Emergence of Parafilaria bovicola in Austria
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Laboratory Analysis
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tubangui, M. Nematodes in the collection of the Philippine Bureau of Science, II: Filarioidea. Philipp. J. Sci. 1934, 55, 115–122. [Google Scholar]
- De Jesus, Z. Haemorrhagic filariasis in cattle caused by a new species of Parafilaria. Philipp. J. Sci. 1934, 55, 125–130. [Google Scholar]
- Bech-Nielsen, S.; Hugoson, G.; Wold-Troell, M. Economic evaluation of several control programs for the cattle nematode Parafiliria bovicola using benefit—Cost analysis. Prev. Vet. Med. 1983, 1, 303–320. [Google Scholar] [CrossRef]
- Lundquist, H. Parafilaria bovicola (Tubangui 1934) established in Swedish cattle. Nord. Vet. Med. 1983, 35, 57–68. [Google Scholar]
- Daslakow, P. Beitrag zur Erforschung der Rinderparafilariose. Z. Parasitenkd. 1944, 254–264. [Google Scholar]
- Metianu, T. Considérations sur la parafilariose hémorragique des bovins. Parafilaria bovicola en Roumanie. Ann. Parasitol. Hum. Comp. 1949, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bech-Nielsen, S.; Sjogren, U.; Lundquist, H. Parafilaria bovicola (Tubangui 1934) in cattle: Epizootiology-disease occurrence. Am. J. Vet. Res. 1982, 43, 945–947. [Google Scholar] [PubMed]
- Gibbons, L.M.; Zakrisson, G.; Uggla, A. Redescription of Parafilaria bovicola Tubangui, 1934 (Nematoda: Filarioidea) from Swedish cattle. Acta Vet. Scand. 2000, 41, 85–91. [Google Scholar] [CrossRef]
- Solismaa, M.; Laaksonen, S.; Nylund, M.; Pitkänen, E.; Airakorpi, R.; Oksanen, A. Filarioid nematodes in cattle, sheep and horses in Finland. Acta Vet. Scand. 2008, 50, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losson, B.; Saegerman, C. First isolation of Parafilaria bovicola from clinically affected cattle in Belgium. Nord. Vet. Med. 2009, 164, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Caron, Y.; Groignet, S.; Saegerman, C.; Losson, B.J. Three cases of Parafilaria bovicola infection in Belgium, and a few recent epidemiological observations on this emergent disease. Vet. Rec. 2013, 172, 129. [Google Scholar] [CrossRef] [Green Version]
- Borgsteede, F.H.M.; van Wuijckhuise, L.; Peutz, J.; Roumen, T.; Kock, P. Import of Parafilaria bovicola in The Netherlands. Vet. Parasitol. 2009, 161, 146–149. [Google Scholar] [CrossRef]
- Torgerson, P.; Doherty, M.; Healy, A. Bovine haemorrhagic parafilariosis in an imported Charolais bull. Ir. Vet. J. 1998, 51, 27–29. [Google Scholar]
- Niilo, L. Bovine hemorrhagic filariasis in cattle imported into Canada. Can. Vet. J. 1968, 9, 132–137. [Google Scholar]
- Webster, W.A.; Wilkins, D.B. The recovery of Parafilaria bovicola Tubangui, 1934 from an imported Charolais bull. Can. Vet. J. 1970, 11, 13–14. [Google Scholar]
- Alzieu, J.P.; Bourdenx, L.; Ducos de Lahitte, J.; Schelcher, F. La parafilariose bovine. BULLETIN-GTV 1993, 5, 85–94. [Google Scholar]
- Gamard, N. La Parafilariose Bovine: Synthèse Bibliographique; Université Paul Sabatier-Toulouse III: Toulouse, France, 2001. [Google Scholar]
- Bech-Nielsen, S.; Bornstein, S.; Christensson, D.; Wallgren, T.B.; Zakrisson, G.; Chirico, J. Parafilaria bovicola (Tubangui 1934) in cattle: Epizootiology-vector studies and experimental transmission of Parafilaria bovicola to cattle. Am. J. Vet. Res. 1982, 43, 948–954. [Google Scholar]
- Stevanovic, O.; Babic, R.; Nedic, D.; Nikolic, S.; Dimitric, R.; Borkovic, M.; Paras, S. First record of bovine parafilariosis in Bosnia and Herzegovina, Western Balkans. Rev. Med. Vet. 2014, 165, 323–326. [Google Scholar]
