Use of Infrared Thermography during Ejaculation Process and Its Link with Semen Quality and Freezability in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
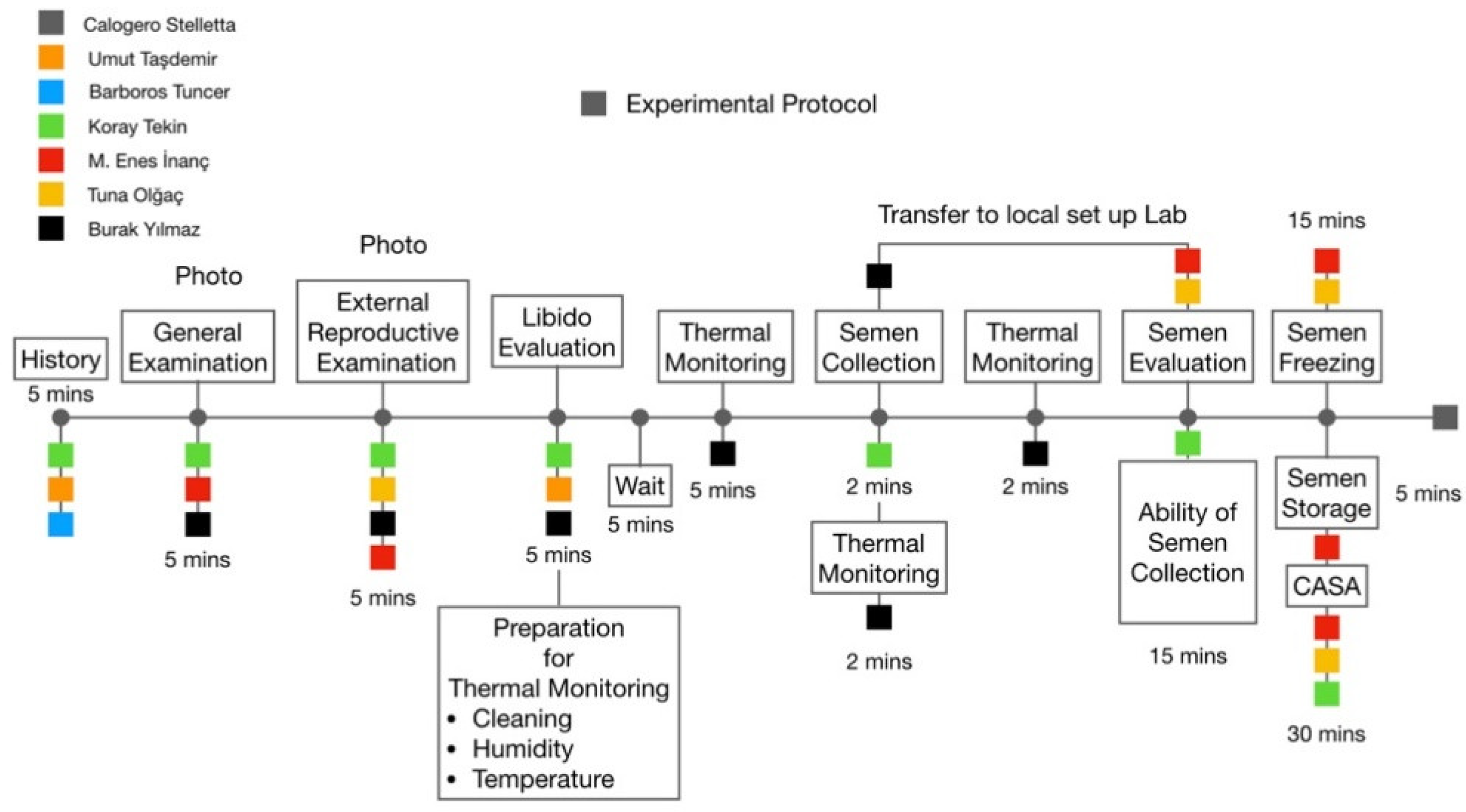
2. Materials and Methods
2.1. Ethics
2.2. Location, Environmental Conditions, and Animals
2.3. External Reproductive Examination
2.4. Semen Collection
2.5. Semen Assessment and Freezing
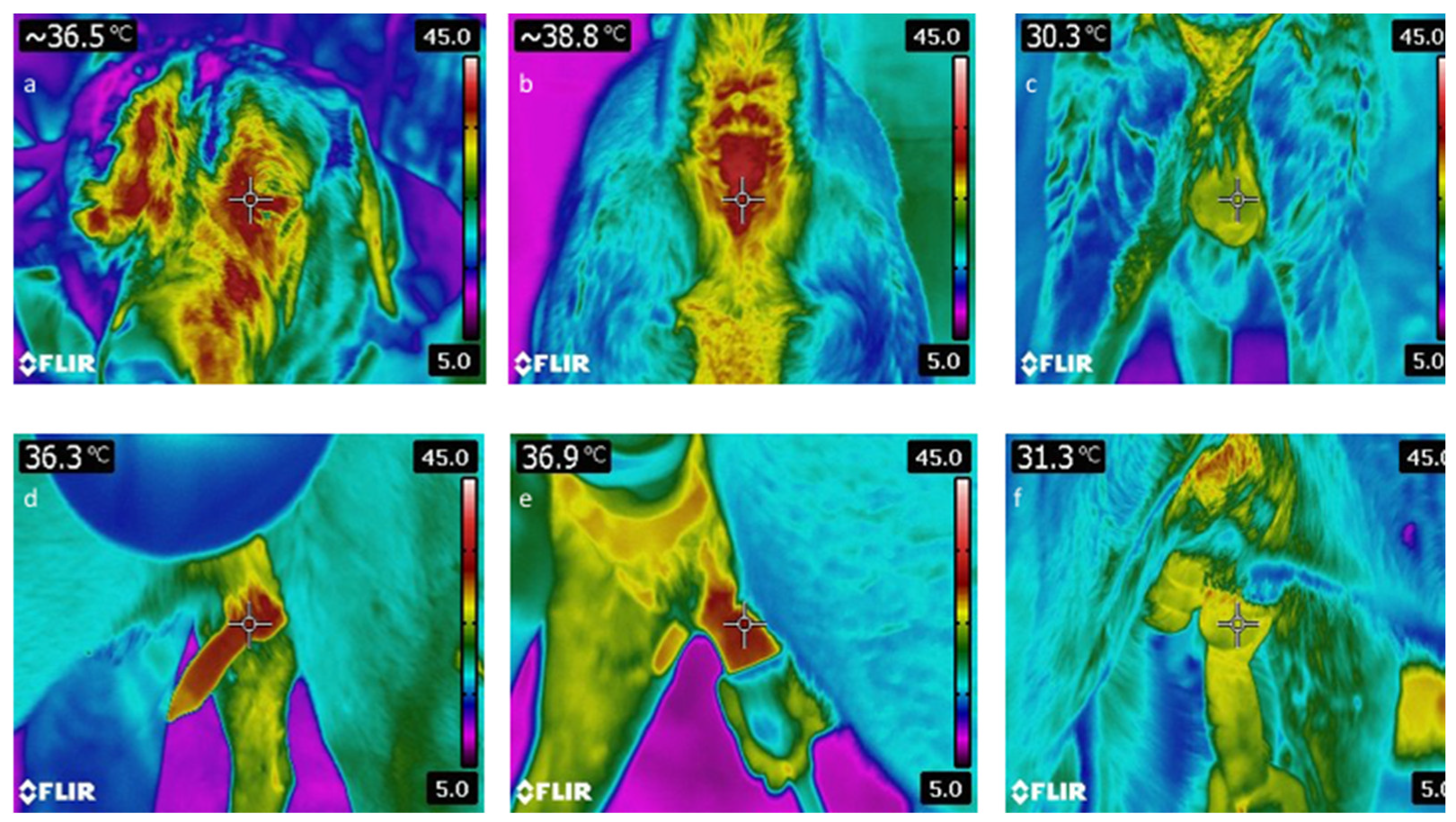
2.6. Thermographic Monitoring
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Redaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Luzi, F. Use of thermography techniques in equines: Principles and applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]
- Martins, R.F.S.; do Prado Paim, T.; de Abreu Cardoso, C.; Dallago, B.S.L.; De Melo, C.B.; Louvandini, H.; McManus, C. Mastitis detection in sheep by infrared thermography. Res. Vet. Sci. 2013, 94, 722–724. [Google Scholar] [CrossRef]
- Waddell, R.E.; Marino, D.J.; Loughin, C.A.; Tumulty, J.W.; Dewey, C.W.; Sackman, J. Medical infrared thermal imaging of cats with hyperthyroidism. Am. J. Vet. Res. 2015, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Loughin, C.A.; Marino, D.J. Evaluation of thermographic imaging of the limbs of healthy dogs. Am. J. Vet. Res. 2007, 68, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Infernuso, T.; Loughin, C.A.; Marino, D.J.; Umbaugh, S.E.; Solt, P.S. thermal imaging of normal and cranial cruciate ligament-deficient stifles in dogs. Vet. Surg. 2010, 39, 410–417. [Google Scholar] [CrossRef]
- Garcia, E.F.V.; Loughin, C.A.; Marino, D.J.; Sackman, J.; Umbaugh, S.E.; Fu, J.; Subedi, S.; Lesser, M.L.; Akerman, M.; Schossler, J.E.W. Medical infrared imaging and orthostatic analysis to determine lameness in the pelvic limbs of dogs. Open Vet. J. 2017, 7, 342–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavelski, M.; Silva, D.M.; Leite, N.C.; Junior, D.A.; De Sousa, R.S.; Guérios, S.D.; Dornbusch, P.T. Infrared thermography in dogs with mammary tumors and healthy dogs. J. Vet. Intern. Med. 2015, 29, 1578–1583. [Google Scholar] [CrossRef]
- Bowers, S.; Gandy, S.; Anderson, B.; Ryan, P.; Willard, S. Assessment of pregnancy in the late-gestation mare using digital infrared thermography. Theriogenology 2009, 72, 372–377. [Google Scholar] [CrossRef]
- Durrant, B.S.; Ravida, N.; Spady, T.; Cheng, A. New technologies for the study of carnivore reproduction. Theriogenology 2006, 66, 1729–1736. [Google Scholar] [CrossRef]
- Stelletta, C.; Tekin, K.; Tirpan, M.B.; Alemdar, H.; Cil, B.; Oztutar Stelletta, F.; Olgac, K.T.; Inanc, M.E.; Daskin, A. Vulvar thermal pattern following synchronization of estrus is linked to fertility after timed artificial insemination in goat. Theriogenology 2017, 103, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tekin, K.; Cil, B.; Alemdar, H.; Olgac, K.T.; Tirpan, M.B.; Daskin, A.; Stelletta, C. Semen collection by trans-rectal digital stimulation and insemination campaign in goat. Andrologia 2020, 52, e13458. [Google Scholar] [CrossRef]
