Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
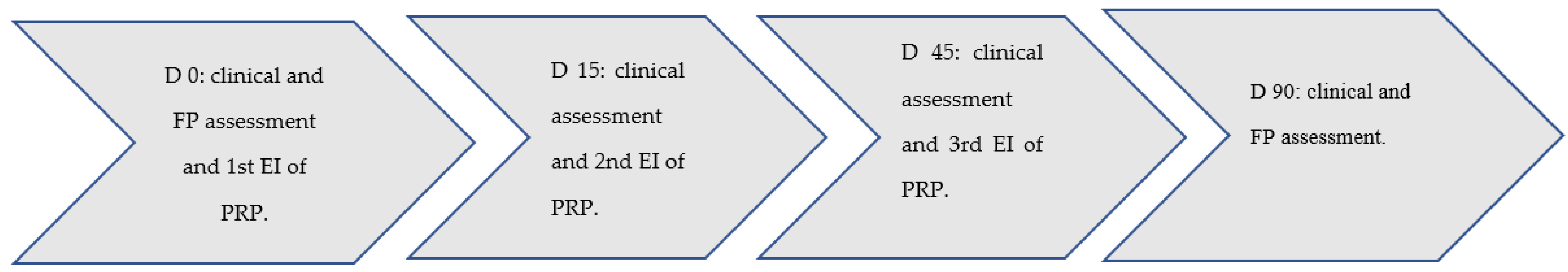
2. Materials and Methods
2.1. Clinical Assessment
2.2. Imaging Confirmation (X-ray and CT Scan)
2.3. FP Assessment
3. Results
3.1. Clinical Parameters
3.2. FP Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worth, A.; Meij, B.; Jeffery, N. Canine Degenerative Lumbosacral Stenosis: Prevalence, Impact And Management Strategies. Vet. Med. 2019, 10, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.A.; Lowrie, M.; Targett, M. Long-term outcome following lateral foraminotomy as treatment for canine degenerative lumbosacral stenosis. Veter-Rec. 2018, 183, 352. [Google Scholar] [CrossRef]
- De Decker, S.; Wawrzenski, L.A.; Volk, H.A. Clinical signs and outcome of dogs treated medically for degenerative lumbosacral stenosis: 98 cases (2004–2012). J. Am. Vet. Med. Assoc. 2014, 4, 408–413. [Google Scholar] [CrossRef]
- Hankin, E.J.; Jerram, R.M.; Walker, A.M.; King, M.D.; Warman, C.G.A. Transarticular Facet Screw Stabilization and Dorsal Laminectomy in 26 Dogs with Degenerative Lumbosacral Stenosis with Instability. Veter-Surg. 2012, 41, 611–619. [Google Scholar] [CrossRef]
- Golini, L.; Kircher, P.R.; Lewis, F.I.; Steffen, F. Transarticular Fixation With Cortical Screws Combined With Dorsal Laminectomy and Partial Discectomy as Surgical Treatment of Degenerative Lumbosacral Stenosis in 17 Dogs: Clinical and Computed Tomography Follow-Up. Veter-Surg. 2014, 43, 405–413. [Google Scholar] [CrossRef]
- Janssens, L.; Beosier, Y.; Daems, R. Lumbosacral degenerative stenosis in the dog. The results of epidural infiltration with methylprednisolone acetate: A retrospective study. Vet. Comp. Orthop. Traumatol. 2009, 22, 486–491. [Google Scholar]
- Gomes, S.; Lowrie, M.; Targett, M. Single dose epidural methylprednisolone as a treatment and predictor of outcome following subsequent decompressive surgery in degenerative lumbosacral stenosis with foraminal stenosis. Veter-J. 2020, 257, 105451. [Google Scholar] [CrossRef]