- Stoll, A.; Pfitzner-Friedrich, A.; Reichmann, F.; Rauschendorfer, J.; Roessler, A.; Rademacher, G.; Knubben-Schweizer, G.; Sauter-Louis, C. Existence of bovine neonatal pancytopenia before the year 2005? Retrospective evaluation of 215 cases of haemorrhagic diathesis in cattle. Vet. J. 2016, 216, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hofer, J. Parafilaria bovicola beim Rind-eine neu auftretende Parasitose in Österreich. Klauentierpraxis 2011, 19, 5–6. [Google Scholar]
- Galuppi, R.; Militerno, G.; Bassi, P.; Nanni, A.; Testoni, S.; Tampieri, M.P.; Gentile, A. Evidence for bovine parafilariosis in Italy: First isolation of Parafilaria bovicola (Tubangui, 1934) from autochthonous cattle. Vet. Parasitol. 2012, 184, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Hamel, D.; Axt, H.; Pfister, K. First report on Parafilaria bovicola (Nematoda: Filaroidea) in Germany. Res. Vet. Sci. 2010, 89, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B 2003, 270 (Suppl. 1), S96–S99. [Google Scholar] [CrossRef] [Green Version]
- Eamsobhana, P.; Song, S.-L.; Yong, H.-S.; Prasartvit, A.; Boonyong, S.; Tungtrongchitr, A. Cytochrome c oxidase subunit I haplotype diversity of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae). Acta Trop. 2017, 171, 141–145. [Google Scholar] [CrossRef]
- Hodžić, A.; Alić, A.; Fuehrer, H.-P.; Harl, J.; Wille-Piazzai, W.; Duscher, G.G. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasites Vectors 2015, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Xie, Z. DAMBE: Software Package for Data Analysis in Molecular Biology and Evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef] [Green Version]
- ZAR. BMNT: Rinderrassenauswertung 2017; Rinderzucht Austria, ZAR: Vienna, Austria, 2018; Available online: https://www.zar.at/Aktuelles/Archiv/2018/20180424_BMNT--Rinderrassenauswertung-2017.html (accessed on 20 September 2021).
- Rostaher, A.; Mueller, R.S.; Majzoub, M.; Schares, G.; Gollnick, N.S. Bovine besnoitiosis in Germany. Vet. Dermatol. 2010, 21, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Basso, W.; Lesser, M.; Grimm, F.; Hilbe, M.; Sydler, T.; Trösch, L.; Ochs, H.; Braun, U.; Deplazes, P. Bovine besnoitiosis in Switzerland: Imported cases and local transmission. Vet. Parasitol. 2013, 198, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Oehm, A.W.; Stoll, A.; Silaghi, C.; Pfitzner-Friedrich, A.; Knubben-Schweizer, G.; Strube, C. Diagnosing bovine parafilariosis: Utility of the cytochrome c oxidase subunit 1 gene and internal transcribed spacer region for PCR detection of Parafilaria bovicola in skin biopsies and serohemorrhagic exudates of cattle. Parasites Vectors 2019, 12, 580. [Google Scholar] [CrossRef]
- Krafsur, E.S.; Moon, R.D. Bionomics of the face fly, Musca automnalis. Annu. Rev. Entomol. 1997, 42, 503–523. [Google Scholar] [CrossRef] [Green Version]
- Swan, G.E.; Soll, M.D.; Gross, S.J. Efficacy of ivermectin against Parafilaria bovicola and lesion resolution in cattle. Vet. Parasitol. 1991, 40, 267–272. [Google Scholar] [CrossRef]
- Viljoen, J.H.; Boomker, J.D. Studies on Parafilaria bovicola Tubangui, 1934, Chemotherapy and pathology. Onderstepoort J. Vet. Res. 1977, 44, 107–112. [Google Scholar] [PubMed]
- Soll, M.D.; Carmichael, I.H.; Barrick, R.A. Ivermectin treatment of feedlot cattle for Parafilaria bovicola. Prev. Vet. Med. 1991, 10, 251–256. [Google Scholar] [CrossRef]
- Spickler, A.R. Parafilariasis; The Center for Food Security and Public Health (CFSPH), Ed.; The Center for Food Security and Public Health (CFSPH): Ames, IA, USA, 2020; Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1098&context=cfsph_factsheets (accessed on 20 July 2021).
