Visual Signaling in the Semi-Fossorial Lizard Pholidobolus montium (Gymnophthalmidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Housing
2.2. Behavioral Observations
2.3. Video Analysis
3. Results
3.1. Ethogram
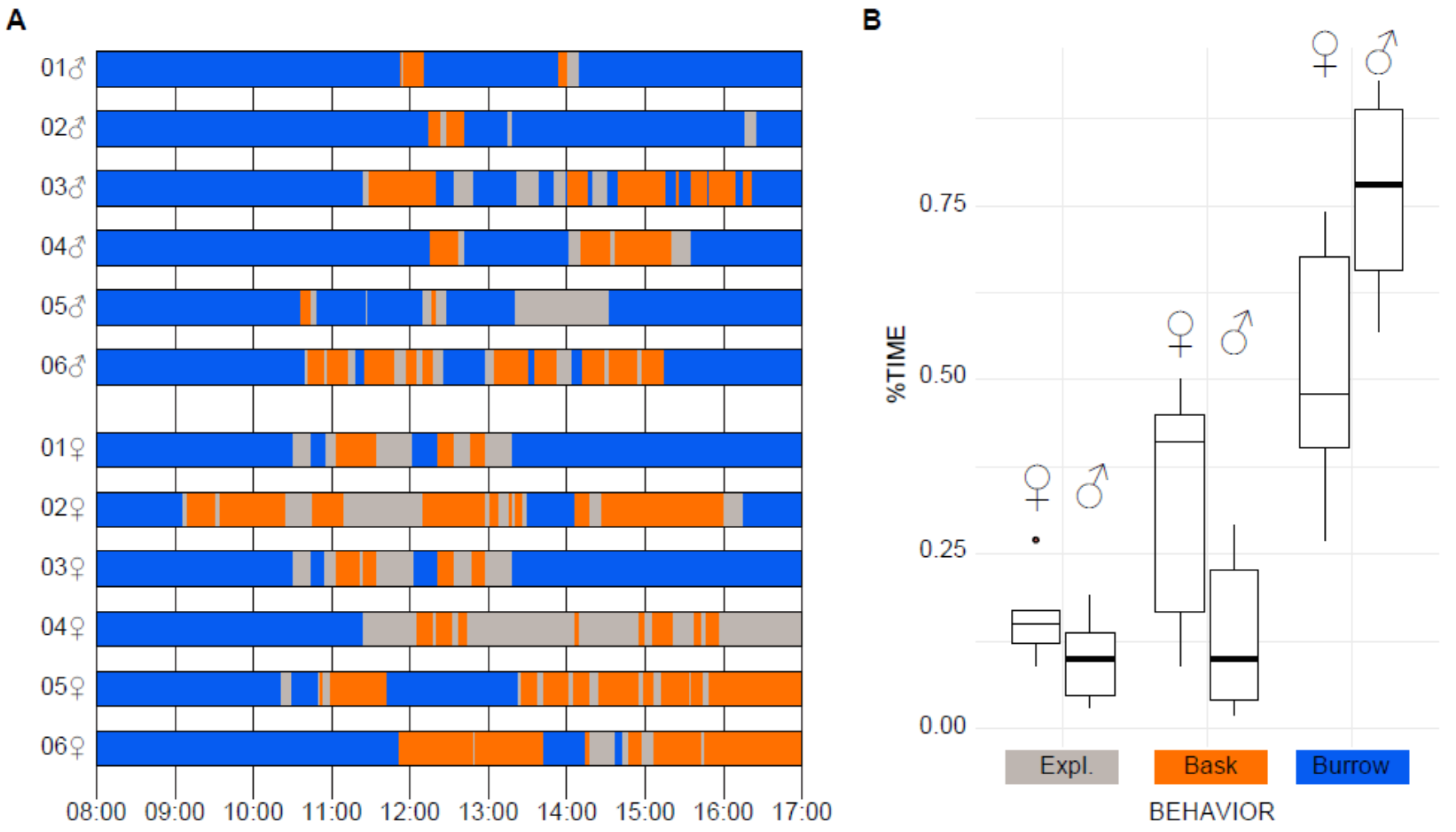
3.2. Focal Solitary Observation and Measurement of Activity during the Day
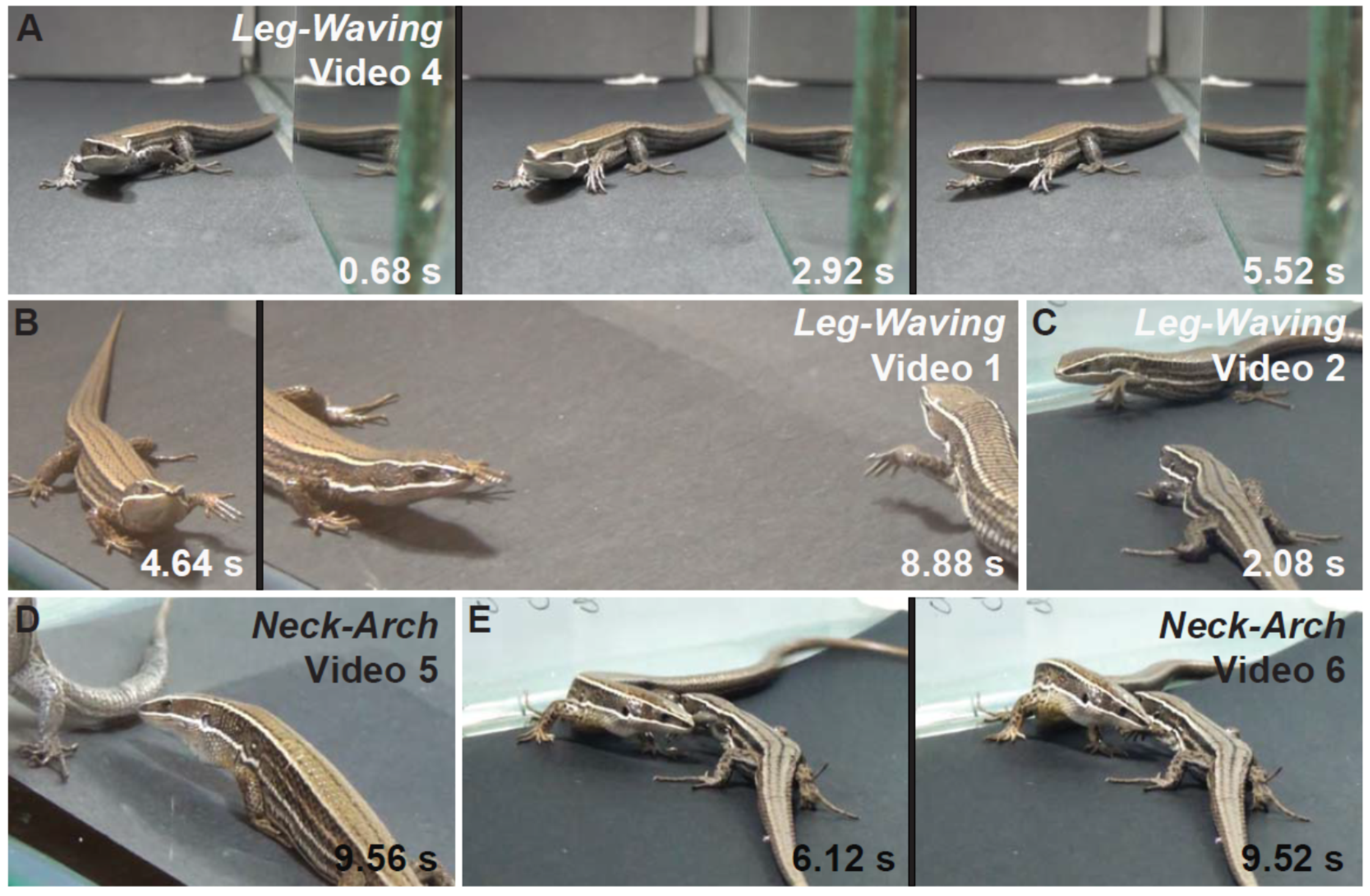
3.3. Conspecific Interactions
4. Discussion
4.1. Visual Signaling in Gymnophtalmidae
4.2. Multimodal Signaling in Lizards
4.3. Ex Situ Behavioral Studies in Pholidobolus Montium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeckens, S.; Driessens, T.; Van Damme, R. Intersexual chemo-sensation in a “visually-oriented” lizard, Anolis sagrei. PeerJ 2016, 4, e1874. [Google Scholar] [CrossRef]
- Martins, E.P.; Bissell, A.N.; Morgan, K.K. Population differences in a lizard communicative display: Evidence for rapid change in structure and function. Anim. Behav. 1998, 4, 1113–1119. [Google Scholar] [CrossRef]
- Radder, R.S.; Saidapur, S.K.; Shine, R.; Shanbhag, B.A. The language of lizards: Interpreting the function of visual displays of the Indian rock lizard, Psammophilus dorsalis (Agamidae). J. Ethol. 2006, 24, 275–283. [Google Scholar] [CrossRef]
- Ramos, J.A.; Peters, R.A. Dragon wars: Movement-based signalling by Australian agamid lizards in relation to species ecology. Austral Ecol. 2016, 41, 302–315. [Google Scholar] [CrossRef]
- Fleishman, L.J.; Font, E. Sensory Processing in Relation to Signaling Behavior. In Behavior of Lizards; Bels, V.L., Russell, A.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–257. [Google Scholar]
- Marcellini, D. Acoustic and visual display behavior of gekkonid lizards. Integr. Comp. Biol. 1977, 17, 251–260. [Google Scholar] [CrossRef]
- Fleishman, L.J.; Ogas, B.; Steinberg, D.; Leal, M. Why do Anolis dewlaps glow? An analysis of a translucent visual signal. Funct. Ecol. 2016, 30, 345–355. [Google Scholar] [CrossRef]
- Mason, R.T.; Gutzke, W.H.N. Sex recognition in the leopard gecko, Eublepharis macularius (Sauria: Gekkonidae) Possible mediation by skin-derived semiochemicals. J. Chem. Ecol. 1990, 16, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mayerl, C.; Van Damme, R.; Baeckens, S. Evolution and role of the follicular epidermal gland system in non-ophidian squamates. Amphib. Reptil. 2015, 36, 185–206. [Google Scholar] [CrossRef]
- Moreira, P.L.; López, P.; Martín, J. Discrimination of conspecific faecal chemicals and spatial decisions in juvenile Iberian rock lizards (Lacerta monticola). Acta Ethol. 2008, 11, 26–33. [Google Scholar] [CrossRef]