- Willems, N.; Mihov, G.; Grinwis, G.C.; van Dijk, M.; Schumann, D.; Bos, C.; Strijkers, G.J.; Dhert, W.J.; Meij, B.P.; Creemers, L.B.; et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Liotta, A.P.; Girod, M.; Peeters, D.; Sandersen, C.; Couvreur, T.; Bolen, G. Clinical effects of computed tomography-guided lumbosacral facet joint, transforaminal epidural, and translaminar epidural injections of methylprednisolone acetate in healthy dogs. Am. J. Vet. Res. 2016, 77, 1132–1139. [Google Scholar] [CrossRef]
- Salmelin, B.; Fitzpatrick, N.; Rose, J.; Driver, C. Safety profile of methylprednisolone acetate epidural injection in dogs treated for lumbosacral disease. In BSAVA Congress Proceedings 2019; British Small Animal Veterinary Association: Birmingham, UK, 2019; p. 546. [Google Scholar]
- Ackerman, W.E.; Ahmad, M. The Efficacy of Lumbar Epidural Steroid Injections in Patients with Lumbar Disc Herniations. Anesth. Analg. 2007, 104, 1217–1222. [Google Scholar] [CrossRef]
- Stolke, D.; Sollmann, W.-P.; Seifert, V. Intra- and Postoperative Complications in Lumbar Disc Surgery. Spine 1989, 14, 56–59. [Google Scholar] [CrossRef]
- McLain, R.F.; Kapural, L.; Mekhail, N.A. Epidural steroid therapy for back and leg pain: Mechanisms of action and efficacy. Spine J. 2005, 5, 191–201. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; de la Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Ahmad, Z.; Howard, D.; Brooks, A.R.; Wardale, J.; Henson, F.; Getgood, A.; Rushton, N. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Ramadan, E.S.; Khattab, M.S.; Salem, N.Y.; Emam, I.A. Corneal Ulcer in Dogs and Cats: Novel Clinical Application of Regenerative Therapy Using Subconjunctival Injection of Autologous Platelet-Rich Plasma. Front. Veter-Sci. 2021, 8, 641265. [Google Scholar] [CrossRef]
