Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. Counting the Inoculated Agar Plates
2.4. Amplification of Parts of the 16S rRNA-Gene Sequence of Grown TAS by PCR
2.5. Statistical Analyses
3. Results and Discussion
3.1. CBP Bedding Material Parameters
3.2. Bacterial Growth/Identification
3.3. PCR and Sequence Analyses
3.4. Effects on the TAS Amount and Concentration
3.4.1. Effects on the Amount of TAS (log10 cfu/g Bedding Material)
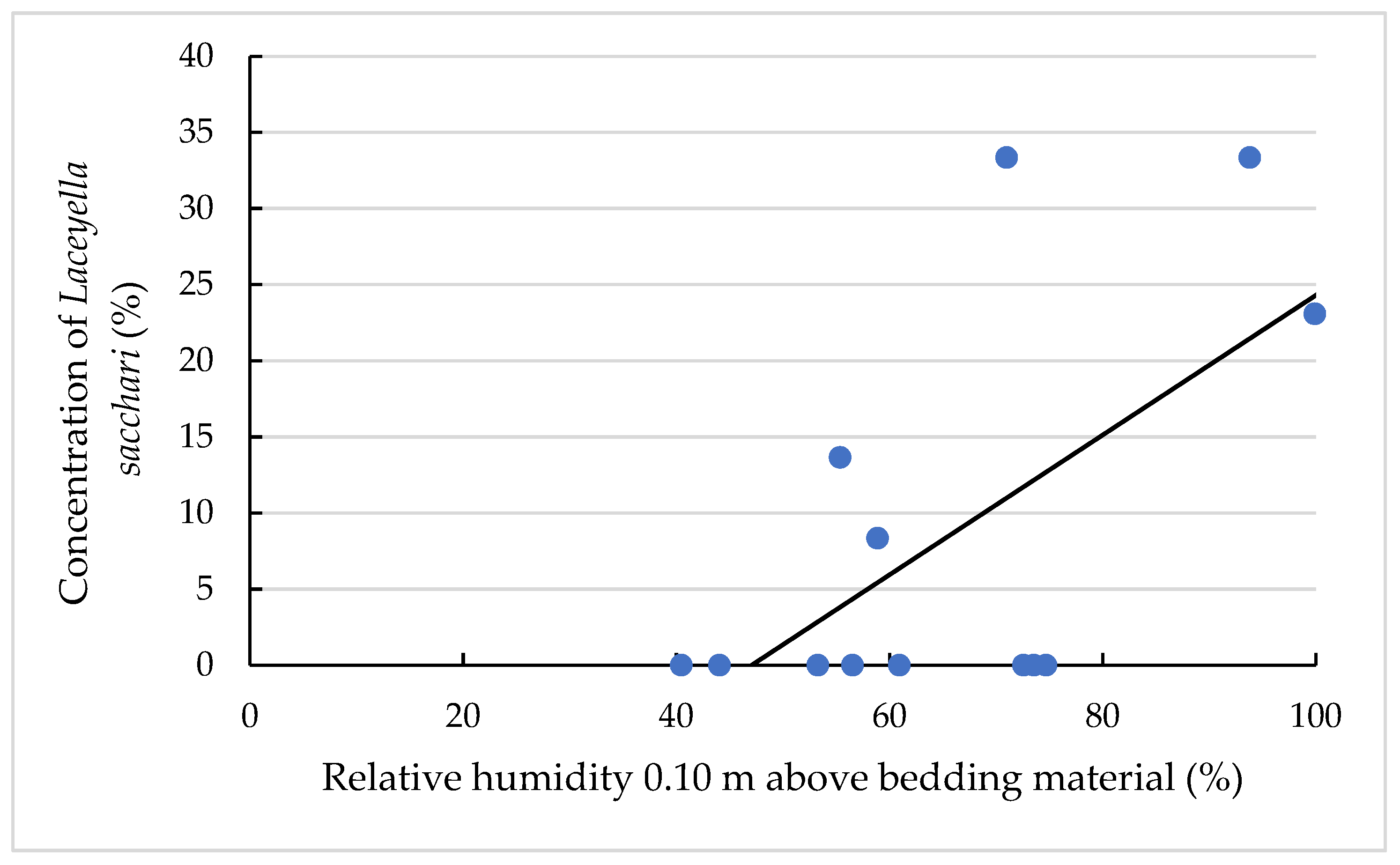
3.4.2. Effects on the TAS Concentrations of the Different TAS Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferraz, P.F.P.; Araújo e Silva Ferraz, G.; Leso, L.; Klopčič, M.; Barbari, M.; Rossi, G. Properties of conventional and alternative bedding materials for dairy cattle. J. Dairy Sci. 2020, 103, 8661–8674. [Google Scholar] [CrossRef]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef] [PubMed]
- Janni, K.A.; Endres, M.I.; Reneau, J.K.; Schoper, W.W. Compost dairy barn layout and management recommendations. Appl. Eng. Agric. 2007, 23, 97–102. [Google Scholar] [CrossRef]
- Bewley, J.M.; Robertson, L.M.; Eckelkamp, E.A. A 100-Year Review: Lactating dairy cattle housing management. J. Dairy Sci. 2017, 100, 10418–10431. [Google Scholar] [CrossRef] [PubMed]
- Eckelkamp, E.A.; Taraba, J.L.; Akers, K.A.; Harmon, R.J.; Bewley, J.M. Understanding compost bedded pack barns: Interactions among environmental factors, bedding characteristics, and udder health. Livest. Sci. 2016, 190, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Galama, P.J.; Bokma, S.; van Dooren, H.J.; Ouweltjes, W.; Smits, M.; Driehuis, F. Prospects for Bedded Pack Barns for Dairy Cattle; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2011. [Google Scholar]
- Blanco-Penedo, I.; Ouweltjes, W.; Ofner-Schröck, E.; Brügemann, K.; Emanuelson, U. Symposium review: Animal welfare in free-walk systems in Europe. J. Dairy Sci. 2020, 103, 5773–5782. [Google Scholar] [CrossRef]
- Kester, E.; Holzhauer, M.; Frankenac, K. Descriptive review of the prevalence and risk factors of hock lesions in dairy cows. Vet. J. 2014, 202, 222–228. [Google Scholar] [CrossRef]
- Schirmann, K.; Chapinal, N.; Weary, D.M.; Heuwieser, W.; von Keyserlingk, M.A.G. Rumination and its relationship to feeding and lying behavior in Holstein dairy cows. J. Dairy Sci. 2012, 95, 3212–3217. [Google Scholar] [CrossRef] [Green Version]
- Barberg, A.E.; Endres, M.I.; Salfer, J.A.; Reneau, J.K. Performance and welfare of dairy cows in an alternative housing system in Minnesota. J. Dairy Sci. 2007, 90, 1575–1583. [Google Scholar] [CrossRef]
