Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Tissue Collection and RNA Extraction
2.3. Real Time RT-qPCR
2.4. Statistical Analysis
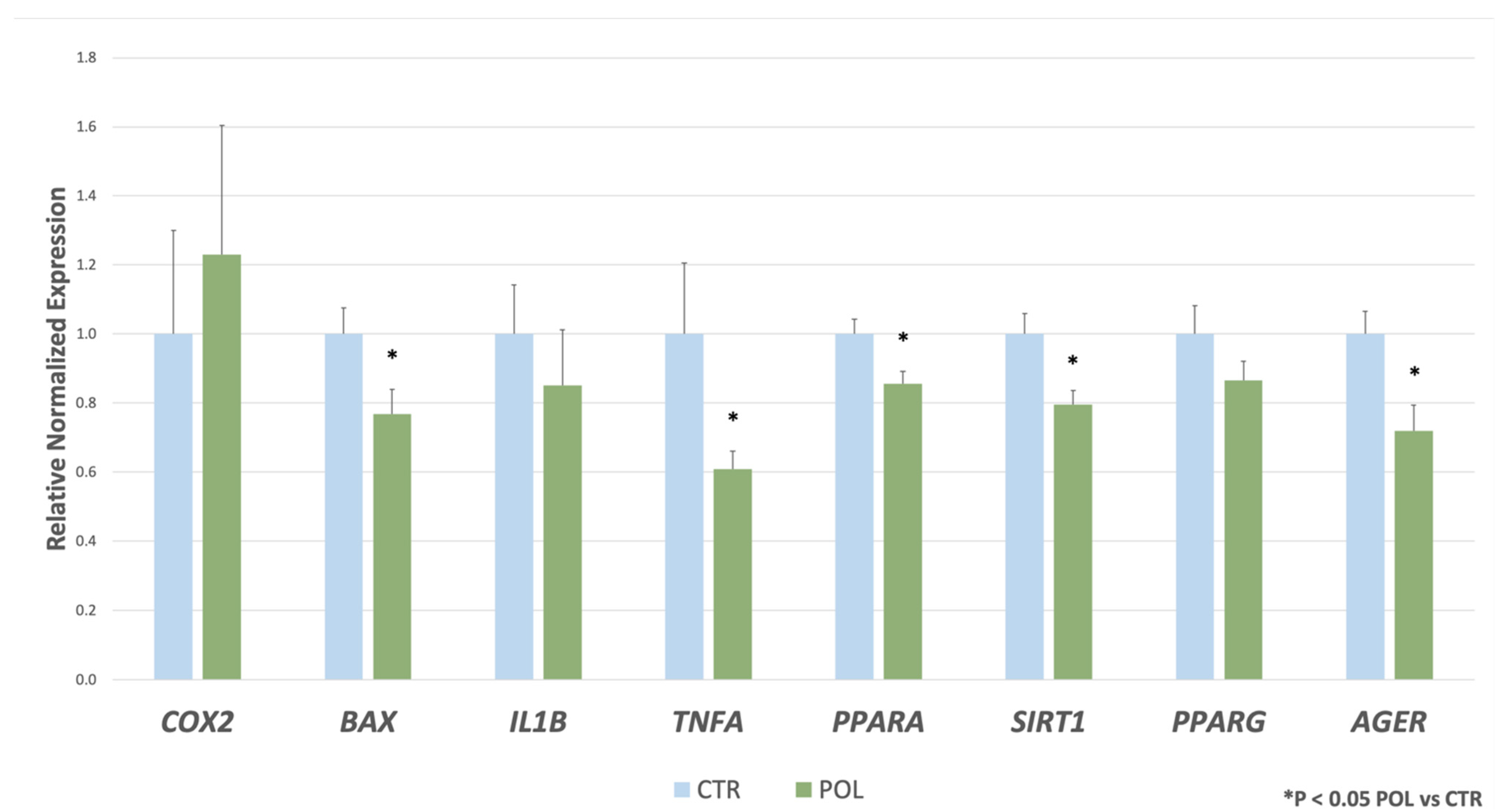
3. Results
Real Time RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- San Martin, D.; Ramos, S.; Zufía, J. Valorisation of Food Waste to Produce New Raw Materials for Animal Feed. Food Chem. 2016, 198, 68–74. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitliagka, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Attia, Y.A. Effect of Citric Acid on the Utilization of Olive Cake Diets for Laying Hens. Ital. J. Anim. Sci. 2015, 14, 3966. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Attia, Y.A. Effect of Citric Acid on the Nutritive Value of Olive Cake in Broiler Diets. Eur. Poult. Sci. 2016, 80. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic Compounds Obtained from Olea Europaea By-Products and Their Use to Improve the Quality and Shelf Life of Meat and Meat Products—A Review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Galarini, R.; Trabalza-Marinucci, M.; Miraglia, D.; Roila, R.; Acuti, G.; Giusepponi, D.; Dal Bosco, A.; Ranucci, D. Effects of Olive Mill Vegetation Water Phenol Metabolites Transferred to Muscle through Animal Diet on Rabbit Meat Microbial Quality. Sustainability 2021, 13, 4522. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative Status and Presence of Bioactive Compounds in Meat from Chickens Fed Polyphenols Extracted from Olive Oil Industry Waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef]
- Sabino, M.; Carmelo, V.A.O.; Mazzoni, G.; Cappelli, K.; Capomaccio, S.; Ajmone-Marsan, P.; Verini-Supplizi, A.; Trabalza-Marinucci, M.; Kadarmideen, H.N. Gene Co-Expression Networks in Liver and Muscle Transcriptome Reveal Sex-Specific Gene Expression in Lambs Fed with a Mix of Essential Oils. BMC Genom. 2018, 19, 236. [Google Scholar] [CrossRef]
- Trocino, A.; Cotozzolo, E.; Zomeño, C.; Petracci, M.; Xiccato, G.; Castellini, C. Rabbit Production and Science: The World and Italian Scenarios from 1998 to 2018. Ital. J. Anim. Sci. 2019, 18, 1361–1371. [Google Scholar] [CrossRef]
- General Directory Commettee on Health and Food Safey. Commercial Rabbit Farming in the European Union: Overview Report; Ufficio delle Pubblicazioni dell’Unione Europea: Luxembourg, 2017; ISBN 978-92-79-43540-9. Available online: https://op.europa.eu/it/publication-detail/-/publication/5029d977-387c-11e8-b5fe-01aa75ed71a1 (accessed on 8 October 2021).
- Abdel-Khalek, A.M. Supplemental Antioxidants in Rabbit Nutrition: A Review. Livest. Sci. 2013, 158, 95–105. [Google Scholar] [CrossRef]