- Johnson, M.A.; Cook, E.G.; Kircher, B.K. Phylogeny and Ontogeny of Display Behavior. In Behavior of Lizards; Bels, V.L., Russell, A.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 259–287. [Google Scholar]
- Burghardt, G.M. Behavioral and Stimulus Correlates of Vomeronasal Functioning in Reptiles: Feeding, Grouping, Sex, and Tongue Use. In Chemical Signals; Springer US: Boston, MA, USA, 1980; pp. 275–301. [Google Scholar]
- Cooper, W.E. Foraging mode, prey chemical discrimination, and phylogeny in lizards. Anim. Behav. 1995, 50, 973–985. [Google Scholar] [CrossRef]
- Schwenk, K. Of tongues and noses: Chemoreception in lizards and snakes. Trends Ecol. Evol. 1995, 10, 7–12. [Google Scholar] [CrossRef]
- Vitt, L.J.; Pianka, E.R.; Cooper, W.E., Jr.; Schwenk, K. History and the Global Ecology of Squamate Reptiles. Am. Nat. 2003, 162, 44–60. [Google Scholar] [CrossRef] [PubMed]
- García-Roa, R.; Jara, M.; Baeckens, S.; López, P.; Van Damme, R.; Martín, J.; Pincheira-Donoso, D. Macroevolutionary diversification of glands for chemical communication in squamate reptiles. Sci. Rep. 2017, 7, 9288. [Google Scholar] [CrossRef] [PubMed]
- García-Roa, R.; Jara, M.; López, P.; Martín, J.; Pincheira-Donoso, D. Heterogeneous tempo and mode of evolutionary diversification of compounds in lizard chemical signals. Ecol. Evol. 2017, 7, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, K. The Evolution of Chemoreception in Squamate Reptiles: A Phylogenetic Approach. Brain Behav. Evol. 1993, 41, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Recoder, R.S.; Camacho, A.; De Sena, M.A.; Navas, C.A.; Rodrigues, M.T. A new species of Bachia Gray, 1845 (Squamata: Gymnophthalmidae) from the Eastern Brazilian Cerrado, and data on its ecology, physiology and behavior. Zootaxa 2013, 3616, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Frost, D.R.; De la Riva, I.; Pellegrino, K.C.M.; Sites, J.; Rodrigues, M.T.; Padial, J.M. Molecular systematics of teioid lizards (Teioidea/Gymnophthalmoidea: Squamata) based on the analysis of 48 loci under tree-alignment and similarity-alignment. Cladistics 2016, 32, 624–671. [Google Scholar] [CrossRef]
- Parra, V.; Nunes, P.M.S.; Torres-Carvajal, O. Systematics of Pholidobolus lizards (Squamata, Gymnophthalmidae) from southern Ecuador, with descriptions of four new species. Zookeys 2020, 954, 109–156. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.C.H. Über Cercosaura und Die Mit Dieser Gattung verwandten Eidechsen aus Südamerica; Kessinger Publishing, LLC: Berlin, Germany, 1863. [Google Scholar]
- Hillis, D.M. Evolutionary Genetics of The Andean Lizard Genus Pholidobolus (Sauria: Gymnophthalmidae): Phylogeny, Biogeography, And Comparison Of Tree Construction Techniques. Syst. Biol. 1985, 34, 109–126. [Google Scholar] [CrossRef]
