Mathematical Models for the Skin to Lumbosacral Epidural Distance in Dogs: A Cadaveric Computed-Tomography Study
Abstract
:Simple Summary
Abstract
1. Introduction
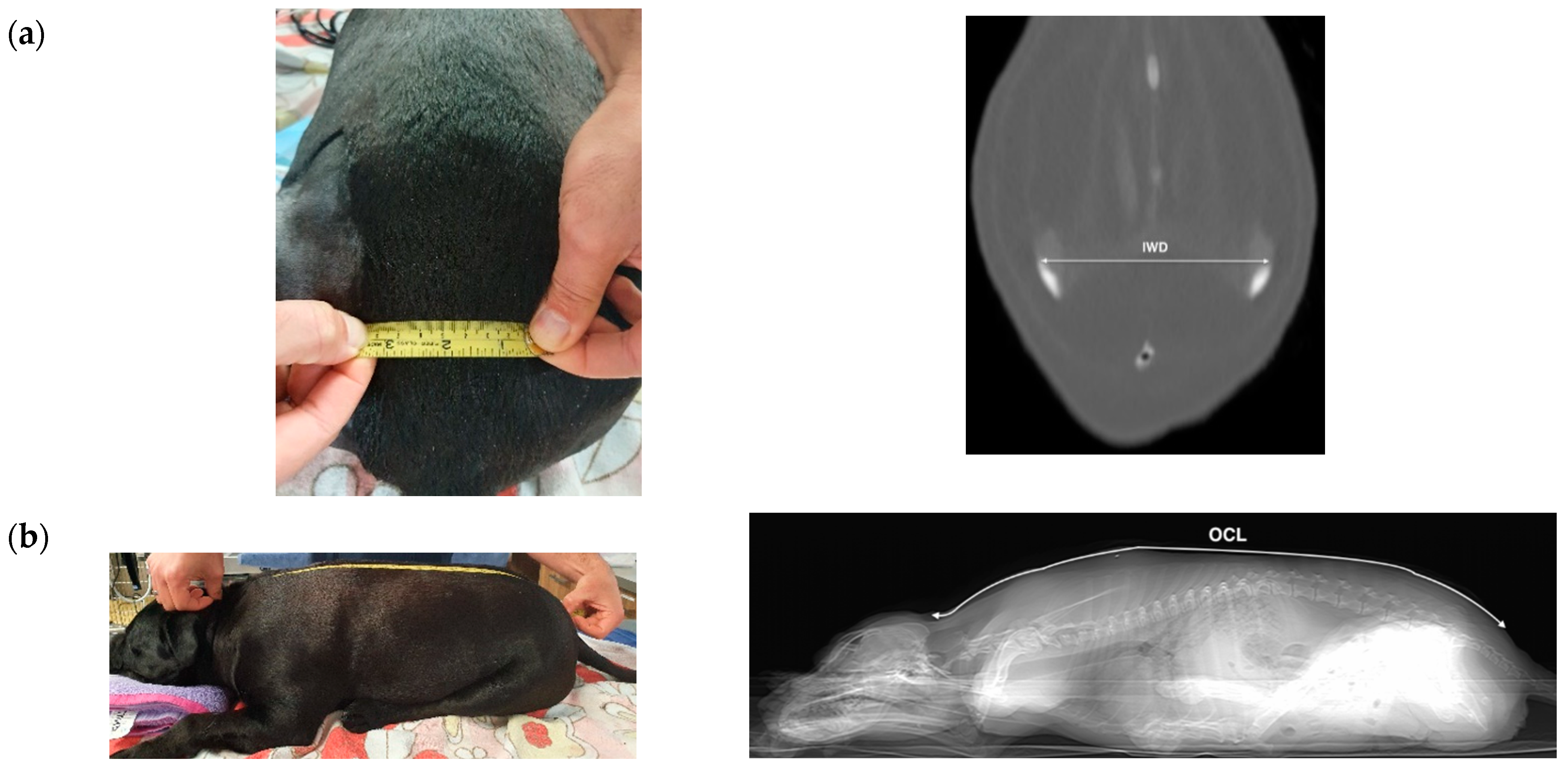
2. Materials and Methods
2.1. Cadavers
2.2. Procedure
2.2.1. Phase 1
2.2.2. Phase 2
2.3. Statistical Analysis
3. Results
3.1. Phase 1
3.2. Phase 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.; Song, S.Y.; Kim, B.J. Predicting the difficulty in performing a neuraxial blockade. Korean J. Anesthesiol. 2011, 61, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, M.; Atallah, M.M.; Elkharboutly, W.S.; Allakkany, N.S.; AbdelKhalek, M. Does Preprocedural Ultrasound Increase the First-Pass Success Rate of Epidural Catheterization Before Cesarean Delivery? A Randomized Controlled Trial. Anesthesia Analg. 2017, 124, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Eley, V.; Chin, A.; Sekar, R.; Donovan, T.; Krepska, A.; Lawrence, M.; Bell, S.; Ralfe, K.; McGrath, S.; Webb, L.; et al. Increasing body mass index and abdominal subcutaneous fat thickness are associated with increased skin-to-epidural space distance in pregnant women. Int. J. Obstet. Anesthesia 2019, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.K.; Kaul, T.K.; Kathuria, S.; Gupta, S.; Khurana, S. Distance from Skin to Epidural Space: Correlation with Body Mass Index (BMI). J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 39–42. [Google Scholar] [PubMed]
- Adachi, Y.U.; Sanjo, Y.; Sato, S. The epidural space is deeper in elderly and obese patients in the Japanese population. Acta Anaesthesiol. Scand. 2007, 51, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Swinson, A.K.; Hughes, C.; Mokashi, S.; Russell, R. Effect of ethnicity and body mass index on the distance from skin to lumbar epidural space in parturients. Anaesthesia 2011, 66, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Moka, E.; Siafaka, I.; Argyra, E.; Vadalouca, A. Prediction of the Distance from the Skin to the Lumbar Epidural Space in the Greek Population, Using Mathematical Models. Pain Pract. 