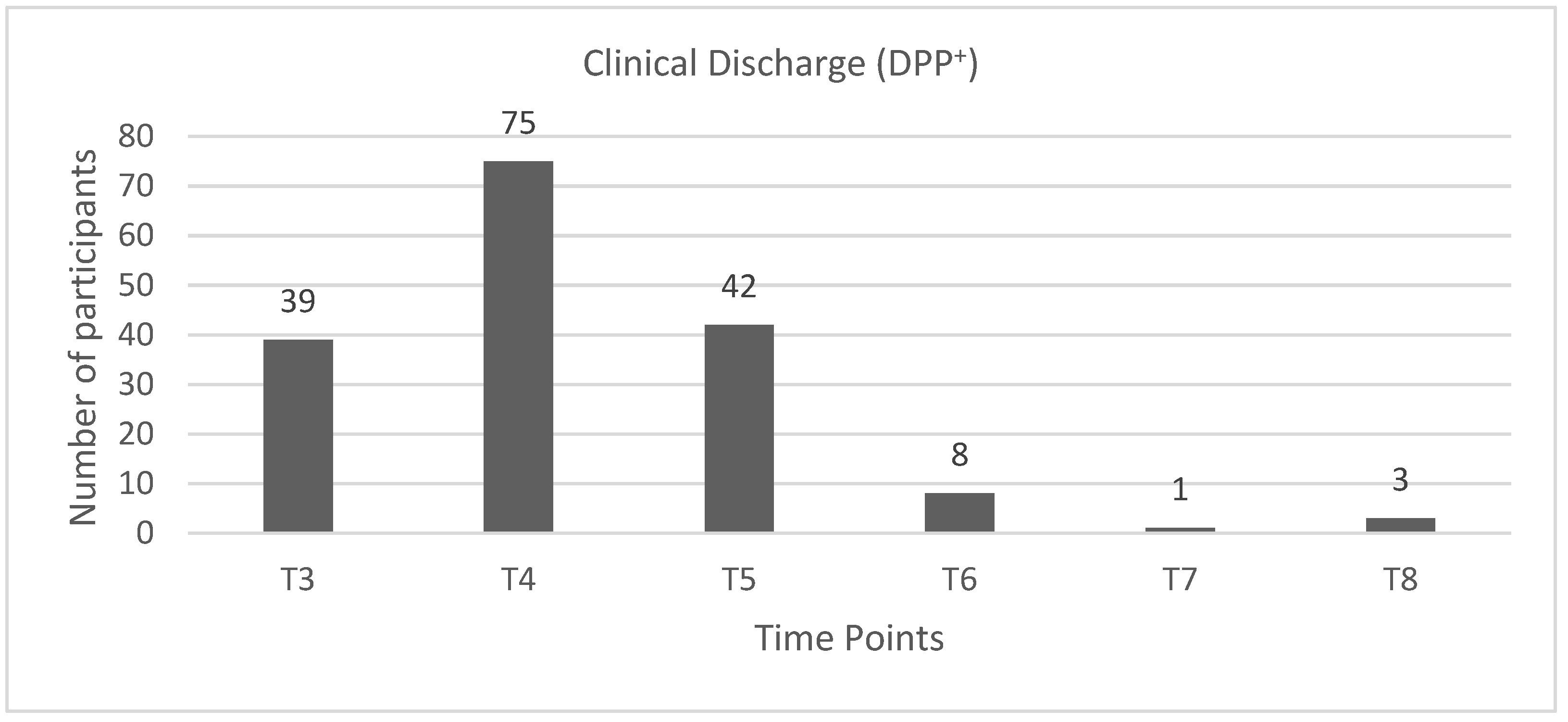
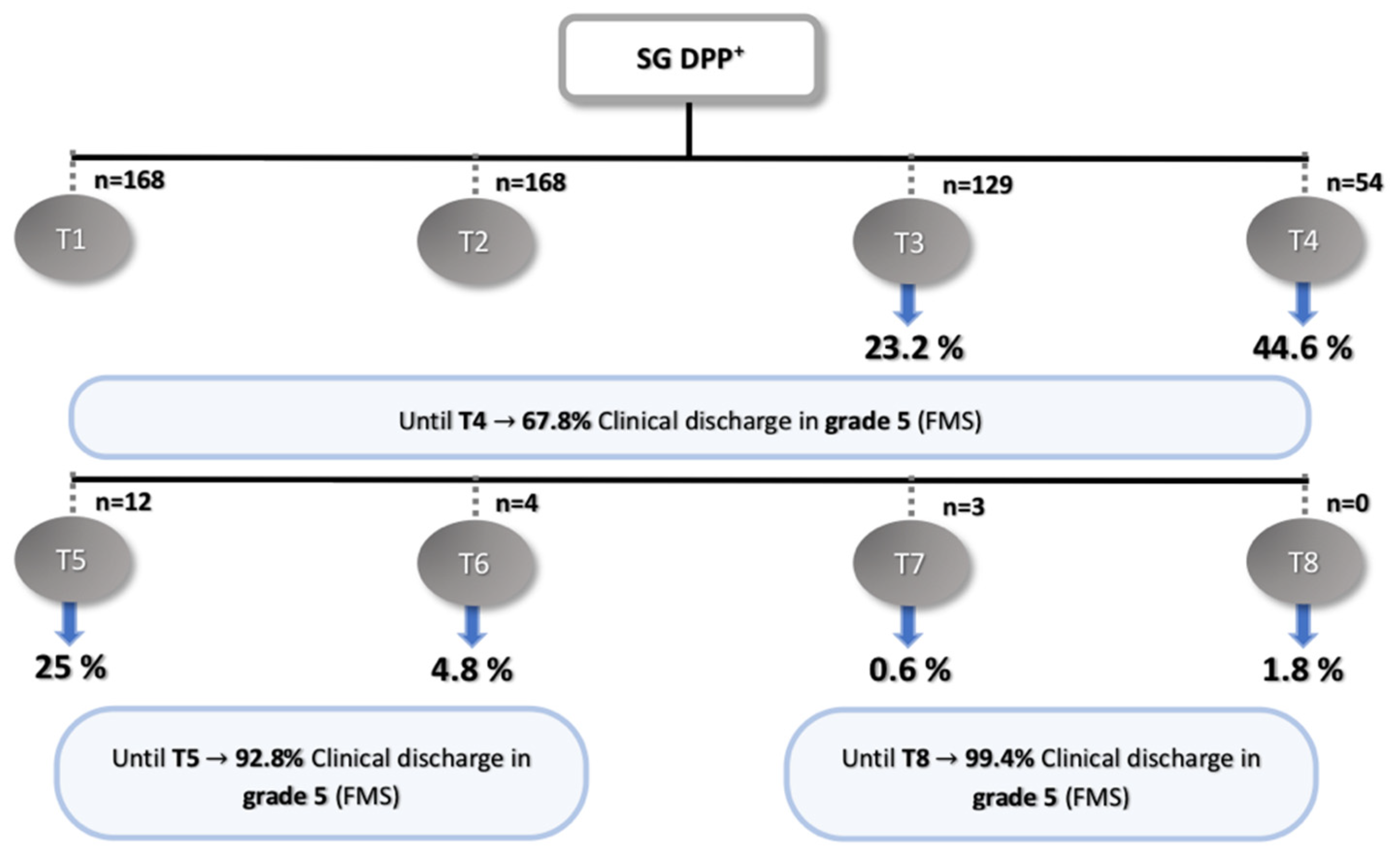
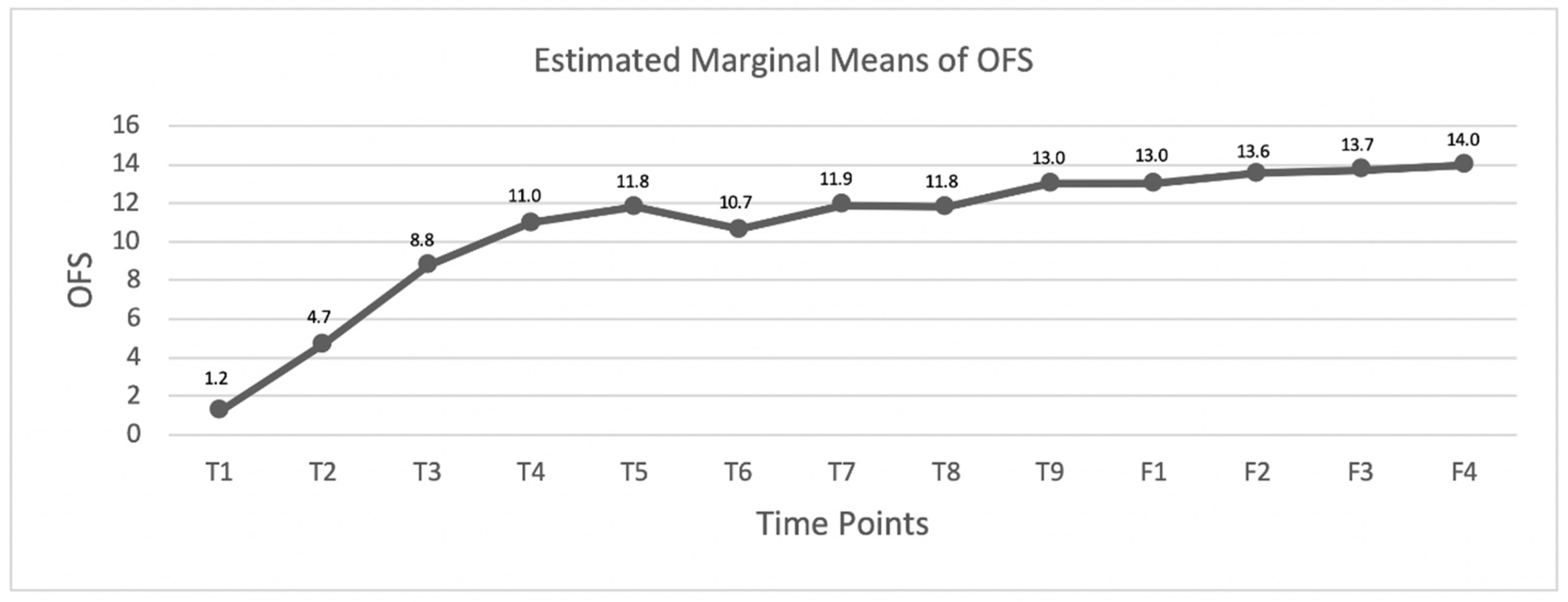
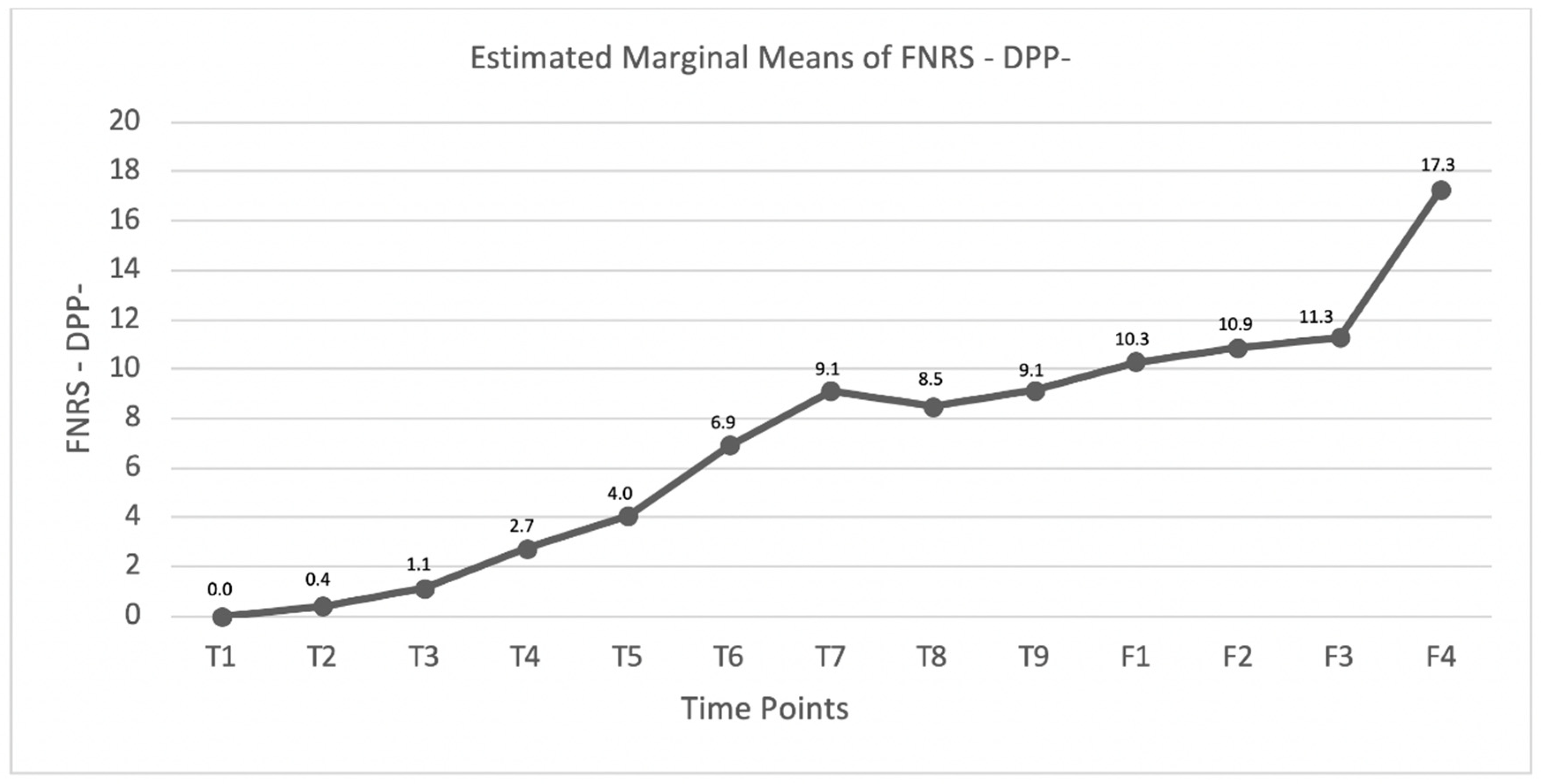
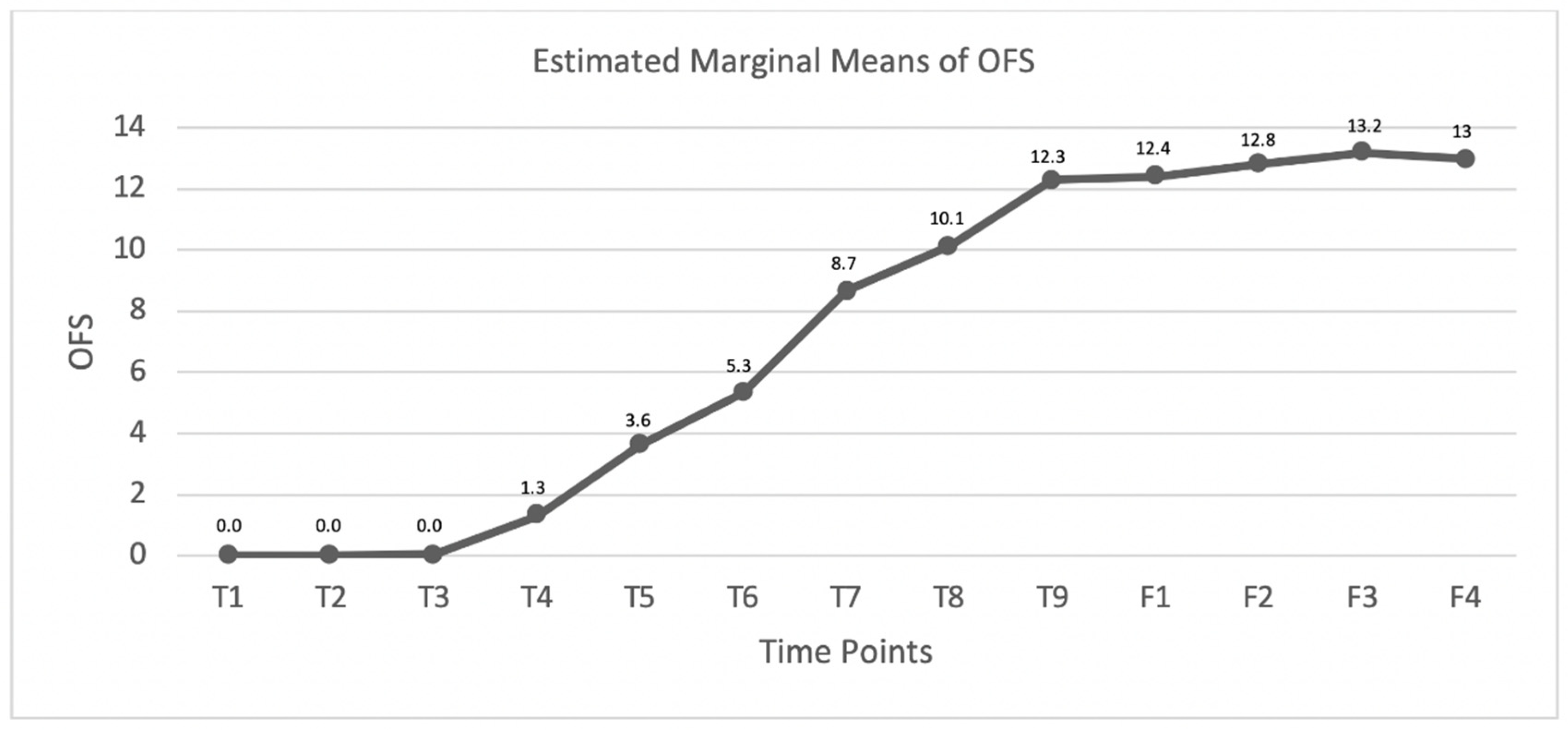
3.1. DPP+ Dogs
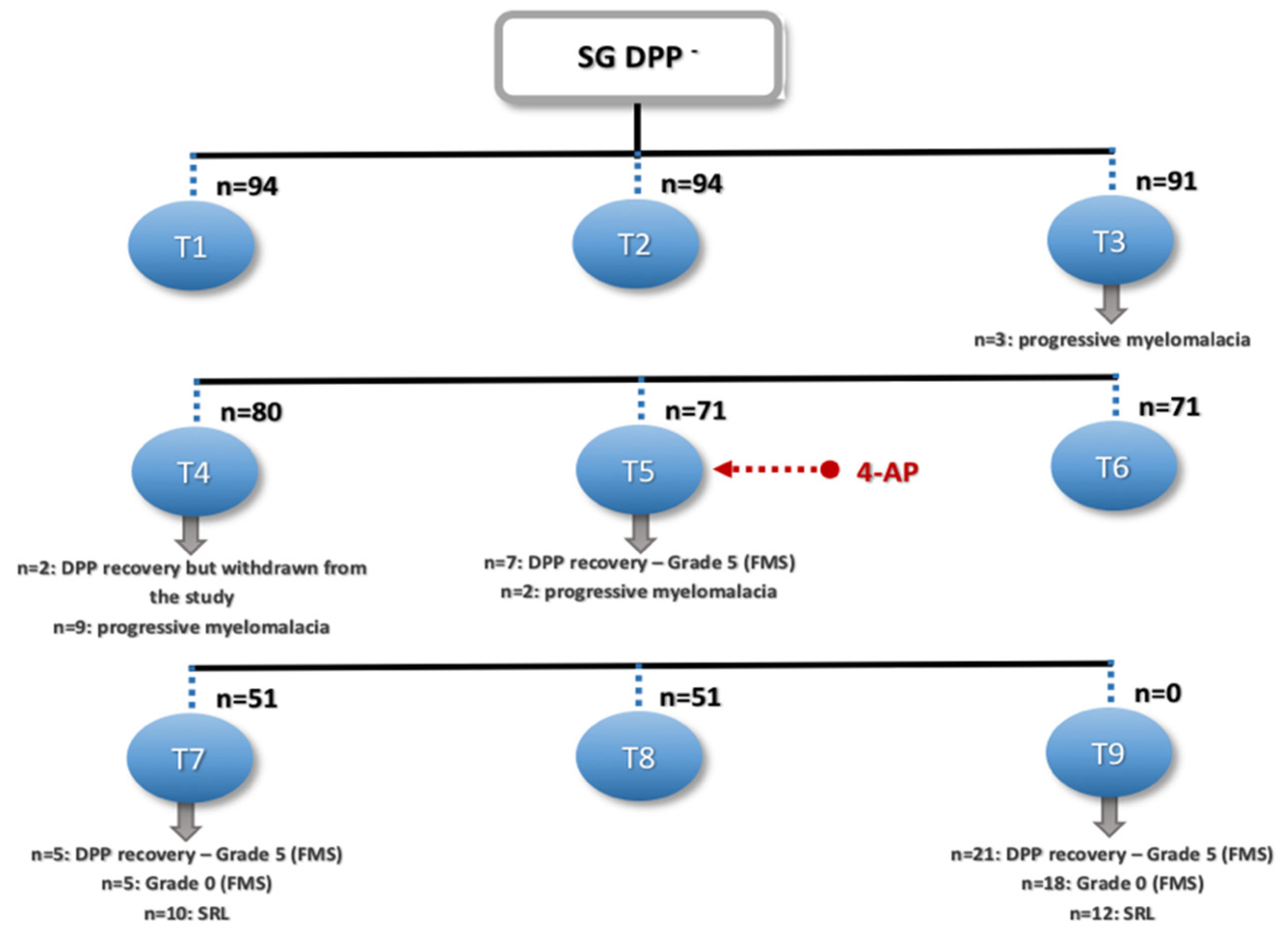
3.2. DPP− Dogs
4. Discussion
4.1. DPP+ Dogs
4.2. DPP− Dogs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fakhoury, M. Spinal cord injury: Overview of experimental approaches used to restore locomotor activity. Rev. Neurosci. 2015, 26, 397–405. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J.; The Canine Spinal Cord Injury Consortium (CANSORT-SCI). Ambulation in Dogs with Absent Pain Perception after Acute Thoracolumbar Spinal Cord Injury. Front. Veter.-Sci. 2020, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Tansey, K.E. Neural Plasticity and Locomotor Recovery After Spinal Cord Injury. PM&R 2010, 2, S220–S226. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R. Residual motor function after spinal cord injury. In Restorative Neurology of Spinal Cord Injury, 1st ed.; Dimitrijevic, M.R., Kakulas, B.A., McKay, W.B., Vrbová, G., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 1–9. [Google Scholar]
- Kakulas, B.A.; Kaelan, C. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin. Neurol. Neurosurg. 2015, 129, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.-P.; Murray, M.; Lemay, M.A. Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure. J. Neurotrauma 2017, 34, 1841–1857. [Google Scholar] [CrossRef] [Green Version]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Raineteau, O.; Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.R.; Danner, S.M.; Mayr, W. Neurocontrol of Movement in Humans with Spinal Cord Injury. Artif. Organ. 2015, 39, 823–833. [Google Scholar] [CrossRef]
- Van de Crommert, H.W.; Mulder, T.; Duysens, J. Neural control of locomotion: Sensory control of the central pattern generator and its relation to treadmill training. Gait. Post. 1998, 7, 251–263. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.-P. Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- Barrière, G.; Leblond, H.; Provencher, J.; Rossignol, S. Prominent Role of the Spinal Central Pattern Generator in the Recovery of Locomotion after Partial Spinal Cord Injuries. J. Neurosci. 2008, 28, 3976–3987. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Wang, Y.; Chen, X.Y.; Wolpaw, J.R. Why New Spinal Cord Plasticity Does Not Disrupt Old Motor Behaviors. J. Neurosci. 2017, 37, 8198–8206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgerton, V.R.; De Leon, R.D.; Harkema, S.J.; Hodgson, J.A.; London, N.; Reinkensmeyer, D.J.; Roy, R.R.; Talmadge, R.J.; Tillakaratne, N.J.; Timoszyk, W.; et al. Retraining the injured spinal cord. J. Physiol. 2001, 533, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wirz, M.; Zemon, D.H.; Rupp, R.; Scheel, A.; Colombo, G.; Dietz, V.; Hornby, T.G. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch. Phys. Med. Rehabil. 2005, 86, 672–680. [Google Scholar] [CrossRef]
- Knikou, M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin. Neurophysiol. 2010, 121, 1655–1668. [Google Scholar] [CrossRef]
- Behrman, A.L.; Harkema, S.J. Locomotor Training After Human Spinal Cord Injury: A Series of Case Studies. Phys. Ther. 2000, 80, 688–700. [Google Scholar] [CrossRef]
- Harbeau, H.; Fung, J.; Leroux, A.; Ladouceur, M. Chapter 2 A review of the adaptability and recovery of locomotion after spinal cord injury. Prog Brain Res. 2002, 137, 9–25. [Google Scholar] [CrossRef]
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of Spinal Cord Reflexes After a Complete Transection in Adult Rats: Relationship to Stepping Ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef]
