Engrafting Horse Immune Cells into Mouse Hosts for the Study of the Acute Equine Immune Responses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Peripheral Blood Mononuclear Cell Engraftment
2.3. Bone Marrow Engraftment
2.4. Assessment of Engraftment
2.5. Cell Culture
2.6. Statistics
3. Results
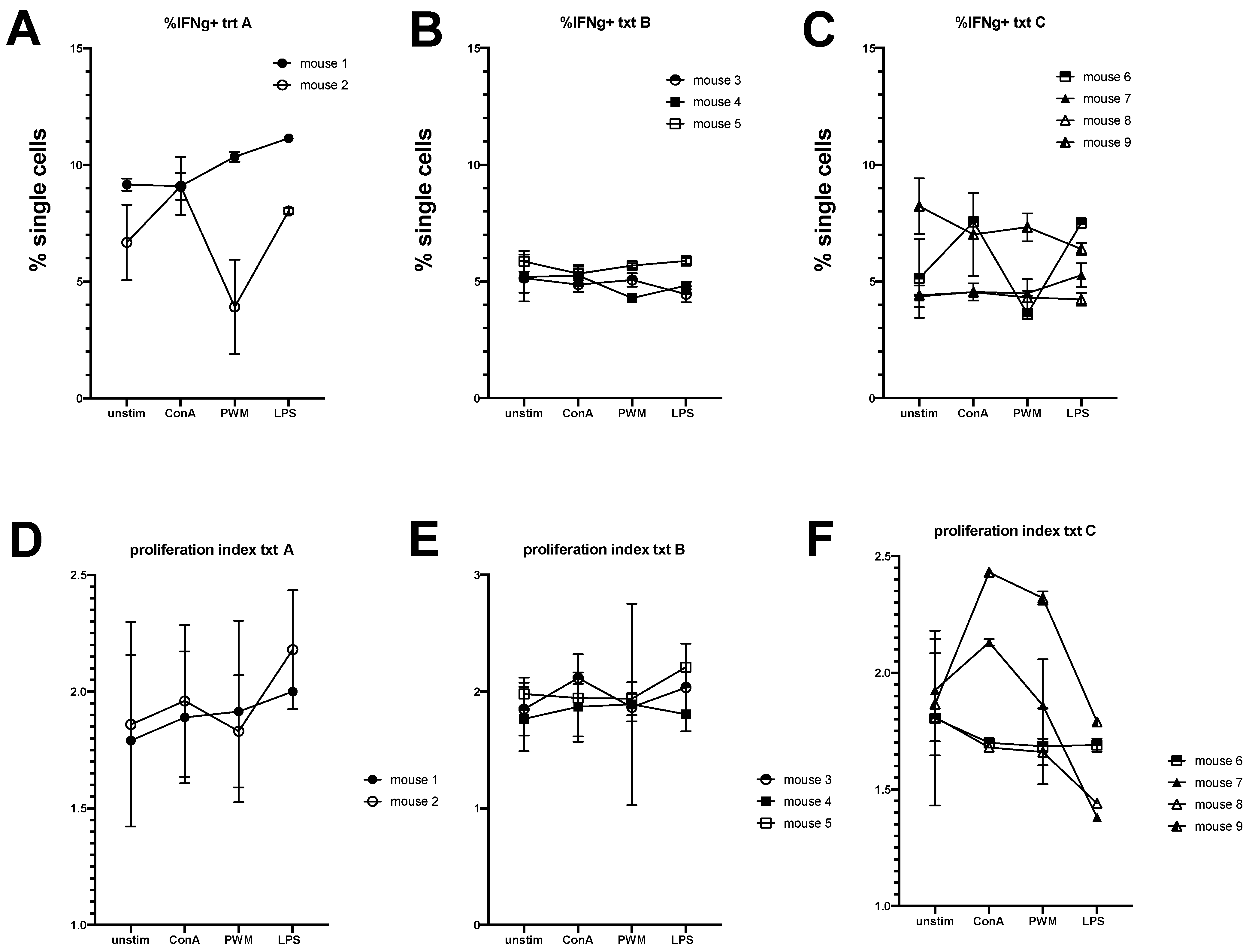
3.1. Equine Peripheral Blood Leukocytes Persist in NSG Recipient Mice up to 4 Weeks Post-Transfer
3.2. Engraftment of Equine Bone Marrow into NSG Mice Is Variable Depending on Transplantation Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foote, J.B.; Kabir, F.M.L.; Graff, E.C.; Cattley, R.C.; DeInnocentes, P.; Smith, B.F.; Bird, R.C. Engraftment of canine peripheral blood lymphocytes into nonobese diabetic-severe combined immune deficient IL-2R common gamma chain null mice. Vet. Immunol. Immunopathol. 2014, 157, 131–141. [Google Scholar] [CrossRef]
- Greenwood, J.D.; Croy, B.A. A study on the engraftment and trafficking of bovine peripheral blood leukocytes in severe combined immunodeficient mice. Vet. Immunol. Immunopathol. 1993, 38, 21–44. [Google Scholar] [CrossRef]
- Greenwood, J.D.; Croy, B.A.; Trout, D.R.; Wilcock, B.P. Xenogeneic (bovine) peripheral blood leukocytes engrafted into severe combined immunodeficient mice retain primary immune function. Vet. Immunol. Immunopathol. 1997, 59, 93–112. [Google Scholar] [CrossRef]
- Petznek, H.; Kleiter, M.; Tichy, A.; Fuchs-Baumgartinger, A.; Hohenadl, C. Murine xenograft model demonstrates significant radio-sensitising effect of liposomal doxorubicin in a combination therapy for Feline Injection Site Sarcoma. Res. Vet. Sci. 2014, 97, 386–390. [Google Scholar] [CrossRef]
- Balson, G.A.; Croy, B.A.; Ross, T.L.; Yager, J.A. Demonstration of equine immunoglobulin in sera from severe combined immunodeficiency/beige mice inoculated with equine lymphocytes. Vet. Immunol. Immunopathol. 1993, 39, 315–325. [Google Scholar] [CrossRef]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Laboratory, T.J. Frequently Asked NSG Questions. Available online: https://www.jax.org/jax-mice-and-services/find-and-order-jax-mice/nsg-portfolio/frequently-asked-nsg-questions (accessed on 30 August 2021).
- Watanabe, S.; Terashima, K.; Ohta, S.; Horibata, S.; Yajima, M.; Shiozawa, Y.; Dewan, M.Z.; Yu, Z.; Ito, M.; Yamamoto, N.; et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 2007, 109, 212–228. [Google Scholar] [CrossRef]