2005, 5, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Kirthinanda, D.; Loh, S.K.N.; Sng, B.L.; Dinoo, K. Strategies to reduce neuraxial analgesia failure during labour. Trends Anaesth. Crit. Care 2016, 7–8, 41–46. [Google Scholar] [CrossRef]
- Singh, S.; Wirth, K.M.; Phelps, A.L.; Badve, M.H.; Shah, T.H.; Sah, N.; Vallejo, M.C. Epidural Catheter Placement in Morbidly Obese Parturients with the Use of an Epidural Depth Equation prior to Ultrasound Visualization. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Iseri, T.; Nishimura, R.; Nagahama, S.; Nakagawa, T.; Fujimoto, Y.; Sasaki, N. Distance between the skin and the lumbosacral epidural space in dogs. Jpn. J. Vet. Res. 2019, 67, 35–40. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Pacheco, P.F.; Sellera, F.; Futema, F.; Cortopassi, S. The use of ultrasound to assist epidural injection in obese dogs. Vet. Anaesth. Analg. 2020, 47, 137–140. [Google Scholar] [CrossRef]
- Sun, T.C.; Makara, M.; Martinez-Taboada, F. Computed Tomography-Derived Occipital–Coccygeal Length and Ilium Wing Distance Correlates with Skin to Epidural and Intrathecal Depths in Dogs. Vet. Sci. 2020, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Martinez-Taboada, F.; Redondo, J.I. Comparison of the hanging-drop technique and running-drip method for identifying the epidural space in dogs. Vet. Anaesth. Analg. 2017, 44, 329–336. [Google Scholar] [CrossRef]
- Pita Fernández, S. Clinical Epidemiology and Biostatistics Unit. Complexo Hospitalario Universitario de A Coruña. Available online: https://www.fisterra.com/mbe/investiga/9muestras/9muestras2.asp (accessed on 19 July 2021).