- Kretzmann, P.M.; Wallace, H.G.; Weaver, D.B. Manifestations of bovine parafilariasis. J. S. Afr. Vet. Assoc. 1984, 55, 127–129. [Google Scholar] [PubMed]
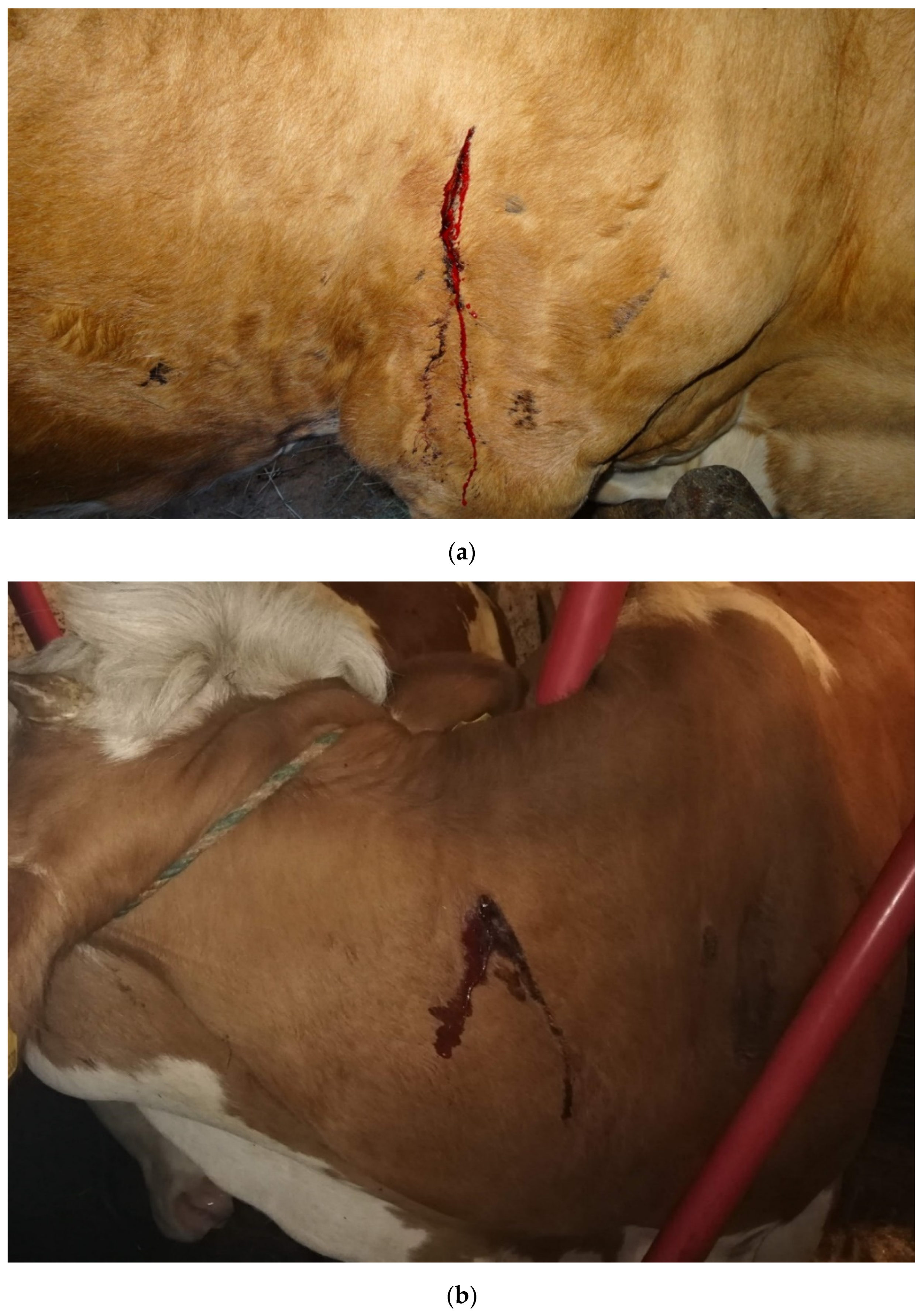
| History Question |
|---|
| What is the type of farm: dairy, suckler cow, or fattening? How many cattle are housed at the affected farm? How many cattle show the symptoms typical of parafilariosis (skin bleeding)? Did you observe any changes in behavior or a decrease in production in the affected cattle? If yes, please describe. Do the affected animals have access to pasture or an outdoor pen? Have you observed the symptoms in the past? If yes, since when? Are you using fly control on the farm? If yes, what do you use? Do you deworm the animals on your farm? If yes, what do you use? Have you submitted samples of the bleeding lesions? If yes, what was the result? |