- Okamoto-Okubo, C.E.; Cassu, R.N.; Joaquim, J.G.F.; Mesquita, L.D.R.; Rahal, S.C.; Oliveira, H.S.S.; Takahira, R.; Arruda, I.; Maia, L.; Landim, F.D.C.; et al. Chronic pain and gait analysis in dogs with degenerative hip joint disease treated with repeated intra-articular injections of platelet-rich plasma or allogeneic adipose-derived stem cells. J. Veter-Med Sci. 2021, 83, 881–888. [Google Scholar] [CrossRef]
- López, S.; Vilar, J.M.; Sopena, J.J.; Damià, E.; Chicharro, D.; Carrillo, J.M.; Cuervo, B.; Rubio, M.; Rubio, A.M. Assessment of the Efficacy of Platelet-Rich Plasma in the Treatment of Traumatic Canine Fractures. Int. J. Mol. Sci. 2019, 20, 1075. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, G.; Robb, S.; Budsberg, S.; Volstad, N. The evaluation of limb symmetry indices using ground reaction forces collected with one or two force plates in healthy dogs. Veter-Comp. Orthop. Traumatol. 2017, 30, 54–58. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef]
- Vilar, J.M.; Rubio, M.; Carrillo, J.M.; Domínguez, A.M.; Mitat, A.; Batista, M. Biomechanic characteristics of gait of four breeds of dogs with different conformations at walk on a treadmill. J. Appl. Anim. Res. 2015, 44, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Ness, M.G. Degenerative lumbosacral stenosis in the dog: A review of 30 cases. J. Small Anim. Pr. 1994, 35, 185–190. [Google Scholar] [CrossRef]
- Martínez-Martínez, A.; Ruiz-Santiago, F.; García-Espinosa, J. Plasma rico en plaquetas: ¿mito o realidad? Radiología 2018, 60, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Willems, N.; Tellegen, A.R.; Bergknut, N.; Creemers, L.B.; Wolfswinkel, J.; Freudigmann, C.; Benz, K.; Grinwis, G.; Tryfonidou, M.A.; Meij, B.P. Inflammatory profiles in canine intervertebral disc degeneration. BMC Veter. Res. 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Risio, L.; Sharp, N.J.; Olby, N.J.; Muñana, K.R.; Thomas, W.B. Predictors of outcome after dorsal decompressive laminectomy for degenerative lumbosacral stenosis in dogs: 69 cases (1987–1997). J. Am. Vet. Med. Assoc. 2001, 5, 624–628. [Google Scholar] [CrossRef]