- Akkina, R.; Berges, B.K.; Palmer, B.E.; Remling, L.; Neff, C.P.; Kuruvilla, J.; Connick, E.; Folkvord, J.; Gagliardi, K.; Kassu, A.; et al. Humanized Rag1−/− gammac−/− mice support multilineage hematopoiesis and are susceptible to HIV-1 infection via systemic and vaginal routes. PLoS ONE 2011, 6, e20169. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.F.; Pek, E.A.; Chenoweth, M.J.; Coombes, B.K.; Ashkar, A.A. Humanized mice for Salmonella typhi infection: New tools for an old problem. Virulence 2011, 2, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Libby, S.J.; Brehm, M.A.; Greiner, D.L.; Shultz, L.D.; McClelland, M.; Smith, K.D.; Cookson, B.T.; Karlinsey, J.E.; Traci, L.; Porwollik, K.S.; et al. Humaniz. Nonobese diabet. -scid il2rgammanull mice are susceptible lethal salmonella typhi infect. Proc. Natl. Acad. Sci. USA 2010, 107, 15589–15594. [Google Scholar] [CrossRef]
- Dorner, M.; Rice, C.M.; Ploss, A. Study of hepatitis C virus entry in genetically humanized mice. Methods 2013, 59, 249–257. [Google Scholar] [CrossRef][Green Version]
- Brehm, M.A.; Powers, A.C.; Shultz, L.D.; Greiner, D.L. Advancing animal models of human type 1 diabetes by engraftment of functional human tissues in immunodeficient mice. Cold Spring Harb. Perspect. Med. 2012, 2, a007757. [Google Scholar] [CrossRef][Green Version]
- Marx, J.O.; Vudathala, D.; Murphy, L.; Rankin, S.; Hankenson, F.C. Antibiotic administration in the drinking water of mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 301–306. [Google Scholar]
- Leeth, C.M.; Racine, J.; Chapman, H.D.; Arpa, B.; Carrillo, J.; Carrascal, J.; Wang, Q.; Ratiu, J.; Mendikute, L.E.; Rosell-Mases, E.; et al. B-lymphocytes expressing an Ig specificity recognizing the pancreatic ss-cell autoantigen peripherin are potent contributors to type 1 diabetes development in NOD mice. Diabetes 2016, 65, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Institute of Laboratory Animal Resources (U.S.). Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. In NIH Publication; U.S. Department of Health and Human Services, Public Health Service: Bethesda, MD, USA; Washington, DC, USA, 1986. [Google Scholar]
- Zhu, J.; Hay, A.N.; Potter, A.A.; Richwine, M.W.; Sproule, T.; LeRoith, T.; Wilson, J.; Hasham, M.G.; Roopenian, D.C.; Leeth, C.M. Abrogated aid function prolongs survival and diminishes renal pathology in the bxsb mouse model of systemic lupus erythematosus. J. Immunol. 2020, 204, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, J.J.; Racine, J.J.; Hasham, M.G.; Wang, Q.; Branca, J.A.; Chapman, H.D.; Zhu, J.; Donghia, N.; Philip, V.; Schott, W.H.; et al. Genetic and small molecule disruption of the aid/rad51 axis similarly protects nonobese diabetic mice from type 1 diabetes through expansion of regulatory b lymphocytes. J. Immunol. 2017, 198, 4255–4267. [Google Scholar] [CrossRef]