- Black, R.A.; Taraba, J.L.; Day, G.B.; Damasceno, F.A.; Bewley, M. Compost bedded pack dairy barn management, performance, and producer satisfaction. J. Dairy Sci. 2013, 96, 8060–8074. [Google Scholar] [CrossRef]
- Biasato, I.; D’Angelo, A.; Bertone, I.; Odore, R.; Bellino, C. Compost bedded-pack barn as an alternative housing system for dairy cattle in Italy: Effects on animal health and welfare and milk and milk product quality. Ital. J. Anim. Sci. 2019, 18, 1142–1153. [Google Scholar] [CrossRef]
- Galama, P.J.; de Boer, H.C.; van Dooren, J.H.C.; Ouweltjes, W.; Driehuis, F. Sustainability Aspects of Ten Bedded Pack Dairy Barns in The Netherlands; Livestock Research Report 873; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2015. [Google Scholar]
- Galama, P.J. On Farm Development of Bedded Pack Dairy Barns in the Netherlands; Livestock Research Report 707; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2014. [Google Scholar]
- Imbeah, M. Composting piggery waste: A review. Bioresour. Technol. 1998, 63, 197–203. [Google Scholar] [CrossRef]
- Beffa, T.; Blanc, M.; Marilley, L.; Fischer, J.L.; Lyon, P.-F.; Aragno, M. Taxonomic and metabolic microbial diversity during composting. In The Science of Composting; de Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Blackies Academic and Professional: Glasgow, Scotland, 1995; Volume 1, pp. 149–161. [Google Scholar]
- Bewley, J.M.; Taraba, J.L.; McFarland, D.; Garrett, P.; Graves, R.; Holmes, B.; Kammel, D.; Porter, J.; Tyson, J.; Weeks, S.; et al. Guidelines for Managing Compost Bedded-Pack Barns; The Dairy Practices Council: Ritchboro, PA, USA, 2013. [Google Scholar]
- Stentiford, E.I. Composting control: Principles and practice. In The Science of Composting, Part 1.; de Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Blackie Academic and Professional: London, UK, 1996; pp. 49–59. [Google Scholar]
- NRAES-54. On-Farm Composting Handbook; North-east Regional Agricultural Engineering Service: Ithaca, NY, USA, 1992. [Google Scholar]
- Leso, L.; Ferraz, P.F.P.; Ferraz, G.A.S.; Rossi, G.; Barbari, M. Factors affecting evaporation of water from cattle bedding materials. Biosyst. Eng. 2021, 205, 164–173. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Reuter, T.; Alexander, T.W.; McAllister, T.A. Viability of Bacillus licheniformis and Bacillus thuringiensis spores as a model for predicting the fate of Bacillus anthracis spores during composting of dead livestock. Appl. Environ. Microbiol. 2011, 77, 1588–1592. [Google Scholar] [CrossRef] [Green Version]
- Rössler, D.; Ludwig, W.; Schleifer, K.H.; Lin, C.; Mcgill, T.J.; Wisotzkey, J.D.; Jurtshuk, P., Jr.; Fox, G.E. Phylogenetic diversity in the genus Bacillus as seen by 16S rRNA sequencing studies. Syst. Appl. Microbiol. 1991, 14, 266–269. [Google Scholar] [CrossRef]