- Olğaç, K.T.; Akçay, E.; Çil, B.; Uçar, B.M.; Daşkın, A. The use of infrared thermography to detect the stages of estrus cycle and ovulation time in anatolian shepherd dogs. J. Anim. Sci. Technol. 2017, 59, 1–6. [Google Scholar] [CrossRef]
- Stelletta, C.; Vencato, J.; Fiore, E.; Gianesella, M. Infrared thermography in reproduction. Thermogr. Curr. Status Adv. Livest. Anim. Vet. Med. 2013, 113–125. Available online: https://www.researchgate.net/profile/Calogero-Stelletta/publication/235978392_INFRARED_THERMOGRAPHY_IN_REPRODUCTION/links/0046351af1a684f45e000000/INFRARED-THERMOGRAPHY-IN-REPRODUCTION.pdf (accessed on 15 October 2021).
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Menegassi, S.R.O.; Barcellos, J.O.J.; Dias, E.A.; Koetz, C.; Pereira, G.R.; Peripolli, V.; McManus, C.; Canozzi, M.E.A.; Lopes, F.G. Scrotal infrared digital thermography as a predictor of seasonal effects on sperm traits in Braford bulls. Int. J. Biometeorol. 2015, 59, 357–364. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Rizzoto, G. Thermoregulation of the Testes, Bovine Reproduction, 2nd ed.; Richard, M.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; Volume 4, pp. 40–46. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Cook, R.B.; Pierson, R.A.; Coulter, G.H. Relationships among scrotal and testicular characteristics, sperm production, and seminal quality in 129 beef bulls. Can. J. Vet. Res. 2001, 65, 111–115. [Google Scholar] [PubMed]
- Menegassi, S.R.O.; Pereira, G.R.; Dias, E.A.; Rocha, M.K.; Carvalho, H.R.; Koetz, C., Jr.; Barcellos, J.O.J. Infrared thermography as a noninvasive method to assess scrotal insulation on sperm production in beef bulls. Andrologia 2018, 50, e12904. [Google Scholar] [CrossRef]
- Capraro, G.A.; Coughlin, B.F.; Mader, T.J.; Smithline, H.A. Testicular cooling associated with testicular torsion and its detection by infrared thermography: An experimental study in sheep. J. Urol. 2008, 180, 2688–2693. [Google Scholar] [CrossRef]
- Neto, C.R.; Monteiro, G.A.; Delfiol, D.J.Z.; Farras, M.C.; Dell’aqua, J.A.Ô.; Papa, F.O.; Alvarenga, M.A. The relationships between scrotal surface temperature, age and sperm quality in stallions. Livest. Sci. 2013, 157, 358–363. [Google Scholar] [CrossRef]
- Zaid, U.B.; Zhang, X.; Lue, T.F. Physiology of penile erection. In Male Sexual Dysfunction: A Clinical Guide; Minhas, S., Mulhall, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 3, pp. 14–21. [Google Scholar]