- Lin, L.I.-K. Assay Validation Using the Concordance Correlation Coefficient. Biometrics 1992, 48, 599. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research. In Relation between Two Continuous Variables; Chapman & Hall/CRC: London, UK, 2006; pp. 317–318. [Google Scholar]
- Campoy, L. Epidural and spinal anaesthesia in the dog. Practice 2004, 26, 262–269. [Google Scholar] [CrossRef]
- Jones, R. Epidural Analgesia in the Dog and Cat. Vet. J. 2001, 161, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panti, A.; Greenhalgh, S.N.; Longo, M.; Liuti, T. The effect of recumbency and hindlimb position on the lumbosacral interlaminar distance in dogs: A cadaveric computed tomography study. Vet. Anaesth. Analg. 2018, 45, 802–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Concetto, S.; Mandsager, R.E.; Riebold, T.W.; Stieger-Vanegas, S.M.; Killos, M. Effect of hind limb position on the craniocaudal length of the lumbosacral space in anesthetized dogs. Vet. Anaesth. Analg. 2012, 39, 99–105. [Google Scholar] [CrossRef]
- Puggioni, A.; Arnett, R.; Clegg, T.; Glyde, M.; Tobin, E.; McAllister, H. Influence Of Patient Positioning On The L5–L6 Mid-Laminar Distance. Vet. Radiol. Ultrasound 2006, 47, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Kawalilak, L.T.; Tucker, R.L.; Greene, S.A. Use Of Contrast-Enhanced Computed Tomography To Study The Cranial Migration Of A Lumbosacral Injectate In Cadaver Dogs. Vet. Radiol. Ultrasound 2015, 56, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Bartynski, W.S.; Grahovac, S.Z.; Rothfus, W.E. Incorrect Needle Position during Lumbar Epidural Steroid Administration: Inaccuracy of Loss of Air Pressure Resistance and Requirement of Fluoroscopy and Epidurography during Needle Insertion. Am. J. Neuroradiol. 2005, 26, 502–505. [Google Scholar] [PubMed]
- Johnson, B.A.; Schellhas, K.P.; Pollei, S.R. Epidurography and Therapeutic Epidural Injections: Technical Considerations and Experience with 5334 Cases. Am. J. Neuroradiol. 1999, 20, 697–705. [Google Scholar]
- Liotta, A.P.; Girod, M.; Peeters, D.; Sandersen, C.; Couvreur, T.; Bolen, G. Clinical effects of computed tomography–guided lumbosacral facet joint, transforaminal epidural, and translaminar epidural injections of methylprednisolone acetate in healthy dogs. Am. J. Vet. Res. 2016, 77, 1132–1139. [Google Scholar] [CrossRef]
- Scarabelli, S.; Cripps, P.; Rioja, E.; Alderson, B. Adverse reactions following administration of contrast media for diagnostic imaging in anaesthetized dogs and cats: A retrospective study. Vet. Anaesth. Analg. 2016, 43, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Ziemer, L.S.; Shofer, F.S.; Steinberg, S.A. Risk factors associated with development of seizures after use of iohexol for myelography in dogs: 182 cases (1998). J. Am. Vet. Med. Assoc. 2002, 220, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Adami, C.; Gendron, K. What is the evidence? The issue of verifying correct needle position during epidural anaesthesia in dogs. Vet. Anaesth. Analg. 2017, 44, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sarotti, D.; Rabozzi, R.; Franci, P. Comparison of epidural versus intrathecal anaesthesia in dogs undergoing pelvic limb orthopaedic surgery. Vet. Anaesth. Analg. 2015, 42, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Taboada, F.; Otero, P.E.; Laredo, F.; Belda, E. Identification of the sacrococcygeal epidural space using the nerve stimulation test or the running-drip method in dogs. Vet. Anaesth. Analg. 2020, 47, 385–390. [Google Scholar] [CrossRef]
- Adegboye, M.; Bolaji, B.; Ibraheem, G. The Correlation between Body Mass Index on The Length From Skin To Lumbar Epidural Space In Nigerian Adults. J. West Afr. Coll. Surg. 2017, 7, 113–127. [Google Scholar] [PubMed]