- El-Ratel, I.T.; Wafa, W.M.; El-Nagar, H.A.; Aboelmagd, A.M.; El-Kholy, K.H. Amelioration of Sperm Fertilizability, Thyroid Activity, Oxidative Stress, and Inflammatory Cytokines in Rabbit Bucks Treated with Phytogenic Extracts. Anim. Sci. J. 2021, 92, e13560. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Bosco, A.D.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary Fish Oil and Flaxseed for Rabbit Does: Fatty Acids Distribution and Δ6-Desaturase Enzyme Expression of Different Tissues. Animal 2019, 13, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Castellini, C.; Dall’Aglio, C.; Petrucci, L.; Mattioli, S.; Boiti, C.; Zerani, M. Effects of PUFAs on Animal Reproduction: Male and Female Performances and Endocrine Mechanisms. Phytochem. Rev. 2018, 17, 801–814. [Google Scholar] [CrossRef]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Mechanisms of Action of Phenolic Compounds in Olive. J. Diet. Suppl. 2012, 9, 96–109. [Google Scholar] [CrossRef]
- Owczarek, K.; Lewandowska, U. The Impact of Dietary Polyphenols on COX-2 Expression in Colorectal Cancer. Nutr. Cancer 2017, 69, 1105–1118. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kang, J.-S.; Park, J.H.Y.; Lee, Y.-J.; Choi, J.-S.; Kang, Y.-H. Polyphenolic Flavonoids Differ in Their Antiapoptotic Efficacy in Hydrogen Peroxide-Treated Human Vascular Endothelial Cells. J. Nutr. 2003, 133, 985–991. [Google Scholar] [CrossRef]
- King, R.E.; Kent, K.D.; Bomser, J.A. Resveratrol Reduces Oxidation and Proliferation of Human Retinal Pigment Epithelial Cells via Extracellular Signal-Regulated Kinase Inhibition. Chem. Biol. Interact. 2005, 151, 143–149. [Google Scholar] [CrossRef]
- Dudley, J.; Das, S.; Mukherjee, S.; Das, D.K. Resveratrol, a Unique Phytoalexin Present in Red Wine, Delivers Either Survival Signal or Death Signal to the Ischemic Myocardium Depending on Dose. J. Nutr. Biochem. 2009, 20, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Thichanpiang, P.; Wongprasert, K. Green Tea Polyphenol Epigallocatechin-3-Gallate Attenuates TNF-α-Induced Intercellular Adhesion Molecule-1 Expression and Monocyte Adhesion to Retinal Pigment Epithelial Cells. Am. J. Chin. Med. 2015, 43, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, J.; Park, H.; Kim, J. Investigating the Role of Sirtuins in Cell Reprogramming. BMB Rep. 2018, 51, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. Current Role of Mammalian Sirtuins in DNA Repair. DNA Repair 2019, 80, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.L.C.; Ramasamy, T.S. Role of Sirtuin1-P53 Regulatory Axis in Aging, Cancer and Cellular Reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.-G.; Yang, Y. Sirtuins in Glucose and Lipid Metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol Protects from Aging Process via AMPK and Autophagy; a Review of Its Effects on Cancer, Metabolic Syndrome, Osteoporosis, Immune-Mediated and Neurodegenerative Diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Alsharif, K.F.; Almalki, A.A.; Al-Amer, O.; Mufti, A.H.; Theyab, A.; Lokman, M.S.; Ramadan, S.S.; Almeer, R.S.; Hafez, M.M.; Kassab, R.B.; et al. Oleuropein Protects against Lipopolysaccharide-Induced Sepsis and Alleviates Inflammatory Responses in Mice. IUBMB Life 2020, 72, 2121–2132. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Constantinou, M.; Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Kamber, E.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. The Olive Constituent Oleuropein Exhibits Anti-Ischemic, Antioxidative, and Hypolipidemic Effects in Anesthetized Rabbits. J. Nutr. 2006, 136, 2213–2219. [Google Scholar] [CrossRef]