| Federal State | Samples Subm. | Animals Subm. | Herds Subm. | Samples Positive | Animals Positive | Herds Positive | Haplotypes |
|---|---|---|---|---|---|---|---|
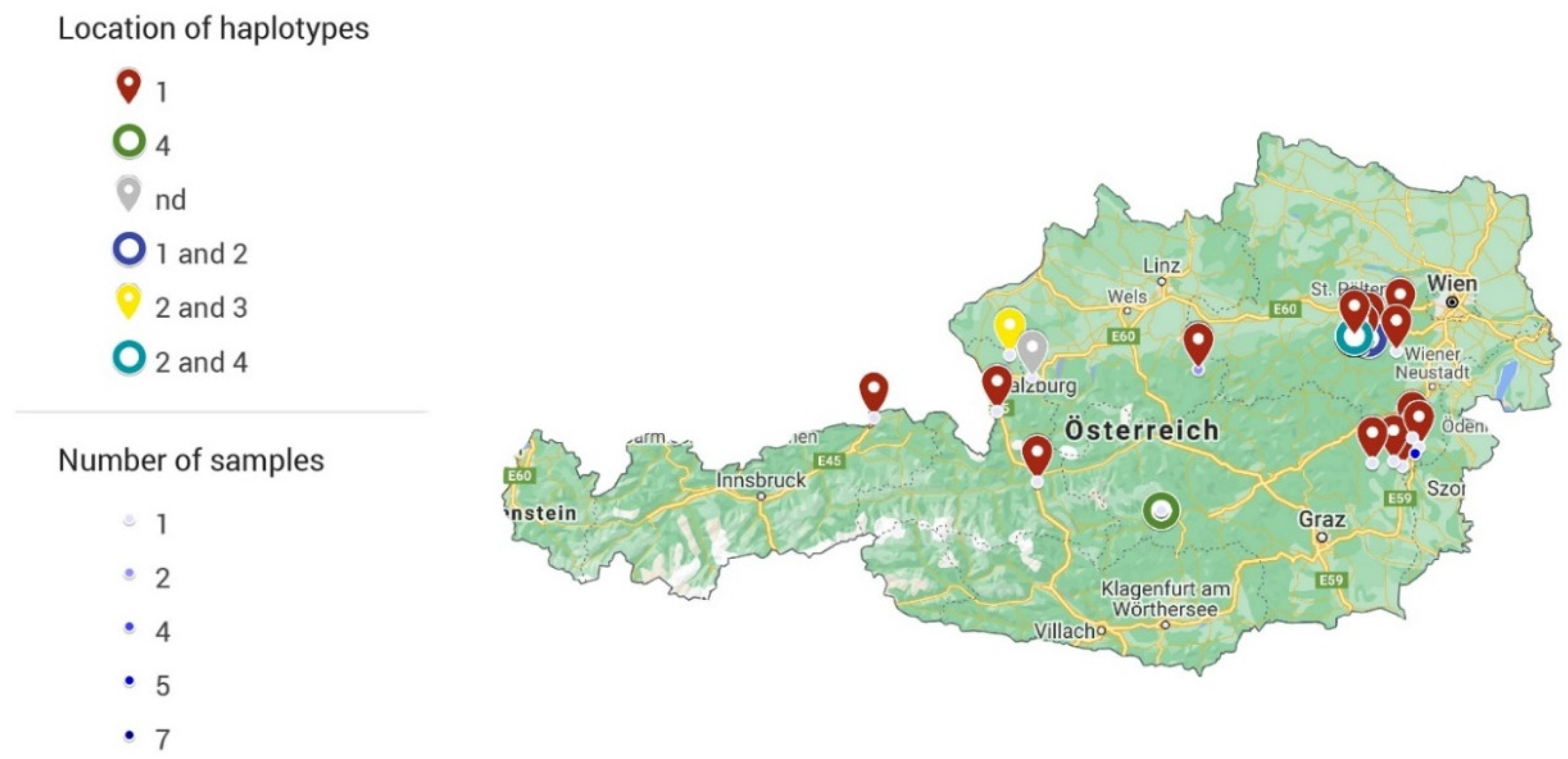
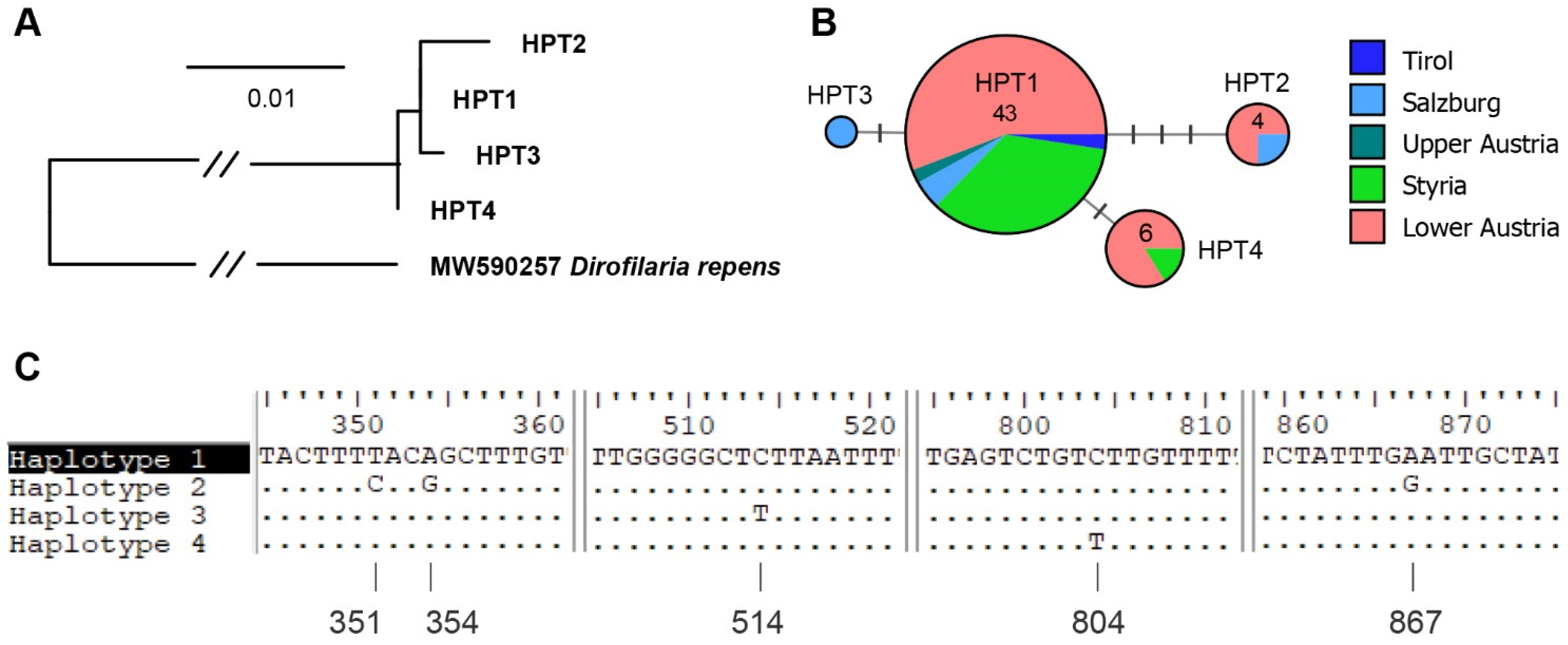
| Lower Austria | 34 | 23 | 9 | 32 | 22 | 8 | 1, 2, 4 |
| Styria | 23 | 15 | 13 | 18 | 12 | 11 | 1, 4 |
| Upper Austria | 11 | 8 | 7 | 2 | 2 | 2 | 1 |
| Salzburg | 13 | 11 | 7 | 4 | 3 | 3 | 1, 2, 3 |
| Carinthia | 3 | 3 | 3 | 0 | 0 | 0 | n.d. |
| Tyrol | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| Total | 86 | 62 | 41 | 57 | 40 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hund, A.; Reithofer, J.; Barogh, B.S.; Unterköfler, M.S.; Harl, J.; Fuehrer, H.-P. Emergence of Parafilaria bovicola in Austria. Animals 2021, 11, 2966. https://doi.org/10.3390/ani11102966
Hund A, Reithofer J, Barogh BS, Unterköfler MS, Harl J, Fuehrer H-P. Emergence of Parafilaria bovicola in Austria. Animals. 2021; 11(10):2966. https://doi.org/10.3390/ani11102966
Chicago/Turabian StyleHund, Alexandra, Johannes Reithofer, Bita Shahi Barogh, Maria Sophia Unterköfler, Josef Harl, and Hans-Peter Fuehrer. 2021. "Emergence of Parafilaria bovicola in Austria" Animals 11, no. 10: 2966. https://doi.org/10.3390/ani11102966
APA StyleHund, A., Reithofer, J., Barogh, B. S., Unterköfler, M. S., Harl, J., & Fuehrer, H.-P. (2021). Emergence of Parafilaria bovicola in Austria. Animals, 11(10), 2966. https://doi.org/10.3390/ani11102966