- Kao, M.; Tsai, S.; Chang, W.; Liu, H.; Hsieh, Y.; Hu, J.; Mok, M. Prediction of the distance from skin to epidural space for low-thoracic epidural catheter insertion by computed tomography. Br. J. Anaesth. 2004, 92, 271–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, G.P.; Shaw, M.; Broom, M.A. Predicting epidural space depth in an obstetric population using patient demographics: An observational study of 1534 patients. Eur. J. Anaesthesiol. 2021, 38, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Recku, G.; Fluder, E.; Corsino, A. The distance from the skin to the epidural space in morbidly obese patients undergoing bariatric surgery. Reg. Anaesth. Pain Med. 2003, 28, A4. [Google Scholar]
- Clinkscales, C.; Greenfield, M.; Vanarase, M.; Polley, L. An observational study of the relationship between lumbar epidural space depth and body mass index in Michigan parturients. Int. J. Obstet. Anesthesia 2007, 16, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cima, D.S.; Credie, L.D.F.G.A.; Futema, F.; Luna, S.P.L. Lumbar Epidural: Anatomical and Clinical Study in Dogs Submitted to Ovariohysterectomy. Front. Vet. Sci. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]

| ID | Sex | Weight (kg) | BCS | Breed | Phase 1 (In Millimetres) | Phase 2 (In Millimetres) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| /9 | CLED | OCL | IWD | Equation (1) | Equation (2) | SLED | ||||||
| Caudal | Neutral | Cranial | Cadaver/CT | |||||||||
| 1 | M | 15 | 5 | Staffordshire bull terrier | 43 | 43 | 34 | 565/510 | 80/59 | 35.6 | 48.3 | 34 |
| 2 | F | 23.5 | 7 | Staffordshire bull terrier | 59 | 53 | 46 | 630/593 | 90/63 | 55.3 | 70.5 | 49 |
| 3 | F | 20.3 | 6 | Staffordshire bull terrier | 57 | 62 | 52 | 580/524 | 95/61 | 52.8 | 73.3 | 51 |
| 4 | M | 29.9 | 4 | Greyhound | 36 | 36 | 29 | 950/941 | 96/102 | 54.8 | 57.3 | 30 |
| 5 | F | 34.1 | 7 | English bulldog | 59 | 63 | 56 | 760/687 | 85/72 | 61.8 | 67.7 | 61 |
| 6 | M | 14.1 | 4 | Beagle | 42 | 41 | 36 | 640/609 | 70/66 | 39.3 | 42.7 | 41 |
| 7 | M | 9.6 | 2 | Crossbred | 22 | 22 | 17 | 560/543 | 60/56 | 35.3 | 37.1 | 24 |
| 8 | M | 14.1 | 4 | Beagle | 34 | 34 | 28 | 670/615 | 80/66 | 40.8 | 48.3 | 31 |
| 9 | F | 9.2 | 5 | French bulldog | 33 | 33 | 29 | 455/454 | 88/53 | 30.1 | 52.8 | 32 |
| 10 | M | 11.2 | 5 | Kelpie | 41 | 40 | 32 | 550/527 | 65/66 | 34.8 | 39.9 | 33 |
| 11 | M | 17.5 | 4 | Staffordshire bull terrier | 40 | 37 | 30 | 730/645 | 90/63 | 43.8 | 53.9 | 29 |
| SLED Prediction | r, 95% CI | Pr | R2 | Intercept | β | F | PR2 | CCC, 95% CI | c.b |
|---|---|---|---|---|---|---|---|---|---|
| Equation (1) | 0.7196, 0.2107–0.9217 | 0.0125 | 0.5179 | 19.0621 | 0.6620 | 9.668 | 0.0125 | 0.6061, 0.1471–0.8503 | 0.8422 |
| Equation (2) | 0.7590, 0.2913–0.9340 | 0.0068 | 0.5755 | 22.7683 | 0.8225 | 12.2 | 0.0068 | 0.3763, 0.0590–0.6246 | 0.4960 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.C.; Schier, M.; Lau, M.P.Y.; Martinez-Taboada, F. Mathematical Models for the Skin to Lumbosacral Epidural Distance in Dogs: A Cadaveric Computed-Tomography Study. Animals 2021, 11, 2974. https://doi.org/10.3390/ani11102974
Sun TC, Schier M, Lau MPY, Martinez-Taboada F. Mathematical Models for the Skin to Lumbosacral Epidural Distance in Dogs: A Cadaveric Computed-Tomography Study. Animals. 2021; 11(10):2974. https://doi.org/10.3390/ani11102974
Chicago/Turabian StyleSun, Tsim Christopher, Mara Schier, Michelle Pui Yan Lau, and Fernando Martinez-Taboada. 2021. "Mathematical Models for the Skin to Lumbosacral Epidural Distance in Dogs: A Cadaveric Computed-Tomography Study" Animals 11, no. 10: 2974. https://doi.org/10.3390/ani11102974
APA StyleSun, T. C., Schier, M., Lau, M. P. Y., & Martinez-Taboada, F. (2021). Mathematical Models for the Skin to Lumbosacral Epidural Distance in Dogs: A Cadaveric Computed-Tomography Study. Animals, 11(10), 2974. https://doi.org/10.3390/ani11102974