- Röszer, T. Transcriptional Control of Apoptotic Cell Clearance by Macrophage Nuclear Receptors. Apoptosis 2017, 22, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Su, D.; Altomonte, J.; Kamagate, A.; He, J.; Perdomo, G.; Tse, T.; Jiang, Y.; Dong, H.H. PPARα Mediates the Hypolipidemic Action of Fibrates by Antagonizing FoxO1. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E421–E434. [Google Scholar] [CrossRef] [PubMed]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and Expression of a Cell Surface Receptor for Advanced Glycosylation End Products of Proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Khazaei, M.; Karimi, J.; Sheikh, N.; Goodarzi, M.T.; Saidijam, M.; Khodadadi, I.; Moridi, H. Effects of Resveratrol on Receptor for Advanced Glycation End Products (RAGE) Expression and Oxidative Stress in the Liver of Rats with Type 2 Diabetes. Phytother. Res. 2016, 30, 66–71. [Google Scholar] [CrossRef]
- Maranesi, M.; Dall’Aglio, C.; Acuti, G.; Cappelli, K.; Trabalza Marinucci, M.; Galarini, R.; Suvieri, C.; Zerani, M. Effects of Dietary Polyphenols from Olive Mill Waste Waters on Inflammatory and Apoptotic Effectors in Rabbit Ovary. Animals 2021, 11, 1727. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Sabino, M.; Capomaccio, S.; Cappelli, K.; Verini-Supplizi, A.; Bomba, L.; Ajmone-Marsan, P.; Cobellis, G.; Olivieri, O.; Pieramati, C.; Trabalza-Marinucci, M. Oregano Dietary Supplementation Modifies the Liver Transcriptome Profile in Broilers: RNASeq Analysis. Res. Vet. Sci. 2018, 117, 85–91. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Ortenzi, R.; Roila, R.; Trabalza-Marinucci, M.; Servili, M.; Papa, P.; Galarini, R.; Valiani, A. Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter Spp. Prevalence in Broiler Chickens. Sustainability 2016, 8, 837. [Google Scholar] [CrossRef]
- Stiuso, P.; Bagarolo, M.L.; Ilisso, C.P.; Vanacore, D.; Martino, E.; Caraglia, M.; Porcelli, M.; Cacciapuoti, G. Protective Effect of Tyrosol and S-Adenosylmethionine against Ethanol-Induced Oxidative Stress of Hepg2 Cells Involves Sirtuin 1, P53 and Erk1/2 Signaling. Int. J. Mol. Sci. 2016, 17, 622. [Google Scholar] [CrossRef]
- Nutrition and Healthy Ageing: Calorie Restriction or Polyphenol-Rich “MediterrAsian” Diet? Available online: https://www.hindawi.com/journals/omcl/2013/707421/ (accessed on 25 August 2021).
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [PubMed]