- Possover, M. Recovery of Sensory and Supraspinal Control of Leg Movement in People with Chronic Paraplegia: A Case Series. Arch. Phys. Med. Rehab. 2014, 95, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Kugler, J.; Pohl, M. Locomotor training for walking after spinal cord injury. Cochr. Datab. Syst. Rev. 2008, 11, CD006676. [Google Scholar] [CrossRef] [Green Version]
- Mehrholz, J.; Harvey, L.A.; Thomas, S.; Elsner, B. Author Correction: Is body-weight supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. 2018, 56, 412. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, H.; Norman, K.; Fung, J.; Visintin, M.; Ladouceur, M. Does neurorehabilitation play a role in the recovery of walking in neurological populations? Ann. N. Y. Acad. Sci. 1998, 860, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Tennissen, A.M. Activity-Dependent Spinal Cord Plasticity in Health and Disease. Annu. Rev. Neurosci. 2001, 24, 807–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, K.C. The Use of 4-Aminopyridine (Fampridine) in Demyelinating Disorders. CNS Drug Rev. 2006, 10, 295–316. [Google Scholar] [CrossRef]
- Hayes, K.C. Fampridine-SR for multiple sclerosis and spinal cord injury. Expert Rev. Neurother. 2007, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.J.; Roy, R.R.; Ichiyama, R.; Lavrov, I.; Courtine, G.; Gerasimenko, Y.; Tai, Y.; Burdick, J.; Edgerton, V.R. Recovery of control of posture and locomotion after a spinal cord injury: Solutions staring us in the face. Biol. Basis Mind Body Interact. 2009, 175, 393–418. [Google Scholar] [CrossRef] [Green Version]
- Guertin, P.A. Central Pattern Generator for Locomotion: Anatomical, Physiological, and Pathophysiological Considerations. Front. Neurol. 2013, 3, 183. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.J.; Laber, E.; Olby, N. Predictors of Response to 4-Aminopyridine in Chronic Canine Spinal Cord Injury. J. Neurotr. 2019, 36, 1428–1434. [Google Scholar] [CrossRef]
- Pearson, K. Role of sensory feedback in the control of stance duration in walking cats. Brain Res. Rev. 2008, 57, 222–227. [Google Scholar] [CrossRef]
- Rossignol, S.; Frigon, A. Recovery of Locomotion After Spinal Cord Injury: Some Facts and Mechanisms. Annu. Rev. Neurosci. 2011, 34, 413–440. [Google Scholar] [CrossRef] [Green Version]
- Edgerton, V.R.; Harkema, S. Epidural stimulation of the spinal cord in spinal cord injury: Current status and future challenges. Expert Rev. Neurother. 2011, 11, 1351–1353. [Google Scholar] [CrossRef] [Green Version]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal Rhythm Generation by Step-Induced Feedback and Transcutaneous Posterior Root Stimulation in Complete Spinal Cord–Injured Individuals. Neurorehabilit. Neur. Rep. 2016, 30, 233–243. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S. Spinal Cord Stimulation and Augmentative Control Strategies for Leg Movement after Spinal Paralysis in Humans. CNS Neurosci. Ther. 2016, 22, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Hofer, C.; Kern, H.; Danner, S.M.; Mayr, W.; Dimitrijevic, M.R.; Minassian, K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. Eng. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knikou, M.; Mummidisetty, C.K. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J. Neurophysiol. 2014, 111, 2264–2275. [Google Scholar] [CrossRef] [Green Version]