- Korngold, R.; Sprent, J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J. Exp. Med. 1978, 148, 1687–1698. [Google Scholar] [CrossRef]
- Morgan, R.A. Human tumor xenografts: The good, the bad, and the ugly. Mol. Ther. 2012, 20, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.L.; Chang, Y.C.; Huang, Y.T.; Liao, J.W.; Kao, P.L.; Chen, Y.F.; Lin, B.-Y.; Lin, Y.-L.; Chen, T.-H.; Wang, Y.-Y. Establishment and characterization of feline mammary tumor patient-derived xenograft model. Animals 2021, 11, 2380. [Google Scholar] [CrossRef]
- Koga, Y.; Ochiai, A. Systematic review of patient-derived xenograft models for preclinical studies of anti-cancer drugs in solid tumors. Cells 2019, 8, 418. [Google Scholar] [CrossRef]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.N.; Chen, M.; Modiano, J.F.; et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef]
- Pearson, T.; Greiner, D.L.; Shultz, L.D. Creation of “humanized” mice to study human immunity. Curr. Protoc. Immunol. 2008, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.; Chou, F.S.; Sexton, C.; Presicce, P.; Chougnet, C.A.; Aliberti, J.; Mulloy, J.C. Improved multilineage human hematopoietic reconstitution and function in NSGS mice. PLoS ONE 2018, 13, e0209034. [Google Scholar]
- Collymore, C.; Giuliano, F.; Banks, E.K. Head tilt in immunodeficient mice due to contamination of drinking water by burkholderia gladioli. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Champlin, R. T-cell depletion to prevent graft-versus-host disease after bone marrow transplantation. Hematol. Oncol. Clin. N. Am. 1990, 4, 687–698. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leeth, C.; Adkins, J.; Hay, A.; Bogers, S.; Potter, A.; Witonsky, S.; Zhu, J. Engrafting Horse Immune Cells into Mouse Hosts for the Study of the Acute Equine Immune Responses. Animals 2021, 11, 2962. https://doi.org/10.3390/ani11102962
Leeth C, Adkins J, Hay A, Bogers S, Potter A, Witonsky S, Zhu J. Engrafting Horse Immune Cells into Mouse Hosts for the Study of the Acute Equine Immune Responses. Animals. 2021; 11(10):2962. https://doi.org/10.3390/ani11102962
Chicago/Turabian StyleLeeth, Caroline, Janie Adkins, Alayna Hay, Sophie Bogers, Ashley Potter, Sharon Witonsky, and Jing Zhu. 2021. "Engrafting Horse Immune Cells into Mouse Hosts for the Study of the Acute Equine Immune Responses" Animals 11, no. 10: 2962. https://doi.org/10.3390/ani11102962
APA StyleLeeth, C., Adkins, J., Hay, A., Bogers, S., Potter, A., Witonsky, S., & Zhu, J. (2021). Engrafting Horse Immune Cells into Mouse Hosts for the Study of the Acute Equine Immune Responses. Animals, 11(10), 2962. https://doi.org/10.3390/ani11102962