- Linn, L.L.; Bartels, K.E.; Rochat, M.C.; Payton, M.E.; Moore, G.E. Lumbosacral stenosis in 29 military working dogs: Epidemiologic findings and outcome after surgical intervention (1990–1999). Veter-Surg. 2003, 32, 21–29. [Google Scholar] [CrossRef]
- Smolders, L.A.; Voorhout, G.; Van De Ven, R.; Bergknut, N.; Grinwis, G.; Hazewinkel, H.A.W.; Meij, B.P. Pedicle Screw-Rod Fixation of the Canine Lumbosacral Junction. Veter-Surg. 2012, 41, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Tohidnezhad, M.; Bayer, A.; Rasuo, B.; Hock, J.V.P.; Kweider, N.; Fragoulis, A.; Sönmez, T.T.; Jahr, H.; Pufe, T.; Lippross, S. Platelet-Released Growth Factors Modulate the Secretion of Cytokines in Synoviocytes under Inflammatory Joint Disease. Mediat. Inflamm. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Singla, V.; Batra, Y.K.; Bharti, N.; Goni, V.G.; Marwaha, N. Steroid vs. Platelet-Rich Plasma in Ultrasound-Guided Sacroiliac Joint Injection for Chronic Low Back Pain. Pain Pr. 2016, 17, 782–791. [Google Scholar] [CrossRef]
- Wu, J.; Du, Z.; Lv, Y.; Zhang, J.; Xiong, W.; Wang, R.; Liu, R.; Zhang, G.; Liu, Q. A New Technique for the Treatment of Lumbar Facet Joint Syndrome Using Intra-articular Injection with Autologous Platelet Rich Plasma. Pain Physician 2016, 19, 617–625. [Google Scholar]
- Yung, Y.-L.; Fu, S.C.; Cheuk, Y.C.; Qin, L.; Ong, M.T.Y.; Chan, K.-M.; Yung, P.S.-H. Optimisation of platelet concentrates therapy: Composition, localisation, and duration of action. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 7, 27–36. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G. The importance of understanding what is platelet-rich growth factor (PRGF) and what is not. J. Shoulder Elb. Surg. 2011, 20, e23–e24. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.E.A.; Lee, D.V.; Case, H.N.; Todhunter, R.J. Comparison of the trotting gaits of Labrador Retrievers and Greyhounds. Am. J. Veter-Res. 2000, 61, 832–838. [Google Scholar] [CrossRef]