- Dávila-Jativa, M.; Cisneros-Heredia, D. Use of human-made buildings by Stenocercus lizards (Iguania, Tropiduridae). Herpetol. Notes 2017, 10, 517–519. [Google Scholar]
- Montanucci, R.R. Systematics and evolution of the andean lizard genus Pholidobolus (Sauria: Teiidae). Univ. Kansas Museum Nat. Hist. Misc. Publ. 1973, 59, 1–52. [Google Scholar]
- Ramírez-Jaramillo, S. Nidos de Pholidobolus montium en una área intervenida de Mulaló, Cotopaxi-Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2016, 37, 29–33. [Google Scholar]
- Bustard, R. Egg laying and incubation of the striped mountain lizard Pholidobolus montium (Teiidae) with notes on an incubator. Br. J. Herpetol. 1964, 3, 163–164. [Google Scholar]
- Doody, J.S.; Freedberg, S.; Keogh, J.S. Communal egg-laying in reptiles and amphibians: Evolutionary patterns and hypotheses. Q. Rev. Biol. 2009, 84, 229–252. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Heredia, D.F. Pholidobolus montium (Errata Version Published in 2017). Available online: https://www.iucnredlist.org/species/44578680/115386433 (accessed on 11 October 2019).
- ASIH Guidelines for Live Amphibians and Reptiles in Field and Laboratory Research. Available online: https://asih.org/sites/default/files/2018-05/guidelines_herps_research_2004.pdf (accessed on 6 June 2021).
- Martin, P.; Bateson, P.P.G. Measuring Behaviour: An Introductory Guide, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; p. 222. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-6. 2019. Available online: https://cran.microsoft.com/snapshot/2019-12-24/web/packages/vegan/index.html (accessed on 6 June 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Barter, R.L.; Yu, B. Superheat: An R package for creating beautiful and extendable heatmaps for visualizing complex data. J. Comput. Graph. Stat. 2018, 27, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Torres-Carvajal, O.; Lobos, S.E.; Venegas, P.J.; Chávez, G.; Aguirre-Peñafiel, V.; Zurita, D.; Echevarría, L.Y. Phylogeny and biogeography of the most diverse clade of South American gymnophthalmid lizards (Squamata, Gymnophthalmidae, Cercosaurinae). Mol. Phylogenet. Evol. 2016, 99, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Etzold, T. The EMBL/EBI Reptile Database. Herpetol. Rev. 1996, 27, 174–175. [Google Scholar]
- Cooper, W.E. Chemical discrimination by tongue-flicking in lizards: A review with hypotheses on its origin and its ecological and phylogenetic relationships. J. Chem. Ecol. 1994, 20, 439–487. [Google Scholar] [CrossRef]