- Prieto, D. Physiological regulation of penile arteries and veins. Int. J. Impot. Res. 2008, 20, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, U.; García-Sacristán, A.; Prieto, D. Penile arteries and erection. J. Vasc. Res. 2002, 39, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Larry, B.M.; Myles, L.M.; Al-Dahir, M.A. Cremasteric Reflex; StatPearls-NCBI Bookshelf: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shafik, A.; Shafik, A.A.; Shafik, I.A.; El Sibai, O. Physiological considerations of the morphologic changes of the testicles during erection and ejaculation: A canine study. Urol. Int. 2007, 79, 262–266. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Hori, T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom. Med. 2001, 63, 476–486. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic ReticulumStress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef]
- Vianna, D.M.L.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef]
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014, 20, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; de Boer, S.F.; Coppens, C.M.; Buwalda, B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front. Neuroendocrinol. 2010, 31, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Schaefer, A.L.; Haley, D.B.; Colyn, J.; Cook, N.J.; Stafford, K.J.; Webster, J.R. Infrared thermography as a non-invasive method for detecting fear-related responses of cattle to handling procedures. Anim. Welf. 2008, 17, 387–393. [Google Scholar]
- Valera, M.; Bartolomé, E.; Sánchez, M.J.; Molina, A.; Cook, N.; Schaefer, A. Changes in eye temperature and stress assessment in horses during show jumping competitions. J. Equine Vet. Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Gabry, K.E.; Chrousos, G.; Gold, P.W. The hypothalamic-pituitary-adrenal (HPA) axis: A major mediator of the adaptive responses to stress. NeuroImmune Biol. 2003, 3, 379–414. [Google Scholar] [CrossRef]
- Romero, L.M. Fight or flight responses. In Encyclopedia of Animal Behavior, 2nd ed.; Michael, D.B., Moore, J., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 547–552. [Google Scholar]
- Johnston, S.D.; Root Kustritz, M.V.; Olson, P.N.S. Semen collection, evaluation, and preservation. In Canine and Feline Theriogenology, 1st ed.; Kersey, R., Le Melledo, D., Eds.; W. B. Saunders Company: Philadelphia, PA, USA, 2001; pp. 287–306. [Google Scholar]
- Sellier, N.; Guettier, E.; Staub, C. A review of methods to measure animal body temperature in precision farming. Am. J. Agric. Sci. Technol. 2014, 2, 74–99. [Google Scholar] [CrossRef]
- McCafferty, D.J. The value of infrared thermography for research on mammals: Previous applications and future directions. Mamm. Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Hilsberg-Merz, S. Infrared thermography in zoo and wild animals. In Zoo and Wild Animal Medicine; Publisher: Location, UK, 2008; pp. 20–32. ISBN 9781416040477. [Google Scholar]