- Adziguel, A.; Ozkan, H.; Baris, O.; Inan, K.; Gulluce, M.; Sahin, F. Identification and characterization of thermophilic bacteria isolated from hot springs in Turkey. J. Microbiol. Methods 2009, 79, 321–328. [Google Scholar] [CrossRef]
- Charbonneau, D.M.; Meddeb-Mouelhi, F.; Boissinot, M.; Sirois, M.; Beauregard, M. Identification of thermophilic bacterial strains producing thermotolerant hydrolytic enzymes from manure compost. Indian J. Microbiol. 2012, 52, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driehuis, F.; Lucas-van de Bos, E.; Wagendorp, A.; Wells-Bennik, M.H.J. Sporen van Thermofiele, Aerobe Sporenvormers in Compost en Andere Beddingmaterialien bij Melkveebedrijven Met een Vrijloop- of Ligboxenstal; NIZO-RAPPORT E2014/045 Mai; NIZO: Ede, The Netherlands, 2014. [Google Scholar]
- Wang, C.-M.; Shyu, C.-L.; Ho, S.-H.; Chiou, S.-H. Species diversity and substrate utilization patterns of thermophilic bacterial communities in hot aerobic poultry and cattle manure composts. Microb. Ecol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, C.M.; Neuman, C.; Katouli, M.; Kurtböke, D.I. Detection of Thermoactinomyces species in selected agricultural substrates from Queensland. Microb. Ecol. 2014, 67, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Te Giffel, M.C.; Wagendorp, A.; Herrewegh, A.; Driehuis, F. Bacterial spores in silage and raw milk. Antonie van Leeuwenhoek 2002, 81, 625–630. [Google Scholar] [CrossRef]
- Scheldemann, P.; Pil, A.; Herman, L.; De Vos, P.; Heyndrickx, M. Incidence and diversity of potentially highly heat-resistant spores isolated at dairy farms. Appl. Environ. Microbiol. 2005, 71, 1480–1494. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.I.; Kent, D.; Martin, N.H.; Evanowski, R.L.; Patel, K.; Godden, S.M.; Wiedmann, M. Bedding and bedding management practices are associated with mesophilic and thermophilic spore levels in bulk tank raw milk. J. Dairy Sci. 2019, 102, 6885–6900. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Lucas-van de Bos, E.; Wells-Bennik, M.H.J. Risico’s van Microbiële Contaminanten van Strooisels: Compost, Gescheiden Mest, Paardenmest en Vrtijloopstallen; NIZO-RAPPORT E2012/119 December; NIZO: Ede, The Netherlands, 2012. [Google Scholar]
- Ronimus, R.S.; Parker, L.E.; Turner, N.; Poudel, S.; Rückert, A.; Morgan, H.W. A RAPD-based comparison of thermophilic bacilli from milk powders. Int. J. Food Microbiol. 2003, 85, 45–61. [Google Scholar] [CrossRef]