- Duran Filho, C.; Molina, F.B. O Comportamento de Acasalamento de Calyptommatus leiolepis Rdorigues, 1992 em Cativerio (Sauria, Gymnophthalmidae: Observacoes Preliminares. Rev. Etol. 2002, 4, 11–15. [Google Scholar]
- Clause, A.; Capaldi, E. Caudal Autotomy and Regeneration in Lizards. J. Exp. Zool. 2006, 305, 965–973. [Google Scholar] [CrossRef]
- Torr, G.A.; Shine, R. An ethogram for the small scincid lizard Lampropholis guichenoti. Amphibia-Reptilia 1994, 5, 21–34. [Google Scholar]
- Van Dyk, D.A.; Evans, C.S. Opponent assessment in lizards: Examining the effect of aggressive and submissive signals. Behav. Ecol. 2008, 19, 895–901. [Google Scholar] [CrossRef]
- Font, E.; Barbosa, D.; Sampedro, C.; Carazo, P. Social behavior, chemical communication, and adult neurogenesis: Studies of scent mark function in Podarcis wall lizards. Gen. Comp. Endocrinol. 2012, 177, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ord, T.J.; Peters, R.A.; Evans, C.S.; Taylor, A.J. Digital video playback and visual communication in lizards. Anim. Behav. 2002, 63, 879–890. [Google Scholar] [CrossRef]
- Peters, R.A.; Ramos, J.A.; Hernandez, J.; Wu, Y.; Qi, Y. Social context affects tail displays by Phrynocephalus vlangalii lizards from China. Nat. Publ. Gr. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.E.; Pérez-Mellado, V.; Baird, T.A.; Caldwell, J.P.; Vitt, L.J. Pursuit deterrent signalling by the bonaire whiptail lizard Cnemidophorus murinus. Behaviour 2004, 141, 297–311. [Google Scholar] [CrossRef]
- Langkilde, T.; Schwarzkopf, L.; Alford, R. An ethogram for adult male rainbow skinks, Carlia jarnoldae. Herpetol. J. 2003, 13, 141–148. [Google Scholar]
- Carpenter, C.C.; Ferguson, G.W. Variation and evolution of stereotyped displays in reptiles. Biol. Reptil. Ecol. Behav. 1977, 7, 335–354. [Google Scholar]
- Font, E.; Carazo, P.; Pérez i de Lanuza, G.; Kramer, M. Predator-elicited foot shakes in Wall Lizards (Podarcis muralis): Evidence for a Pursuit-Deterrent function. J. Comp. Psychol. 2012, 126, 87–96. [Google Scholar] [CrossRef]
- Torr, G.A.; Shine, R. Patterns of Dominance in the Small Scincid Lizard Lampropholis guichenoti. J. Herpetol. 1996, 30, 230–237. [Google Scholar] [CrossRef]
- Bro-Jørgensen, J. Dynamics of multiple signalling systems: Animal communication in a world in flux. Trends Ecol. Evol. 2010, 25, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N. An Ethogram of the Blue Spiny Lizard, Sceloporus cyanogenys (Reptilia, Lacertilia, Iguanidae). J. Herpetol. 1977, 11, 177–195. [Google Scholar] [CrossRef]
- Qi, Y.; Li, S.; Suo, L.; Li, H.; Wang, Y. An ethogram of the toad-headed lizard Phrynocephalus vlangalii during the breeding season. Asian Herpetol. Res. 2011, 2, 110–116. [Google Scholar] [CrossRef]