- Alexander, R.M.c.N.; Jayes, S.A. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 1983, 201, 135–152. [Google Scholar] [CrossRef]
- Hof, A.L. Scaling gait data to body size. Gait Posture 1996, 4, 222–223. [Google Scholar] [CrossRef]
- Budsberg, S.C.; Jevens, D.J.; Brown, J.; Foutz, T.L.; De Camp, E.C.; Reece, L. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am. J. Veter-Res. 1993, 54, 1569–1574. [Google Scholar]
- Bockstahler, B.A.; Vobornik, A.; Müller, M.; Peham, C. Compensatory load redistribution in naturally occurring osteoarthritis of the elbow joint and induced weight-bearing lameness of the forelimbs compared with clinically sound dogs. Veter-J. 2009, 180, 202–212. [Google Scholar] [CrossRef]
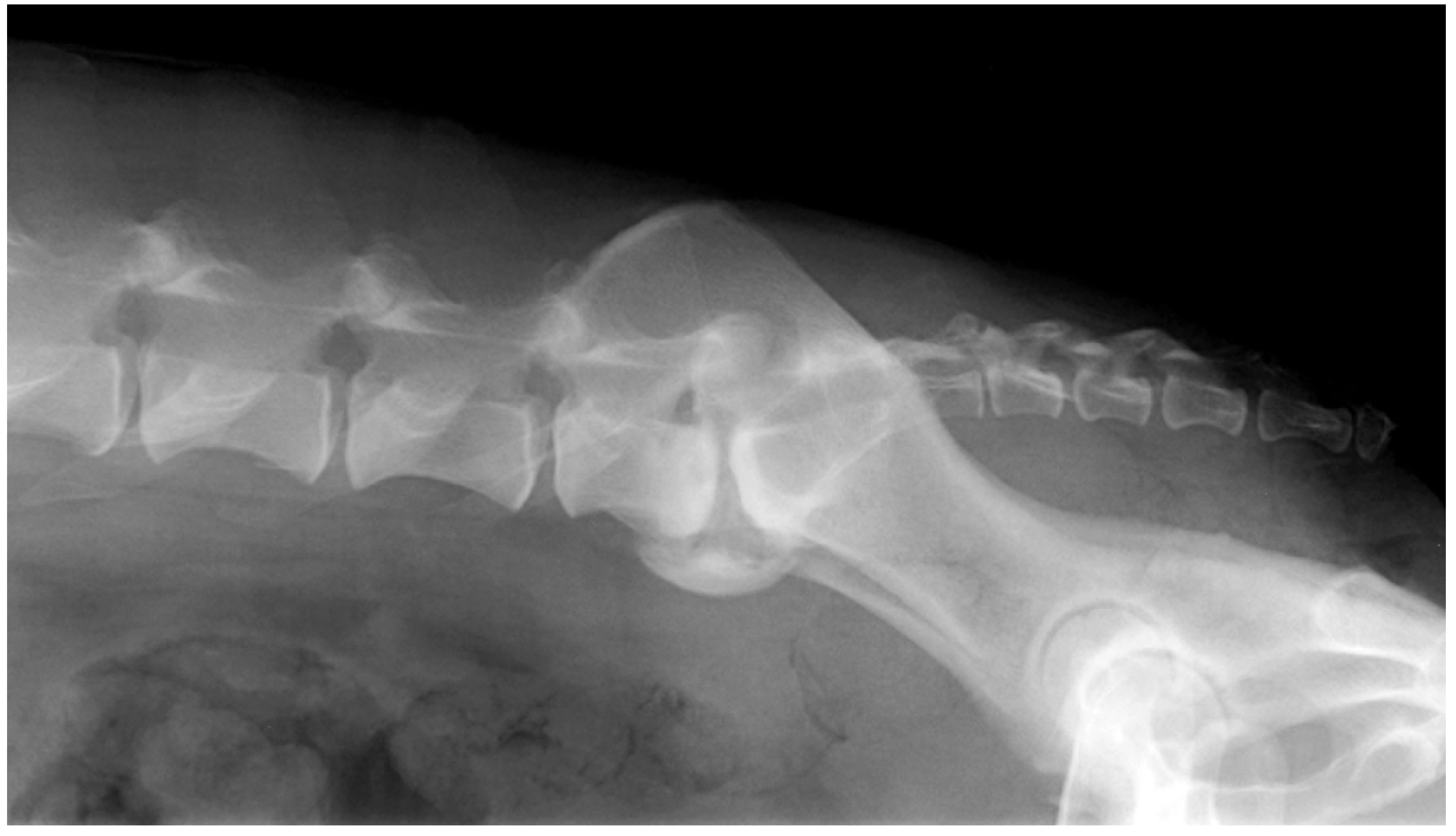
| Hypertrophy of the ligaments stabilizing the LS junction (dorsal longitudinal ligament ventrally and ligamentum flavum dorsally). |
| Degeneration of the lumbosacral disc, with protrusion of the disc annulus. |
| Degenerative joint disease of the articular processes, with modification of the shape of the articular surface, periarticular new bone formation and hypertrophy of the joint capsule. |
| Lateral spondylosis deformans at the lumbosacral junction and sacroiliac joint, which can impinge into the exit zone of the L7-S1 intervertebral foramen and compress the L7 intervertebral neurovascular bundle. |
| Dynamic compression of the cauda equina caused by the ventral displacement of the sacrum in relation to L7 (step lesion = retrolisthesis). |
| Dynamic narrowing of the L7-S1 lateral intervertebral foramen during extension of the lumbosacral joint (telescoping). |
| Congenital stenosis of the vertebral canal at the LS junction. |
| Transitional vertebral anomaly. |
| Osteochondrosis-like lesion of L7 or S1. |
| Item | D0 | D15 | D45 | D90 |
|---|---|---|---|---|
| Paresis/and weakness of the pelvic limbs | 12 | 2 | 2 | 0 |