- Gouletsou, P.G.; Galatos, A.D.; Leontides, L.S. Comparison between ultrasonographic and caliper measurements of testicular volume in the dog. Anim. Reprod. Sci. 2008, 108, 1–12. [Google Scholar] [CrossRef]
- Inanc, M.E.; Tekin, K.; Olgac, K.T.; Yilmaz, B.; Cil, B.; Tasdemir, U.; Tuncer, P.B.; Buyukleblebici, S.; Durmaz, E.; Uysal, O. Effect of cholesterol loaded cyclodextrin on semen cryopreservation of Aksaray Malakli shepherd dogs of different ages. Anim. Reprod. Sci. 2018, 193, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kastelic, J.P.; Cook, R.B.; Coulter, G.H.; Saacke, R.G. Insulating the scrotal neck affects semen quality and scrotal/testicular temperatures in the bull. Theriogenology 1996, 45, 935–942. [Google Scholar] [CrossRef]
- Vencato, J.; Cestaro, L.; Vazzana, I.; Carrer, G.; Carlo, E.; Dara, S.; Stelletta, C. Integrated evaluation of scrotal temperature and testosteronemia after GnRH administration in young bulls with low semen production. Reprod. Domest. Anim. 2014, 49, 481–486. [Google Scholar] [CrossRef]
- Brito, L.F.C.; Silva, A.E.D.F.; Barbosa, R.T.; Kastelic, J.P. Testicular thermoregulation in Bos indicus, crossbred and Bos taurus bulls: Relationship with scrotal, testicular vascular cone and testicular morphology, and effects on semen quality and sperm production. Theriogenology 2004, 61, 511–528. [Google Scholar] [CrossRef]
- Rahman, M.B.; Vandaele, L.; Rijsselaere, T.; Maes, D.; Hoogewijs, M.; Frijters, A.; Noordman, J.; Granados, A.; Dernelle, E.; Shamsuddin, M.; et al. Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in Holstein-Friesian and Belgian Blue bulls. Theriogenology 2011, 76, 1246–1257. [Google Scholar] [CrossRef]
- Henning, H.; Masal, C.; Herr, A.; Wolf, K.; Urhausen, C.; Beineke, A.; Beyerbach, M.; Kramer, S.; Günzel-Apel, A.R. Effect of short-term scrotal hyperthermia on spermatological parameters, testicular blood flow and gonadal tissue in dogs. Reprod. Domest. Anim. 2014, 49, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.M.; Romano, J.E.; Troedsson, M.H.T.; Crabo, B.G. Effect of scrotal insulation on clusterin-positive cells in ram semen and their relationship to semen quality. J. Androl. 2001, 22, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Arman, C.; Quintana Casares, P.I.; Sanchez-Partida, L.G.; Setchell, B.P. Ram sperm motility after intermittent scrotal insulation evaluated by manual and computer-assisted methods. Asian J. Androl. 2006, 8, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, A.; Gauly, M.; Erhardt, G.; Bergmann, M. Changes in testicular histology and sperm quality in llamas (Lama glama), following exposure to high ambient temperature. Theriogenology 2007, 67, 1316–1323. [Google Scholar] [CrossRef]