- Whiting, M.J.; Miles, D.B. Behavioral Ecology of Aggressive Behavior in Lizards. In Behavior of Lizards; Bels, V.L., Russell, A.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 280–320. [Google Scholar]
- Kabir, M.S.; Venkatesan, R.; Thaker, M. Multiple sensory modalities in diurnal geckos is associated with the signalling environment and evolutionary constraints. Integr. Org. Biol. 2020, 2. [Google Scholar] [CrossRef]
- Reedy, A.M.; Pope, B.D.; Kiriazis, N.M.; Giordano, C.L.; Sams, C.L.; Warner, D.A.; Cox, R.M. Female anoles display less but attack more quickly than males in response to territorial intrusions. Behav. Ecol. 2017, 28, 1323–1328. [Google Scholar] [CrossRef]
- López, P.; Martín, J.; Cuadrado, M. Pheromone-Mediated Intrasexual Aggression in Male Lizards, Podarcis hispanicus. Aggress. Behav. 2002, 28, 154–163. [Google Scholar] [CrossRef]
- Martin, J.; Salvador, A. Effects of Tail Loss on the Time-Budgets, Movements, and Spacing Patterns of Iberian Rock Lizards, Lacerta monticola. Herpetologica 1997, 53, 117–125. [Google Scholar]
- Miles, D.B.; Losos, J.B.; Irschick, D.J. Morphology, performance, and foraging mode. In Lizard Ecology; Miles, D.B., McBrayer, L.B., Reilly, S.M., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 49–93. ISBN 9780521833585. [Google Scholar]
- Cooper, W.E. The foraging mode controversy: Both continuous variation and clustering of foraging movements occur. J. Zool. 2005, 267, 179–190. [Google Scholar] [CrossRef]
- Martínez Silvestre, A. How to assess stress in reptiles. J. Exot. Pet Med. 2014, 23, 240–243. [Google Scholar] [CrossRef]
- Warwick, C.; Arena, P.; Lindley, S.; Jessop, M.; Steedman, C. Assessing reptile welfare using behavioural criteria. In Pract. 2013, 35, 123–131. [Google Scholar] [CrossRef]
- Murphy, J.B.; Mitchell, L.A. Ritualized Combat Behavior of the Pygmy Mulga Monitor Lizard, Varanus gilleni (Sauria: Varanidae). Herpetologica 1974, 30, 90–97. [Google Scholar]
- Quirola, D.R.; Mármol, A.; Torres-Carvajal, O.; Narváez, A.E.; Ayala-Varela, F.; Moore, I.T. Use of a rostral appendage during social interactions in the Ecuadorian Anolis proboscis. J. Nat. Hist. 2017, 51, 1625–1638. [Google Scholar] [CrossRef]
- Brandt, Y. Lizard threat display handicaps endurance. Proc. R. Soc. B Biol. Sci. 2003, 270, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Whiting, M.J.; Fu, J.; Qi, Y. The driving forces behind female-female aggression and its fitness consequence in an Asian agamid lizard. Behav. Ecol. Sociobiol. 2019, 73, 73. [Google Scholar] [CrossRef]