- Preyat, N.; Leo, O. Sirtuin Deacylases: A Molecular Link between Metabolism and Immunity. J. Leukoc. Biol. 2013, 93, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S. SIRT1 Promotes the Central Adaptive Response to Diet Restriction through Activation of the Dorsomedial and Lateral Nuclei of the Hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Rahman, S.U.; Huang, Y.; Zhu, L.; Chu, X.; Junejo, S.A.; Zhang, Y.; Khan, I.M.; Li, Y.; Feng, S.; Wu, J.; et al. Tea Polyphenols Attenuate Liver Inflammation by Modulating Obesity-Related Genes and down-Regulating COX-2 and INOS Expression in High Fat-Fed Dogs. BMC Vet. Res. 2020, 16, 234. [Google Scholar] [CrossRef]
- Duszka, K.; Wahli, W. Peroxisome Proliferator-Activated Receptors as Molecular Links between Caloric Restriction and Circadian Rhythm. Nutrients 2020, 12, 3476. [Google Scholar] [CrossRef]
- Ayissi, V.B.O.; Ebrahimi, A.; Schluesenner, H. Epigenetic Effects of Natural Polyphenols: A Focus on SIRT1-Mediated Mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Li Volti, G.; Raffaele, M.; Distefano, A.; Palmeri, R.; Parafati, L.; Licari, M.; Zingales, V.; Avola, R.; Vanella, L. The Effects of Olive Leaf Extract from a Sicilian Cultivar in an Experimental Model of Hepatic Steatosis. Rend. Fis. Acc. Lincei 2017, 28, 643–650. [Google Scholar] [CrossRef]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.J.; Cheng, H.L.; Alt, F.W.; Guarente, L. Tissue-Specific Regulation of SIRT1 by Calorie Restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef]
- González, E.; Tejeda, J.F. Effects of Dietary Incorporation of Different Antioxidant Extracts and Free-Range Rearing on Fatty Acid Composition and Lipid Oxidation of Iberian Pig Meat. Animal 2007, 1, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tang, Y.; Kang, Q.; Feng, Y.; Chen, A. Curcumin Inhibits Gene Expression of Receptor for Advanced Glycation End-Products (RAGE) in Hepatic Stellate Cells in Vitro by Elevating PPARγ Activity and Attenuating Oxidative Stress. Br. J. Pharmacol. 2012, 166, 2212–2227. [Google Scholar] [CrossRef]
- Diao, J.-X.; Ou, J.-Y.; Dai, H.; Li, H.-Y.; Huang, W.; Hua, H.-Y.; Xie, T.; Wang, M.; Yang, Y.-G. Antioxidant and Antiapoptotic Polyphenols from Green Tea Extract Ameliorate CCl4-Induced Acute Liver Injury in Mice. Chin. J. Integr. Med. 2020, 26, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Shen, X.; Ai, Y.; Han, X. Tea Polyphenols Protect against Ischemia/Reperfusion-Induced Liver Injury in Mice through Anti-Oxidative and Anti-Apoptotic Properties. Exp. Ther. Med. 2016, 12, 3433–3439. [Google Scholar] [CrossRef][Green Version]
- El-Hack, M.E.A.; Elnesr, S.S.; Alagawany, M.; Gado, A.; Noreldin, A.E.; Gabr, A.A. Impact of Green Tea (Camellia Sinensis) and Epigallocatechin Gallate on Poultry. Worlds Poult. Sci. J. 2020, 76, 49–63. [Google Scholar] [CrossRef]