- Hofstoetter, U.S.; Knikou, M.; Guertin, P.A.; Minassian, K. Probing the Human Spinal Locomotor Circuits by Phasic Step-Induced Feedback and by Tonic Electrical and Pharmacological Neuromodulation. Curr. Pharm. Des. 2017, 23, 1805–1820. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Osman, J.; Cortes, M.; Guest, J.; Pascual-Leone, A. A Systematic Review of Experimental Strategies Aimed at Improving Motor Function after Acute and Chronic Spinal Cord Injury. J. Neurotr. 2016, 33, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Gant, K.L.; Nagle, K.G.; Cowan, R.E.; Field-Fote, E.C.; Nash, M.S.; Kressler, J.; Thomas, C.K.; Castellanos, M.; Widerström-Noga, E.; Anderson, K.D. Body System Effects of a Multi-Modal Training Program Targeting Chronic, Motor Complete Thoracic Spinal Cord Injury. J. Neurotr. 2018, 35, 411–423. [Google Scholar] [CrossRef]
- Olby, N.; De Risio, L.; Munana, K.R.; Wosar, M.A.; Skeen, T.M.; Sharp, N.J.; Keene, B.W. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Veter Res. 2001, 62, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Cardoso, A.; Cruz, R.; Gouveia, D.; Pina, R.; Moisés, M. Functional neurorehabilitation scale for dogs with thoracolombar spinal cord injury without deep pain sensation. In Proceedings of the Poster Presentation in 31st Annual Symposium of the ESVN-ECVN, Copenhagen, Denmark, 20–22 September 2018. [Google Scholar]
- Dietz, V. Degradation of neuronal function following a spinal cord injury: Mechanisms and countermeasures. Brain 2004, 127, 2221–2231. [Google Scholar] [CrossRef] [Green Version]
- Millis, D.L.; Ciuperca, I.A. Evidence for Canine Rehabilitation and Physical Therapy. Veter. Clin. North Am. Small Anim. Pr. 2015, 45, 1–27. [Google Scholar] [CrossRef]
- Maier, I.C.; Ichiyama, R.M.; Courtine, G.; Schnell, L.; Lavrov, I.; Edgerton, V.R.; Schwab, M.E. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain 2009, 132, 1426–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.K.; Sureddi, S.; Alam, M.; Zhong, H.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Unique Spatiotemporal Neuromodulation of the Lumbosacral Circuitry Shapes Locomotor Success after Spinal Cord Injury. J. Neurotr. 2016, 33, 1709–1723. [Google Scholar] [CrossRef]
- De Leon, R.D.; Hodgson, J.A.; Roy, R.R.; Edgerton, V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998, 80, 83–91. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Huang, F.; Gates, M.; White, J.; Holmberg, E.G. Tail nerve electrical stimulation induces body weight-supported stepping in rats with spinal cord injury. J. Neurosci. Methods 2010, 187, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Alluin, O.; Delivet-Mongrain, H.; Rossignol, S. Inducing hindlimb locomotor recovery in adult rat after complete thoracic spinal cord section using repeated treadmill training with perineal stimulation only. J. Neurophysiol. 2015, 114, 1931–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, V.; Colombo, G.; Jensen, L.; Baumgartner, L. Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 1995, 37, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Benito-Penalva, J.; Edwards, D.J.; Opisso, E.; Cortes, M.; Lopez-Blazquez, R.; Murillo, N.; Costa, U.; Tormos, J.M.; Vidal-Samsó, J.; Valls-Solé, J.; et al. Gait Training in Human Spinal Cord Injury Using Electromechanical Systems: Effect of Device Type and Patient Characteristics. Arch. Phys. Med. Rehab. 2012, 93, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Meyns, P.; Van De Crommert, H.W.A.A.; Rijken, H.; Van Kuppevelt, D.H.J.M.; Duysens, J. Locomotor training with body weight support in SCI: EMG improvement is more optimally expressed at a low testing speed. Spin. Cord 2014, 52, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Battistuzzo, C.R.; Callister, R.J.; Callister, R.; Galea, M. A Systematic Review of Exercise Training to Promote Locomotor Recovery in Animal Models of Spinal Cord Injury. J. Neurotr. 