| Functional Category | Behavior | Description | Occur during Solitary obs. | Occur during Conspecific Stimulus Experiments | Occur during Mirror Experiments |
|---|---|---|---|---|---|
| Maintenance | |||||
| Adpress * | One or more limbs are raised off the substrate and held against the side of the body. | yes | no | no | |
| Bask ~ | Dorsoventral flattening of the body onto the substrate. | yes | no | no | |
| Thread * | Lizard rolls its body sideways. | yes | no | no | |
| Burrow ~ | Lizard hides inside its burrow. | yes | no | no | |
| Bury ~ | Hide underground by digging down the substrate. | yes | no | no | |
| Cloacal-drag ~ | Full body displacement frontward while keeping the cloacal region in contact to the substrate. | yes | no | no | |
| Dig ~ | Lizard removes the substrate with its forelimbs. | yes | no | no | |
| Drink ~ | Snout placed into the water and tongue is slowly protruded and returned to the mouth. | yes | no | no | |
| Eat ~ | A food item is grasped with the jaws and ingested. | yes | no | no | |
| Tongue-flick * | Tongue is protruded and returned to the mouth. | yes | yes | yes | |
| Scratch * | Hind limb movements used to rapidly scrape the body bowed laterally. | yes | no | no | |
| Slough * | Scrapes its body against stationary objects as to remove sloughed skin. | yes | no | no | |
| Looking-around ~ | Side to side movement of the head, while the body remains motionless. | yes | no | no | |
| General Locomotor patterns | |||||
| Walk ~ | Forward movement with the ventral region of the body in contact with the substrate. | yes | yes | yes | |
| Jump ~ | Fast leap, all four feet are not in contact to the substrate. | yes | no | no | |
| Run ~ | Fast forward movement with the body raised off the substrate. | yes | yes | yes | |
| Stalk ~ | Slow walking movement. | yes | yes | yes | |
| Conspecific elicited locomotor patterns | |||||
| Approach ~ | One lizard moves toward another up to a considerable distance, which does not allow physical interaction. | no | yes | yes | |
| Chase ~ | Rapid pursuit of one lizard to another. | no | yes | no | |
| Flee * | Rapid evacuation when chased by another individual. | no | yes | no | |
| Move-away ~ | One lizard slowly passes over another. | no | yes | yes | |
| Move-over ~ | One lizard moves over the top of another. | no | yes | no | |
| Conspecific elicited positions and movements | |||||
| Bite * | Grip another individual with its jaws. | no | yes | no | |
| Hindlimb-kick * | Push away another individual by using one hindlimb. | no | yes | no | |
| Lateral orientation ~ | Face another individual laterally, the sagittal plane of the entire body or, at least the anterior part, is presented. | no | yes | yes | |