- Garcia-Oliveros, L.N.; de Arruda, R.P.; Batissaco, L.; Gonzaga, V.H.G.; Nogueira, V.J.M.; Florez-Rodriguez, S.A.; Celeghini, E.C.C. Heat stress effects on bovine sperm cells: A chronological approach to early findings. Int. J. Biometeorol. 2020, 64, 1367–1378. [Google Scholar] [CrossRef]
- Fernandes, C.E.; Dode, M.A.N.; Pereira, D.; Silva, A.E.D.F. Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability. Theriogenology 2008, 70, 1560–1568. [Google Scholar] [CrossRef]
- Abdelhamid, M.H.M.; Walschaerts, M.; Ahmad, G.; Mieusset, R.; Bujan, L.; Hamdi, S. Mild experimental increase in testis and epididymis temperature in men: Effects on sperm morphology according to spermatogenesis stages. Transl. Androl. Urol. 2019, 8, 651. [Google Scholar] [CrossRef]
- Paul, C.; Teng, S.; Saunders, P.T.K. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod. 2009, 80, 913–919. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Miola, P.; Caretta, N.; Pizzol, D.; Menegazzo, M.; Bertoldo, A.; Foresta, C. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum. Reprod. 2015, 30, 1006–1013. [Google Scholar] [CrossRef]
- Janevic, T.; Kahn, L.G.; Landsbergis, P.; Cirillo, P.M.; Cohn, B.A.; Liu, X.; Factor-Litvak, P. Effects of work and life stress on semen quality. Fertil. Steril. 2014, 102, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.H. The effect of stress on semen reduction in the marmoset monkey (Callithrix jacchus). Hum. Reprod. 1996, 11, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Beaver, B. Canine Behavior: A Guide for Veterinarians; WB Saunders: Philadelphia, PA, USA, 1999; pp. 150–151. [Google Scholar]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Diverio, S.; Barbato, O.; Cavallina, R.; Guelfi, G.; Iaboni, M.; Zasso, R.; Di Mari, W.; Santoro, M.M.; Knowles, T.G. A simulated avalanche search and rescue mission induces temporary physiological and behavioural changes in military dogs. Physiol. Behav. 2016, 163, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rovira, S.; Munoz, A.; Benito, M. Effect of exercise on physiological, blood and endocrine parameters in search and rescue-trained dogs. Vet. Med. 2008, 53, 333–346. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Cook, N.; Schaefer, A.; Warren, L.; Burwash, L.; Anderson, M.; Baron, V. Adrenocortical and metabolic responses to ACTH injection in horses An assessment by salivary cortisol and infrared thermography of the eye. Can. J. Anim. Sci. 2001, 81, 621. [Google Scholar]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Denham, H.D.C.; Bradshaw, J.W.S.; Rooney, N.J. Repetitive behaviour in kennelled domestic dog: Stereotypical or not? Physiol. Behav. 2014, 128, 288–294. [Google Scholar] [CrossRef]