2012, 29, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; De Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Tillakaratne, N.J.; Duru, P.; Fujino, H.; Zhong, H.; Xiao, M.S.; Edgerton, V.R.; Roy, R.R. Identification of interneurons activated at different inclines during treadmill locomotion in adult rats. J. Neurosci. Res. 2014, 92, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Garcia-Alias, G.; Choe, J.; Gad, P.; Gerasimenko, Y.; Tillakaratne, N.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 2013, 136, 3362–3377. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.; Millis, D.L.; Flocker, J.; Macguire, L. Aquatic therapy. In Canine Rehabilitation and Physical, 2nd ed.; Therapy, D.L., Millis, D.L., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2014; pp. 526–542. [Google Scholar]
- Sims, C.; Waldron, R.; Marcellin-Little, D.J. Rehabilitation and Physical Therapy for the Neurologic Veterinary Patient. Veter. Clin. North Am. Small Anim. Pr. 2015, 45, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Engesser-Cesar, C.; Ichiyama, R.M.; Nefas, A.L.; Hill, M.A.; Edgerton, V.R.; Cotman, C.W.; Anderson, A.J. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 2007, 25, 1931–1939. [Google Scholar] [CrossRef]
- Leech, K.A.; Kinnaird, C.R.; Holleran, C.L.; Kahn, J.; Hornby, T.G. Effects of Locomotor Exercise Intensity on Gait Performance in Individuals with Incomplete Spinal Cord Injury. Phys. Ther. 2016, 96, 1919–1929. [Google Scholar] [CrossRef]
- Sherman & Olby. Nursing and Rehabilitation of the neurological patient. In Canine and Feline Neurology, 3rd ed.; Platt, S.R., Olby, N.J., Eds.; British Small Animal Veterinary Association: England, UK, 2014; pp. 394–407. [Google Scholar]
- Levine, D.; Bockstahler, B. Electrical Stimulation. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D.L., Levine, D., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; pp. 342–358. [Google Scholar]
- Hady, L.L.; Schwarz, P.D. Recovery times for dogs undergoing thoracolumbar hemilaminectomy with fenestration and physical rehabilitation: A review of 113 cases. JVMAH 2015, 7, 278–289. [Google Scholar]
- Kralj, A.; Bajd, T.; Turk, R.; Krajnik, J.; Benko, H. Gait restoration in paraplegic patients: A feasibility demonstration using multichannel surface electrode FES. J. Rehab. R D 1983, 20, 3–20. [Google Scholar]
- Holsheimer, J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spin. Cord. 1998, 36, 531–540. [Google Scholar] [CrossRef]
- Hamid, S.; Hayek, R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur. Spin. J. 2008, 17, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Field-Fote, E.; Lindley, S.D.; Sherman, A.L. Locomotor Training Approaches for Individuals with Spinal Cord Injury. J. Neurol. Phys. Ther. 2005, 29, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapadia, N.; Masani, K.; Craven, B.; Giangregorio, L.; Hitzig, S.L.; Richards, K.; Popovic, M.R. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J. Spin. Cord Med. 2014, 37, 511–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postans, N.J.; Hasler, J.P.; Granat, M.H.; Maxwell, D.J. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2004, 85, 604–610. [Google Scholar] [CrossRef]
- Nooijen, C.F.; ter Hoeve, N.; Field-Fote, E.C. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J. Neuroeng. Rehab. 2009, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hedel, H.J.; Dietz, V. Rehabilitation of locomotion after spinal cord injury. Restor. Neurol. Neurosci. 2010, 28, 123–134. [Google Scholar] [CrossRef]