- Kent, D.J.; Chauhan, K.; Boor, K.J.; Wiedmann, M.; Martin, N.H. Spore test parameters matter: Mesophilic and thermophilic spore counts detected in raw milk and dairy powders differ significantly by test method. J. Dairy Sci. 2016, 99, 5180–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Júnior, J.R.; de Oliveira, A.M.; Silva, F.D.G.; Tamanini, R.; de Oliveira, A.L.M.; Beloti, V. The main spoilage-related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 2018, 101, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansson, A.; Bertilsson, J.; Svensson, B. Bacillus cereus spores in raw milk: Factors affecting the contamination of milk during the grazing period. J. Dairy Sci. 1999, 82, 305–314. [Google Scholar] [CrossRef]
- Mehta, D.S.; Metzger, L.E.; Hassan, A.N.; Nelson, B.K.; Patel, H.A. The ability of spore formers to degrade milk proteins, fat, phospholipids, common stabilizers, and exopolysaccharides. J. Dairy Sci. 2019, 102, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.H.; Ward, L.J.H.; Walker, K.M.R. Functional grouping of thermophilic Bacillus strains using amplification profiles of the 16S–23S internal spacer region. Syst. Appl. Microbiol. 2001, 24, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods. 2007, 69, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Rainey, F.A.; Fritze, D.; Stackebrandt, E. The phylogenetic diversity of thermophilic members of the genus Bacillus as revealed by 16S rDNA analysis. FEMS Microbiol. Lett. 1994, 115, 205–212. [Google Scholar] [CrossRef]
- D’Amore, R.; Zeeshan Ijaz, U.; Schirmer, M.; Kenny, J.G.; Gregory, R.; Darby, A.C.; Shakya, M.; Podar, M.; Quince, C.; Hall, N. A comprehensive benchmarking study of protocols and sequencing platforms for 16S rRNA community profiling. BMC Genom. 2016, 17, 55. [Google Scholar] [CrossRef] [Green Version]
- Leso, L.; Galama, P.; Blanco Penedo, I.; Bruegemann, K.; Zentner, A.; Klopcic, M.; Hovstad, K.; Barbari, M. Bedding management in compost-bedded pack and cubicle barns for dairy cows in Europe. In Proceedings of the 72nd Annual Meeting of the European Federation of Animal Science, Davos, Switzerland, 30 August–3 September 2021; p. 407. [Google Scholar]
- Driehuis, F.; Lucas-van de Bos, E.; Bomhof, E.; Wells-Bennik, M.H.J. Sporen van Thermofiele Sporenvormers in Gescheiden Mest, Gecomposteerde Houtsnippers en Andere Beddingmaterialen; NIZO-RAPPORT E2015/040 April; NIZO: Ede, The Netherlands, 2015. [Google Scholar]
- Bergkessel, M.; Guthrie, C. Chapter Twenty Five—Colony PCR. Meth. Enzymol. 2013, 529, 299–309. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.M. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria 2020. Available online: http://www.r-project.org/index.html (accessed on 3 May 2021).