- Part, C.E.; Kiddie, J.L.; Hayes, W.A.; Mills, D.S.; Neville, R.F.; Morton, D.B.; Collins, L.M. Physiological, physical and behavioural changes in dogs (Canis familiaris) when kennelled: Testing the validity of stress parameters. Physiol. Behav. 2014, 133, 260–271. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Behavioural and hormonal indicators of enduring environmental stress in dogs. Anim. Welf. 2000, 9, 49–62. [Google Scholar]
- Pasing, S.; Von Lewinski, M.; Wulf, M.; Erber, R.; Aurich, C. Influence of semen collection on salivary cortisol release, heart rate, and heart rate variability in stallions. Theriogenology 2013, 80, 256–261. [Google Scholar] [CrossRef]
| Libido Sexualis | Ability of Semen Collection | ||
|---|---|---|---|
| Secretion of 1st fraction immediately | 5 | Non-responsive (<5 min) | 0 |
| Secretion of 1st fraction 0–45 s. | 4 | Responsive (>5 min) | 1 |
| Secretion of 1st fraction 45 s–1.5 min. | 3 | Hypersensitive (>1 min) | 2 |
| Secretion of 1st fraction 1.5–5 min. | 2 | ||
| Secretion of 1st fraction >5 min. | 1 | ||
| Parameters | n | Mean | Standard Deviation | Minimum | Maximum | Median |
|---|---|---|---|---|---|---|
| Libido Score (1–5) | 46 | 4.77 | 0.57 | 2.00 | 5.00 | 5.00 |
| Ability of Semen Collection (0–2) | 46 | 1.60 | 0.73 | 0.00 | 2.00 | 2.00 |
| Semen Volume (mL) | 42 | 11.74 | 6.04 | 4.60 | 26.50 | 9.80 |
| 1st Fraction Volume (mL) | 42 | 2.84 | 1.86 | 0.50 | 10.00 | 2.30 |
| 2nd Fraction Volume (mL) | 42 | 2.45 | 1.25 | 0.80 | 6.00 | 2.00 |
| 3rd Fraction Volume (mL) | 42 | 6.45 | 4.88 | 0.50 | 20.00 | 5.00 |
| 1st Fraction pH | 42 | 5.97 | 0.24 | 5.50 | 6.50 | 5.90 |
| 2nd Fraction pH | 42 | 5.80 | 0.22 | 5.30 | 6.30 | 5.80 |
| 3rd Fraction pH | 42 | 5.84 | 0.23 | 5.40 | 6.80 | 5.80 |
| Total Ejaculation Time (s) | 42 | 823.72 | 109.24 | 703.00 | 1095.00 | 775.00 |
| Dead Spermatozoa (%) | 42 | 17.70 | 7.88 | 7.19 | 34.44 | 15.35 |
| Acrosome Abnormality (%) | 42 | 0.49 | 0.75 | 0.00 | 2.77 | 0.00 |
| Head Abnormality (%) | 42 | 2.39 | 4.84 | 0.00 | 31.27 | 1.60 |
| Midpiece Abnormality (%) | 42 | 2.35 | 3.64 | 0.00 | 17.94 | 1.00 |
| Tail Abnormality (%) | 42 | 7.67 | 7.92 | 0.00 | 32.98 | 5.04 |
| Cytoplasmic Droplet (%) | 42 | 2.70 | 4.38 | 0.00 | 16.60 | 1.03 |
| Total Abnormal Sperm (%) | 42 | 15.62 | 11.88 | 1.04 | 45.09 | 11.65 |
| Fresh Motility (%) | 42 | 86.05 | 4.39 | 75.00 | 90.00 | 88.00 |
| Post-thaw Motility (%) | 42 | 33.86 | 14.78 | 11.20 | 67.51 | 33.11 |
| Delta Motility (%) | 42 | −52.34 | 15.63 | −12.49 | −78.03 | −53.65 |
| Eye Before EJA (°C) | 42 | 34.42 | 2.86 | 27.30 | 40.20 | 34.40 |
| Perianal Before EJA (°C) | 42 | 35.30 | 2.55 | 28.10 | 38.90 | 35.90 |
| Scrotum Before EJA (°C) | 42 | 31.13 | 4.38 | 29.11 | 34.80 | 31.05 |