| Leg-waving * | Lizard elevates and swings a foreleg. | no | yes | yes | |
| Lunge * | One lizard rapidly moves toward and away from another. | no | yes | yes | |
| Mount * | One lizard steps/stands on the antagonist’s dorsum. | no | yes | no | |
| Neck-arch ~ | Lizard raises its body using push up (stretch front legs), while the snout is pointed toward the ground. | no | yes | no | |
| Neck-bite * | Lizard bits the skin on the neck of another. | no | yes | no | |
| Sagittal expansion ~ | Lateral compression of the body and dorsoventral expansion. | no | yes | no | |
| Strobe-motion * | Short rapid jerks. | no | yes | no | |
| Tail-bite * | Grasp another lizard’s tail in its jaws. | no | yes | no | |
| Tail-undulate * | A sinusoidal, horizontal movement of the entire tail. | no | yes | yes | |
| Individual | Exploratory | Basking | Hiding /Burrowing | PTM | TTO (min) |
|---|---|---|---|---|---|
| M1 | 2.81 | 4.08 | 93.11 | 40.76 | 37.21 |
| M2 | 4.14 | 3.99 | 91.91 | 50.94 | 43.65 |
| M3 | 12.73 | 24.97 | 62.36 | 33.77 | 203.6 |
| M4 | 7.06 | 15.72 | 77.23 | 30.98 | 123.01 |
| M5 | 19.5 | 1.58 | 78.94 | 92.5 | 113.81 |
| M6 | 13.91 | 29.13 | 57 | 32.32 | 232.43 |
| M | 10.02 | 13.25 | 76.76 | 46.88 | 125.62 |
| S.E. | 1.77 | 3.26 | 4.08 | 6.48 | 22.08 |
| F1 | 16.57 | 9.36 | 74.09 | 63.91 | 139.99 |
| F2 | 27.49 | 45.87 | 26.69 | 37.47 | 396.11 |
| F3 | 16.87 | 9.36 | 73.86 | 64.33 | 141.64 |
| F4 | 12.19 | 51.34 | 38.2 | 19.18 | 343.04 |
| F5 | 14.14 | 40.81 | 47.68 | 25.73 | 296.7 |
| F6 | 9.13 | 41.65 | 49.24 | 17.98 | 274.18 |
| F | 16.06 | 33.06 | 51.63 | 38.1 | 265.28 |
| S.E. | 1.73 | 5.16 | 5.25 | 5.86 | 28.92 |
| 13.04 | 23.15 | 64.19 | 42.49 | 195.45 | |
| S.E. | 1.98 | 5.25 | 6.04 | 6.32 | 33.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poma-Soto, F.; Narváez, A.E.; Romero-Carvajal, A. Visual Signaling in the Semi-Fossorial Lizard Pholidobolus montium (Gymnophthalmidae). Animals 2021, 11, 3022. https://doi.org/10.3390/ani11113022
Poma-Soto F, Narváez AE, Romero-Carvajal A. Visual Signaling in the Semi-Fossorial Lizard Pholidobolus montium (Gymnophthalmidae). Animals. 2021; 11(11):3022. https://doi.org/10.3390/ani11113022
Chicago/Turabian StylePoma-Soto, Franco, Andrea E. Narváez, and Andrés Romero-Carvajal. 2021. "Visual Signaling in the Semi-Fossorial Lizard Pholidobolus montium (Gymnophthalmidae)" Animals 11, no. 11: 3022. https://doi.org/10.3390/ani11113022
APA StylePoma-Soto, F., Narváez, A. E., & Romero-Carvajal, A. (2021). Visual Signaling in the Semi-Fossorial Lizard Pholidobolus montium (Gymnophthalmidae). Animals, 11(11), 3022. https://doi.org/10.3390/ani11113022