- Jia, P.; Ji, S.; Zhang, H.; Chen, Y.; Wang, T. Piceatannol Ameliorates Hepatic Oxidative Damage and Mitochondrial Dysfunction of Weaned Piglets Challenged with Diquat. Animals 2020, 10, 1239. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Cho, S.-S.; Li, Y.; Bae, C.-S.; Park, K.M.; Park, D.-H. Anti-Inflammatory Effect of Curcuma Longa and Allium Hookeri Co-Treatment via NF-ΚB and COX-2 Pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Taranu, I.; Habeanu, M.; Gras, M.A.; Pistol, G.C.; Lefter, N.; Palade, M.; Ropota, M.; Chedea, V.S.; Marin, D.E. Assessment of the Effect of Grape Seed Cake Inclusion in the Diet of Healthy Fattening-Finishing Pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-Inflammatory Pathways: A Whole Transcriptomic In Vitro Study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Jung, J.-H.; Park, H.; Kim, H. Olive Leaf Extract Suppresses Messenger RNA Expression of Proinflammatory Cytokines and Enhances Insulin Receptor Substrate 1 Expression in the Rats with Streptozotocin and High-Fat Diet–Induced Diabetes. Nutr. Res. 2014, 34, 450–457. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO Regulates Oxidative Stress and NO Production through SIRT1 in Diabetic Mice and Vascular Endothelial Cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Malliou, F.; Andreadou, I.; Gonzalez, F.J.; Lazou, A.; Xepapadaki, E.; Vallianou, I.; Lambrinidis, G.; Mikros, E.; Marselos, M.; Skaltsounis, A.-L.; et al. The Olive Constituent Oleuropein, as a PPARα Agonist, Markedly Reduces Serum Triglycerides. J. Nutr. Biochem. 2018, 59, 17–28. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Wan, B.; Wang, Q.; Wan, X. Green Tea Polyphenols Alter Lipid Metabolism in the Livers of Broiler Chickens through Increased Phosphorylation of AMP-Activated Protein Kinase. PLoS ONE 2017, 12, e0187061. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Douglas, T.D.; Maiyoh, G.K.; Adeli, K.; Theriault, A.G. Green Tea Leaf Extract Improves Lipid and Glucose Homeostasis in a Fructose-Fed Insulin-Resistant Hamster Model. J. Ethnopharmacol. 2006, 104, 24–31. [Google Scholar] [CrossRef]
- Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Hayirli, A.; Sahin, K. Epigallocatechin-3-Gallate Exerts Protective Effects against Heat Stress through Modulating Stress-Responsive Transcription Factors in Poultry. Br. Poult. Sci. 2013, 54, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green Tea Polyphenols Decrease Weight Gain, Ameliorate Alteration of Gut Microbiota, and Mitigate Intestinal Inflammation in Canines with High-Fat-Diet-Induced Obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Tsai, S.H.; Tsai, D.C.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of Inducible Cyclooxygenase and Nitric Oxide Synthase through Activation of Peroxisome Proliferator-Activated Receptor-Gamma by Flavonoids in Mouse Macrophages. FEBS Lett. 2001, 496, 12–18. [Google Scholar] [CrossRef]