| Scrotum After EJA (°C) | 42 | 32.62 | 5.58 | 28.30 | 35.50 | 31.25 |
| Bulbus Before EJA (°C) | 42 | 33.60 | 2.68 | 25.90 | 36.70 | 34.20 |
| Penis Before EJA (°C) | 42 | 31.91 | 3.45 | 24.60 | 36.10 | 33.10 |
| Penis During EJA (°C) | 42 | 29.16 | 3.72 | 18.40 | 34.70 | 29.81 |
| Statistics * | df | F (df1, df2) | p | ||
|---|---|---|---|---|---|
| Model | 3.205 | 5 | 35.44 (35, 170) | <0.001 | |
| Age | 0.987 | 1 | 345.12 (7, 30) | <0.001 | |
| Body Weight | 0.958 | 1 | 98.03 (7, 30) | <0.001 | |
| Total Testicular Volume | 0.923 | 1 | 51.09 (7, 30) | <0.001 | |
| Total Ejaculation Time | 0.905 | 1 | 40.83 (7, 30) | <0.001 | |
| Concentration | 0.954 | 1 | 90.53 (7, 30) | <0.001 | |
| Residual | 36 | ||||
| Total | 41 | ||||
| Dependent Variables | Independent Variables | Category | Margin | Std. Error | dy/dx * | Std. Error | p |
|---|---|---|---|---|---|---|---|
| ∆ Motility | Age | ≤4 | −44.64 | 2.65 | −7.36 | 5.48 | 0.325 |
| >4 | −52.00 | 6.66 | |||||
| BW | ≤75 | −54.27 | 4.39 | 17.66 | 7.81 | 0.024 | |
| >75 | −36.61 | 3.71 | |||||
| TTV | ≤600 | −35.98 | 4.65 | −18.05 | 7.28 | 0.014 | |
| >600 | −54.03 | 4.06 | |||||
| TET | ≤800 | −29.83 | 6.02 | −21.05 | 7.28 | 0.004 | |
| >800 | −50.88 | 2.92 | |||||
| CON | ≤300 | −47.35 | 5.37 | 2.07 | 5.76 | 0.759 | |
| >300 | −45.27 | 3.04 | |||||
| Scrotum Before EJA | Age | ≤4 | 29.18 | 0.36 | −2.2 | 1.01 | 0.029 |
| >4 | 26.98 | 0.89 | |||||
| BW | ≤75 | 33.26 | 0.59 | −9.34 | 1.05 | 0.001 | |
| >75 | 23.92 | 0.63 | |||||
| TTV | ≤600 | 26.60 | 0.62 | 4.03 | 0.98 | 0.001 | |
| >600 | 30.64 | 0.55 | |||||
| TET | ≤800 | 26.57 | 0.81 | 2.93 | 0.98 | 0.003 | |
| >800 | 29.51 | 0.39 | |||||
| CON | ≤300 | 29.71 | 0.72 | −1.26 | 0.91 | 0.166 | |
| >300 | 28.45 | 0.41 | |||||
| Penis During EJA | Age | ≤4 | 30.17 | 0.20 | −0.25 | 0.57 | 0.671 |
| >4 | 29.92 | 0.51 | |||||
| BW | ≤75 | 34.76 | 0.34 | −9.70 | 0.60 | 0.001 | |
| >75 | 25.04 | 0.36 | |||||
| TTV | ≤600 | 26.45 | 0.36 | 6.72 | 0.56 | 0.001 | |
| >600 | 33.18 | 0.31 | |||||
| TET | ≤800 | 26.47 | 0.46 | 4.81 | 0.56 | 0.001 | |
| >800 | 31.27 | 0.22 | |||||
| CON | ≤300 | 27.87 | 0.41 | 3.16 | 0.52 | 0.001 | |
| >300 | 31.03 | 0.23 | |||||
| Eye Before EJA | Age | ≤4 | 36.38 | 0.13 | −2.47 | 0.35 | 0.001 |
| >4 | 33.91 | 0.32 | |||||
| BW | ≤75 | 34.42 | 0.21 | 3.25 | 0.37 | 0.001 | |
| >75 | 37.67 | 0.22 | |||||
| TTV | ≤600 | 37.66 | 0.22 | −3.09 | 0.35 | 0.001 | |
| >600 | 34.57 | 0.19 | |||||
| TET | ≤800 | 38.21 | 0.29 | −2.93 | 0.35 | 0.001 | |
| >800 | 35.27 | 0.13 | |||||
| CON | ≤300 | 36.70 | 0.26 | −1.02 | 0.32 | 0.002 | |
| >300 | 35.68 | 0.14 | |||||
| Perianal Before EJA | Age | ≤4 | 36.79 | 0.24 | 0.03 | 0.69 | 0.967 |
| >4 | 36.82 | 0.62 | |||||