- Rath, M.; Vette, A.H.; Ramasubramaniam, S.; Li, K.; Burdick, J.; Edgerton, V.R.; Gerasimenko, Y.P.; Sayenko, D.G. Trunk Stability Enabled by Noninvasive Spinal Electrical Stimulation after Spinal Cord Injury. J. Neurotr. 2018, 35, 2540–2553. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Floyd, T.C.; Gorodnichev, R.M.; Moshonkina, T.R.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 2015, 609, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayenko, D.G.; Atkinson, D.A.; Mink, A.M.; Gurley, K.M.; Edgerton, V.R.; Harkema, S.J.; Gerasimenko, Y.P. Vestibulospinal and Corticospinal Modulation of Lumbosacral Network Excitability in Human Subjects. Front. Physiol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Sayenko, D.; Gad, P.; Kozesnik, J.; Moshonkina, T.; Grishin, A.; Pukhov, A.; Moiseev, S.; Gorodnichev, R.; Selionov, V.; et al. Electrical Spinal Stimulation, and Imagining of Lower Limb Movements to Modulate Brain-Spinal Connectomes That Control Locomotor-Like Behavior. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the Human Lumbar Spinal Cord with Implanted and Surface Electrodes: A Computer Simulation Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [Green Version]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organ. 2015, 39, E176–E186. [Google Scholar] [CrossRef]
- Lim, J.-H.; Muguet-Chanoit, A.C.; Smith, D.T.; Laber, E.; Olby, N.J. Potassium Channel Antagonists 4-Aminopyridine and the T-Butyl Carbamate Derivative of 4-Aminopyridine Improve Hind Limb Function in Chronically Non-Ambulatory Dogs; A Blinded, Placebo-Controlled Trial. PLoS ONE 2014, 9, e116139. [Google Scholar] [CrossRef]
- Savin, Z.; Lejbkowicz, I.; Glass-Marmor, L.; Lavi, I.; Rosenblum, S.; Miller, A. Effect of Fampridine-PR (prolonged released 4-aminopyridine) on the manual functions of patients with Multiple Sclerosis. J. Neurol. Sci. 2016, 360, 102–109. [Google Scholar] [CrossRef]
- Tseng, K.; Li, H.; Clark, A.; Sundem, L.; Zuscik, M.; Noble, M.; Elfar, J. 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol. Med. 2016, 8, 1409–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zörner, B.; Filli, L.; Reuter, K.; Kapitza, S.; Lörincz, L.; Sutter, T.; Weller, D.; Farkas, M.; Easthope, C.S.; Czaplinski, A.; et al. Prolonged-release fampridine in multiple sclerosis: Improved ambulation effected by changes in walking pattern. Mult. Scler. J. 2016, 22, 1463–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, Â.; Ferreira, A. Neuroreabilitação funcional em lesões medulares. In Fisiatria em Pequenos Animais, 1st ed; Lopes, R.S., Diniz, R., Eds.; Editora Inteligente: São Paulo, Brazil, 2018; pp. 287–298. [Google Scholar]
- Bennaim, M.; Porato, M.; Jarleton, A.; Hamon, M.; Carroll, J.D.; Gommeren, K.; Balligand, M. Preliminary evaluation of the effects of photobiomodulation therapy and physical rehabilitation on early postoperative recovery of dogs undergoing hemilaminectomy for treatment of thoracolumbar intervertebral disk disease. Am. J. Veter. Res. 2017, 78, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.A. Sample size calculations in randomised trials: Mandatory and mystical. Lancet 2005, 365, 1348–1353. [Google Scholar] [CrossRef]
- Martins, A.; Gouveia, D.; Cardoso, A.; Viegas, I.; Ferreira, A. Intensive Neurorehabilitation of 84 paraplegic dogs affected by spinal cord injury. In Proceedings of the Poster Presentation in 60st International Spinal Cord Society Annual Scientific Meeting (ISCOS), Virtual Congress, 29 September–2 October 2021. [Google Scholar]
- Burns, A.S.; Marino, R.J.; Flanders, A.E.; Flett, H. Clinical diagnosis and prognosis following spinal cord injury. Sports Neurol. 2012, 109, 47–62. [Google Scholar] [CrossRef]