| Lumbosacral pain /Hyperesthesia | ||||
| Dorsal digital pressure | 14 | 12 | 2 | 2 |
| Lifting the tail | 6 | 6 | 4 | 2 |
| Extending hips | 2 | 2 | 0 | 0 |
| Urinary incontinence | 4 | 1 | 2 | 2 |
| Hind leg withdrawal reflex | 8 | 8 | 4 | 0 |
| Proprioceptive deficit | 6 | 6 | 4 | 0 |
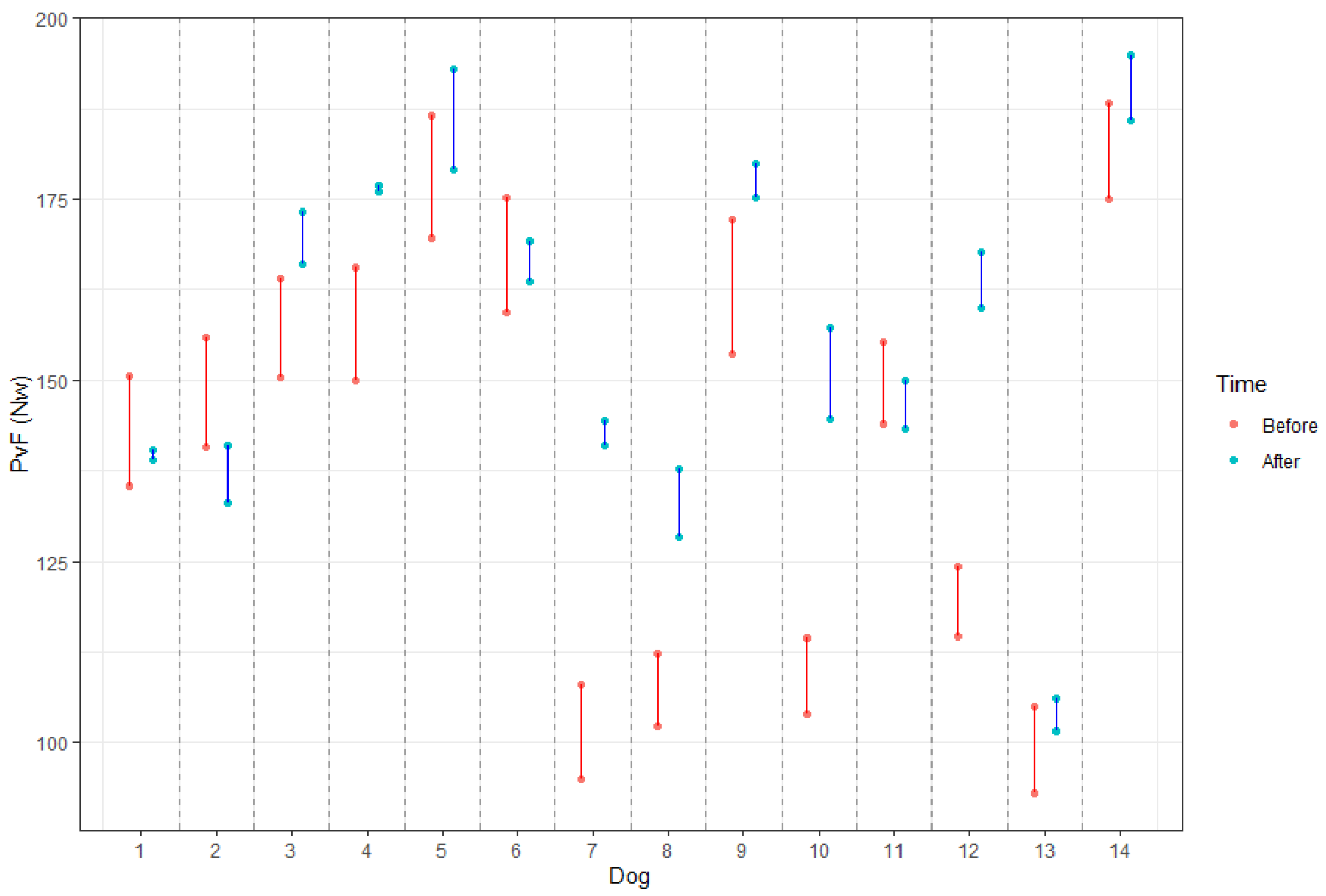
| Dog ID | Pathology | SI before | SI after | Difference |
|---|---|---|---|---|
| 1 | DP + FH + FV | 10.72 | 0.95 | 9.77 |
| 2 | DP + FS | 8.09 | 4.68 | 3.41 |
| 3 | TV | 12.12 | 4.17 | 7.95 |
| 4 | DP | 7.34 | 4.72 | 2.62 |
| 5 | DP + FH + TV | 10.34 | 5.84 | 4.50 |
| 6 | DP + FS | 8.70 | 4.32 | 4.37 |
| 7 | DP + FS | 9.93 | 0.57 | 9.36 |
| 8 | DP | 9.54 | 7.53 | 2.01 |
| 9 | TV | 9.56 | 3.40 | 6.16 |
| 10 | DP + FH + TV | 12.81 | 2.34 | 10.47 |
| 11 | DP | 9.32 | 7.02 | 2.30 |
| 12 | DP + FH + TV | 11.45 | 2.63 | 8.83 |
| 13 | DP | 9.47 | 8.39 | 1.08 |
| 14 | DP + SF | 7.57 | 4.55 | 3.03 |
| Mean | 9.78 | 4.36 | 5.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Guerra, Á.M.; Carrillo, J.M.; Sopena, J.J.; Vilar, J.M.; Peláez, P.; Cuervo, B.; Santana, A.; Rubio, M. Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs. Animals 2021, 11, 2965. https://doi.org/10.3390/ani11102965
Hernández-Guerra ÁM, Carrillo JM, Sopena JJ, Vilar JM, Peláez P, Cuervo B, Santana A, Rubio M. Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs. Animals. 2021; 11(10):2965. https://doi.org/10.3390/ani11102965
Chicago/Turabian StyleHernández-Guerra, Ángel María, José María Carrillo, Joaquín Jesús Sopena, José Manuel Vilar, Pau Peláez, Belén Cuervo, Angelo Santana, and Mónica Rubio. 2021. "Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs" Animals 11, no. 10: 2965. https://doi.org/10.3390/ani11102965
APA StyleHernández-Guerra, Á. M., Carrillo, J. M., Sopena, J. J., Vilar, J. M., Peláez, P., Cuervo, B., Santana, A., & Rubio, M. (2021). Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs. Animals, 11(10), 2965. https://doi.org/10.3390/ani11102965