| BW | ≤75 | 36.72 | 0.41 | 0.17 | 0.72 | 0.811 | |
| >75 | 36.89 | 0.43 | |||||
| TTV | ≤600 | 36.83 | 0.43 | −0.04 | 0.68 | 0.954 | |
| >600 | 36.79 | 0.37 | |||||
| TET | ≤800 | 36.08 | 0.56 | 0.95 | 0.67 | 0.158 | |
| >800 | 37.03 | 0.27 | |||||
| CON | ≤300 | 37.44 | 0.49 | −0.89 | 0.63 | 0.154 | |
| >300 | 36.55 | 0.28 | |||||
| Scrotum After EJA | Age | ≤4 | 29.69 | 0.47 | −2.27 | 1.33 | 0.088 |
| >4 | 27.42 | 1.18 | |||||
| BW | ≤75 | 30.09 | 0.78 | −1.64 | 1.39 | 0.238 | |
| >75 | 28.45 | 0.84 | |||||
| TTV | ≤600 | 30.28 | 0.83 | −1.75 | 1.31 | 0.180 | |
| >600 | 28.52 | 0.72 | |||||
| TET | ≤800 | 30.25 | 1.07 | −1.24 | 1.29 | 0.340 | |
| >800 | 29.02 | 0.52 | |||||
| CON | ≤300 | 32.01 | 0.96 | −3.77 | 1.29 | 0.002 | |
| >300 | 29.69 | 0.47 |
| (n = 42) | Eye before EJA | Perianal before EJA | Scrotum before EJA | Scrotum after EJA | Bulbus Before EJA | Penis before EJA | Penis during EJA |
|---|---|---|---|---|---|---|---|
| Libido scoring | 0.336 * | ||||||
| The ability of semen collection | −0.476 * | −0.508 ** | |||||
| Semen volume | 0.306 * | ||||||
| Dead Spermatozoa | −0.386 * | ||||||
| Acrosome abnormality | −0.440 * | ||||||
| Head abnormality | −0.391 * | −0.576 ** | −0.349 * | ||||
| Total Head Abnormality | −0.714 ** | −0.422 * | −0.476 ** | ||||
| Midpiece abnormality | −0.361 * | −0.537 ** | |||||
| Total Sperm Defects | −0.516 * | ||||||
| Fresh Motility | 0.416 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, K.; İnanç, M.E.; Özen, D.; Cil, B.; Olğaç, K.T.; Yılmaz, B.; Taşdemir, U.; Tuncer, P.B.; Büyükleblebici, S.; Daşkın, A.; et al. Use of Infrared Thermography during Ejaculation Process and Its Link with Semen Quality and Freezability in Dogs. Animals 2021, 11, 3023. https://doi.org/10.3390/ani11113023
Tekin K, İnanç ME, Özen D, Cil B, Olğaç KT, Yılmaz B, Taşdemir U, Tuncer PB, Büyükleblebici S, Daşkın A, et al. Use of Infrared Thermography during Ejaculation Process and Its Link with Semen Quality and Freezability in Dogs. Animals. 2021; 11(11):3023. https://doi.org/10.3390/ani11113023
Chicago/Turabian StyleTekin, Koray, Muhammed Enes İnanç, Doğukan Özen, Beste Cil, Kemal Tuna Olğaç, Burak Yılmaz, Umut Taşdemir, Pürhan Barbaros Tuncer, Serhat Büyükleblebici, Ali Daşkın, and et al. 2021. "Use of Infrared Thermography during Ejaculation Process and Its Link with Semen Quality and Freezability in Dogs" Animals 11, no. 11: 3023. https://doi.org/10.3390/ani11113023
APA StyleTekin, K., İnanç, M. E., Özen, D., Cil, B., Olğaç, K. T., Yılmaz, B., Taşdemir, U., Tuncer, P. B., Büyükleblebici, S., Daşkın, A., Uysal, O., & Stelletta, C. (2021). Use of Infrared Thermography during Ejaculation Process and Its Link with Semen Quality and Freezability in Dogs. Animals, 11(11), 3023. https://doi.org/10.3390/ani11113023