- Burns, A.S.; Marino, R.; Kalsi-Ryan, S.; Middleton, J.W.; Tetreault, L.A.; Dettori, J.R.; Mihalovich, K.E.; Fehlings, M. Type and Timing of Rehabilitation Following Acute and Subacute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 175S–194S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, Â.; Silva, C.; Gouveia, D.; Cardoso, A.; Coelho, T.; Gamboa, Óscar; Marcelino, E.; Ferreira, A. Spinal Locomotion in Cats Following Spinal Cord Injury: A Prospective Study. Animal 2021, 11, 1994. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Mankin, J.M.; Ito, D.; Boudreau, C.E.; Kerwin, S.C.; Levine, J.M.; Krasnow, M.S.; Dvm, M.N.A.; Alcott, C.J.; Granger, N. Extended durotomy to treat severe spinal cord injury after acute thoracolumbar disc herniation in dogs. Veter. Surg. 2020, 49, 884–893. [Google Scholar] [CrossRef]
- Whiteneck, G.; Gassaway, J.; Dijkers, M.; Heinemann, A.; Kreider, S.E.D. Relationship of patient characteristics and rehabilitation services to outcomes following spinal cord injury: The SCIRehab Project. J. Spinal Cord Med. 2012, 35, 484–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornhaber, R.; McLean, L.; Betihavas, V.; Cleary, M. Resilience and the rehabilitation of adult spinal cord injury survivors: A qualitative systematic review. J. Adv. Nurs. 2017, 74, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Zidan, N.; Sims, C.; Fenn, J.; Williams, K.; Griffith, E.; Early, P.J.; Mariani, C.; Munana, K.R.; Guevar, J.; Olby, N.J. A randomized, blinded, prospective clinical trial of postoperative rehabilitation in dogs after surgical decompression of acute thoracolumbar intervertebral disc herniation. J. Veter. Intern. Med. 2018, 32, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, T.; Fujita, H.; Kanazono, S.; Shibata, M.; Yoshigae, Y. Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J. Am. Veter-Med Assoc. 2012, 241, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.J.; Brown, D.C. Prognostic indicators for time to ambulation after surgical decompression in nonambulatory dogs with acute thoracolumbar disk extrusions: 112 cases. Veter. Surg. 2002, 31, 513–518. [Google Scholar] [CrossRef]
- Smith, R.R.; Brown, E.H.; Shum-Siu, A.; Whelan, A.; Burke, D.A.; Benton, R.L.; Magnuson, D. Swim Training Initiated Acutely after Spinal Cord Injury Is Ineffective and Induces Extravasation in and around the Epicenter. J. Neurotr. 2009, 26, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Detloff, M.R.; Smith, E.J.; Molina, D.Q.; Ganzer, P.D.; Houlé, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Fouad, K.; Tetzlaff, W. Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 2012, 235, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Gamboa, Ó.; Millis, D.; Ferreira, A. Nervous system modulation through electrical stimulation in companion animals. Acta Veter. Scand. 2021, 63, 1–12. [Google Scholar] [CrossRef]
- Mushahwar, V.K.; Jacobs, P.L.; Normann, R.A.; Triolo, R.J.; Kleitman, N. New functional electrical stimulation approaches to standing and walking. J. Neural Eng. 2007, 4, S181–S197. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A Comparison Between Body Weight-Supported Treadmill Training and Conventional Over-Ground Training in Dogs with Incomplete Spinal Cord Injury. Front. Veter. Sci. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Olby, N.J.; Tipold, A. Editorial: Canine Intervertebral Disc Disease: The Current State of Knowledge. Front. Veter. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N. Cansort- Canine Spinal Cord Injury Consortium (CANSORT SCI) Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Veter. Sci. 2020, 7, 610. [Google Scholar] [CrossRef]