- Jabalbarezi Hukerdi, Y.; Fathi Nasri, M.H.; Rashidi, L.; Ganjkhanlou, M.; Emami, A. Effects of Dietary Olive Leaves on Performance, Carcass Traits, Meat Stability and Antioxidant Status of Fattening Mahabadi Male Kids. Meat Sci. 2019, 153, 2–8. [Google Scholar] [CrossRef]
- Cimmino, R.; Barone, C.M.A.; Claps, S.; Varricchio, E.; Rufrano, D.; Caroprese, M.; Albenzio, M.; De Palo, P.; Campanile, G.; Neglia, G. Effects of Dietary Supplementation with Polyphenols on Meat Quality in Saanen Goat Kids. BMC Vet. Res. 2018, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Gómez, D.; Yangüela, J.; Roncalés, P.; Ariño, A. Olive Leaves Extract from Algerian Oleaster (Olea Europaea Var. Sylvestris) on Microbiological Safety and Shelf-Life Stability of Raw Halal Minced Beef during Display. Foods 2019, 8, 10. [Google Scholar] [CrossRef] [PubMed]
| Gene | Accession Number and Genomic Coordinates | Primer Forward | Primer Reverse |
|---|---|---|---|
| ACTB | NM_001101683.1 chrUn0180:126294-126816 | CACCTTCTACAACGAGCTGC | TGTTGAACGTCTCGAACATGA |
| RN18S | Maranesi et al. [36] | CGATCAGATACCGTTCGTAGT | TTCCTTTAAGTTTCAGCTTTGC |
| RN18S | NR_033238.1 chrUn0416:102245-103489 | CGTCTGCCCTATCAACTTTCG | AATGGGGTTCAACGGGTTAC |
| AGER | XM_002714315.3 chr12:20967567-20968080 | CCACCCATCCCAACCGTG | GCTAGAGTCCCCAGGCCT |
| BAX | XM_008252361.2 chrUn0331:93,210-93,457 | CCTTTTGCTTCAGGGTTCA | ATCCTCTGCAGCTCCATGTT |
| COX2 | NC_001913.1 chrM:7201+7281 | ACAATGGATGCTCAGGAGGT | TAGGGAGGGCAGCGCAATTA |
| IL1B | NM_001082201.1 chr2:97615039-97616359 | GAATCTGTACCTGTCCTGCGT | TTGGGTAACGGTTGGGGTCT |
| PPARA | XM_002723354.3 chrUn0230:149032+156977 | ATGAACAAGGTCAAAGCCCG | ATGAACAAGGTCAAAGCCCG |
| PPARG | NM_001082148.1 chr9:11539648-11541491 | CCTTTCACCACCGTGGACTT | GGGGATGCAGGTTCCACTTT |
| SIRT1 | XM_017348747.1 chr18:18711449-18715261 | TGCAAGCTCTAGTGACTGGA | TGTTCGAGGATCTGTGCCAA |
| TNFA | NM_001082263.1 chr12:20399776+20400811 | ACTTCAGGGTGATCGGCC | CCTCCACTTGCGGGTTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappelli, K.; Ferlisi, F.; Mecocci, S.; Maranesi, M.; Trabalza-Marinucci, M.; Zerani, M.; Dal Bosco, A.; Acuti, G. Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach. Animals 2021, 11, 2932. https://doi.org/10.3390/ani11102932
Cappelli K, Ferlisi F, Mecocci S, Maranesi M, Trabalza-Marinucci M, Zerani M, Dal Bosco A, Acuti G. Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach. Animals. 2021; 11(10):2932. https://doi.org/10.3390/ani11102932
Chicago/Turabian StyleCappelli, Katia, Flavia Ferlisi, Samanta Mecocci, Margherita Maranesi, Massimo Trabalza-Marinucci, Massimo Zerani, Alessandro Dal Bosco, and Gabriele Acuti. 2021. "Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach" Animals 11, no. 10: 2932. https://doi.org/10.3390/ani11102932
APA StyleCappelli, K., Ferlisi, F., Mecocci, S., Maranesi, M., Trabalza-Marinucci, M., Zerani, M., Dal Bosco, A., & Acuti, G. (2021). Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach. Animals, 11(10), 2932. https://doi.org/10.3390/ani11102932