- Olby, N.; Levine, J.; Harris, T.; Munana, K.; Skeen, T.; Sharp, N. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J. Am. Veter. Med. Assoc. 2003, 222, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, A.; Dragone, L.; Menchetti, M.; Gagliardo, T.; Pietra, M.; Cardinali, M.; Gandini, G. Acquisition of Involuntary Spinal Locomotion (Spinal Walking) in Dogs with Irreversible Thoracolumbar Spinal Cord Lesion: 81 Dogs. J. Veter. Intern. Med. 2017, 31, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jeffery, N.; Granger, N. Somatosensory and motor evoked potentials in dogs with chronic severe thoracolumbar spinal cord injury. Veter. J. 2018, 237, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.J.; Cohen, E.B.; Olby, N.J. Magnetic resonance imaging features of dogs with incomplete recovery after acute, severe spinal cord injury. Spin. Cord 2017, 56, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgerton, V.R.; Tillakaratne, N.; Bigbee, A.J.; de Leon, R.D.; Roy, R.R. Plasticity of the spinal neural circuitry after injury. Ann. Rev. Neurosci. 2004, 27, 145–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AuYong, N.; Lu, D.C. Neuromodulation of the Lumbar Spinal Locomotor Circuit. Neurosurg. Clin. N. Am. 2014, 25, 15–23. [Google Scholar] [CrossRef]
- Branco, É.; Alves, J.G.; Pinheiro, L.L.; Coutinho, L.N.; Gomes, C.R.; Galvão, G.R.; Santos, G.R.D.O.D.; Moreira, L.F.; David, M.B.; Martins, D.M. Can Paraplegia by Disruption of the Spinal Cord Tissue Be Reversed? The Signs of a New Perspective. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 303, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Granger, N.; Jeffery, N.D.; The Canine Spinal Cord Injury Consortium (CANSORT-SCI); Moore, S.A.; Olby, N.J.; Gjessing, K.M.; Davidson, R.M.; Levine, J.M.; McWhorter, H. Emerging and Adjunctive Therapies for Spinal Cord Injury Following Acute Canine Intervertebral Disc Herniation. Front. Veter. Sci. 2020, 7, 73–85. [Google Scholar] [CrossRef]
- Coates, J.R. Intervertebral Disk Disease. Veter.-Clin. N. Am. Small Anim. Pr. 2000, 30, 77–110. [Google Scholar] [CrossRef]
- Moore, S.A.; Early, P.J.; Hettlich, B. Practice patterns in the management of acute intervertebral disc herniation in dogs. J. Small Anim. Pr. 2016, 57, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Costa, R.C. Practical Guide to Canine and Feline Neurology; John Wiley & Sons: Ames, IA, USA, 2015. [Google Scholar]
- Takeoka, A.; Vollenweider, I.; Courtine, G.; Arber, S. Muscle Spindle Feedback Directs Locomotor Recovery and Circuit Reorganization after Spinal Cord Injury. Cell 2014, 159, 1626–1639. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, S.; Martinez, M.; Escalona, M.; Kundu, A.; Delivet-Mongrain, H.; Alluin, O.; Gossard, J.-P. The “beneficial” effects of locomotor training after various types of spinal lesions in cats and rats. Progr. Brain Res. 2015, 218, 173–198. [Google Scholar] [CrossRef]
- Jeong, I.; Piao, Z.; Rahman, M.; Kim, S.; Kim, N. Canine thoracolumbar intervertebral disk herniation and rehabilitation therapy after surgical decompression: A retrospective study. J. Adv. Veter-Anim. Res. 2019, 6, 394–402. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, W.; Liu, Y.; Sheng, C.; So, K.-F.; Zhou, L.; Zhu, H. The effects and potential mechanisms of locomotor training on improvements of functional recovery after spinal cord injury. Int. Rev. Neurobiol. 2019, 147, 199–217. [Google Scholar] [CrossRef]
- Aravind, N.; Harvey, L.A.; Glinsky, J.V. Physiotherapy interventions for increasing muscle strength in people with spinal cord injuries: A systematic review. Spin. Cord 2019, 57, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, I.R. The extensive myelopathy of intervertebral disc protrusions in dogs (‘the ascending syndrome’). J. Small Anim. Pr. 1972, 13, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Barker, A.K.; Hu, H.Z.; Alcott, C.J.; Kraus, K.H.; Scanlin, E.M.; Granger, N.; Levine, J.M. Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J. Am. Veter. Med. Assoc. 2016, 248, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Olby, N.; Muguet-Chanoit, A.; Lim, J.; Davidian, M.; Mariani, C.; Freeman, A.; Platt, S.; Humphrey, J.; Kent, M.; Giovanella, C.; et al. A Placebo-Controlled, Prospective, Randomized Clinical Trial of Polyethylene Glycol and Methylprednisolone Sodium Succinate in Dogs with Intervertebral Disk Herniation. J. Veter. Intern. Med. 2016, 30, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Olby, N.J.; Lim, J.; Wagner, N.; Zidan, N.; Early, P.J.; Mariani, C.; Muñana, K.R.; Laber, E. Time course and prognostic value of serum GFAP, pNFH, and S100β concentrations in dogs with complete spinal cord injury because of intervertebral disc extrusion. J. Veter. Intern. Med. 2019, 33, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blight, A.R.; Toombs, J.P.; Bauer, M.S.; Widmer, W.R. The Effects of 4-Aminopyridine on Neurological Deficits in Chronic Cases of Traumatic Spinal Cord Injury in Dogs: A Phase I Clinical Trial. J. Neurotr. 1991, 8, 103–119. [Google Scholar] [CrossRef]
- Blight, A.R. Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Res. Bull. 1989, 22, 47–52. [Google Scholar] [CrossRef]
- Smith, K.J.; Felts, P.A.; John, G.R. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain 2000, 123, 171–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olby, N.J.; Da Costa, R.C.; Levine, J.M.; Stein, V.M. The Canine Spinal Cord Injury Consortium (CANSORT SCI) Prognostic Factors in Canine Acute Intervertebral Disc Disease. Front. Veter. Sci. 2020, 7, 144–157. [Google Scholar] [CrossRef]
| DPP+ (n = 230) | SG (n = 168) | CG (n = 62) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Median | Mean (SD) | 95% CI | Median | Mean (SD) | 95% CI | Median | |
| Age (years) | 4.07 (1.574) | 3.86–4.27 | 4.00 | 4.07 (1.642) | 3.86–4.27 | 4.00 | 3.76 (1.339) | 3.42–4.10 | 4.00 |
| Bodyweight (kg) | 8.79 (4.032) | 8.27–9.32 | 8.00 | 8.79 (4.484) | 8.27–9.32 | 8.00 | 7.79 (2.159) | 7.24–8.34 | 7.00 |
| DPP− (n = 137) | SG (n = 94) | CG (n = 43) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Median | Mean (SD) | 95% CI | Median | Mean (SD) | 95% CI | Median | |
| Age (years) | 4.03 (1.576) | 3.76–4.30 | 4.00 | 3.90 (1.566) | 3.58–4.23 | 4.00 | 4.30 (1.582) | 3.82–4.79 | 4.00 |
| Body weight (kg) | 8.14 (3.218) | 7.59–8.68 | 8.00 | 8.51 (3.466) | 7.80–9.22 | 8.00 | 7.33 (2.437) | 6.58–8.08 | 7.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Coelho, T.; Silva, C.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A Controlled Clinical Study of Intensive Neurorehabilitation in Post-Surgical Dogs with Severe Acute Intervertebral Disc Extrusion. Animals 2021, 11, 3034. https://doi.org/10.3390/ani11113034
Martins Â, Gouveia D, Cardoso A, Carvalho C, Coelho T, Silva C, Viegas I, Gamboa Ó, Ferreira A. A Controlled Clinical Study of Intensive Neurorehabilitation in Post-Surgical Dogs with Severe Acute Intervertebral Disc Extrusion. Animals. 2021; 11(11):3034. https://doi.org/10.3390/ani11113034
Chicago/Turabian StyleMartins, Ângela, Débora Gouveia, Ana Cardoso, Carla Carvalho, Tiago Coelho, Cátia Silva, Inês Viegas, Óscar Gamboa, and António Ferreira. 2021. "A Controlled Clinical Study of Intensive Neurorehabilitation in Post-Surgical Dogs with Severe Acute Intervertebral Disc Extrusion" Animals 11, no. 11: 3034. https://doi.org/10.3390/ani11113034
APA StyleMartins, Â., Gouveia, D., Cardoso, A., Carvalho, C., Coelho, T., Silva, C., Viegas, I., Gamboa, Ó., & Ferreira, A. (2021). A Controlled Clinical Study of Intensive Neurorehabilitation in Post-Surgical Dogs with Severe Acute Intervertebral Disc Extrusion. Animals, 11(11), 3034. https://doi.org/10.3390/ani11113034