- Lobeck, K.M.; Endres, M.I.; Shane, E.M.; Godden, S.M.; Fetrow, J. Animal welfare in cross-ventilated, compost-bedded pack, and naturally ventilated dairy barns in the upper Midwest. J. Dairy Sci. 2011, 94, 5469–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The prevalence and control of bacillus and related spore-forming bacteria in the dairy industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Taraba, J.L.; Day, G.B.; Damasceno, F.A.; Newman, M.C.; Akers, K.A.; Wood, C.L.; McQuerry, K.J.; Bewley, J.M. The relationship between compost bedded pack performance, management, and bacterial counts. J. Dairy Sci. 2014, 97, 2669–2679. [Google Scholar] [CrossRef] [Green Version]
- Astiz, S.; Sebastian, F.; Fargas, O.; Fernández, M.; Calvet, E. Enhanced udder health and milk yield of dairy cattle on compost bedding systems during the dry period: A comparative study. Livest. Sci. 2014, 159, 161–164. [Google Scholar] [CrossRef]
- Galama, P.J.; Ouweltjes, W.; Endres, M.I.; Sprecher, J.R.; Leso, L.; Kuipers, A.; Klopčič, M. Symposium review: Future of housing for dairy cattle. J. Dairy Sci. 2020, 103, 5759–5772. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Han, X.; Li, J.; Zhao, Z.; Liu, Y.; Xi, O.; Guo, X.; Gun, S. Microbial community and its association with physicochemical factors during compost bedding for dairy cows. Front. Microbiol. 2020, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Langarica-Fuentes, A.; Zafar, U.; Heyworth, A.; Brown, T.; Fox, G.; Robson, G.D. Fungal succession in an in-vessel composting system characterized using 454 pyrosequencing. FEMS Microbiol. Ecol. 2014, 88, 296–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
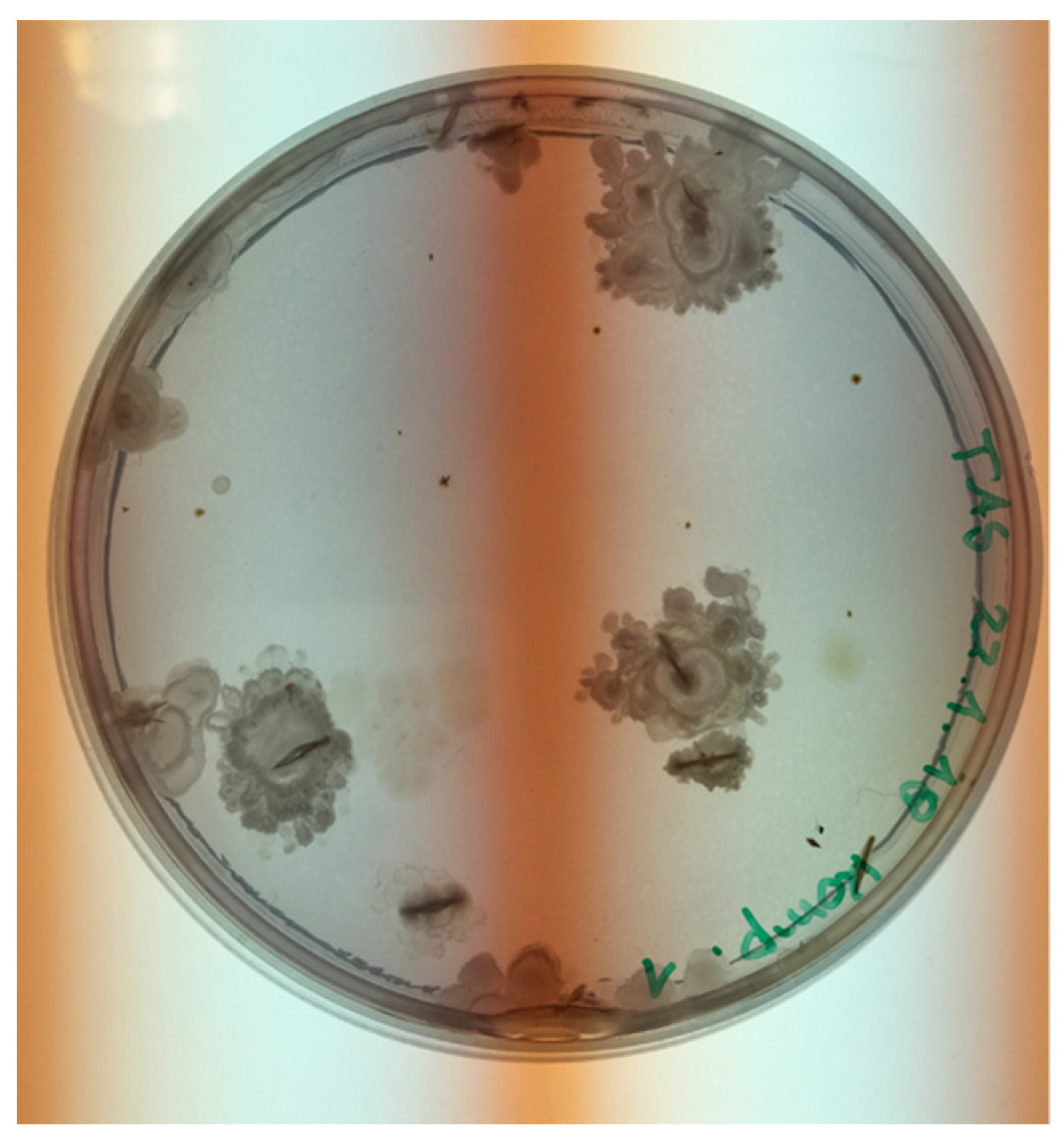


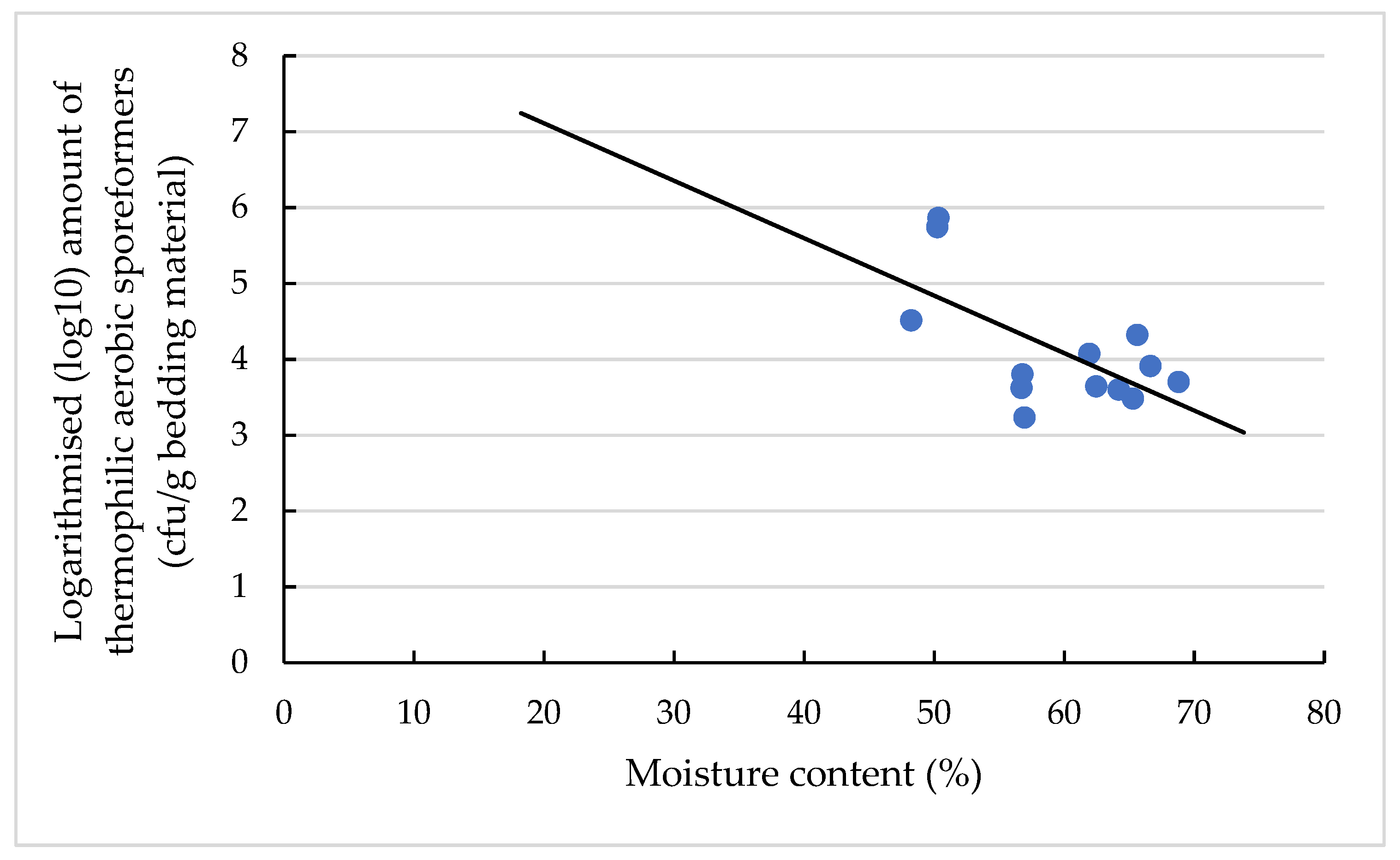
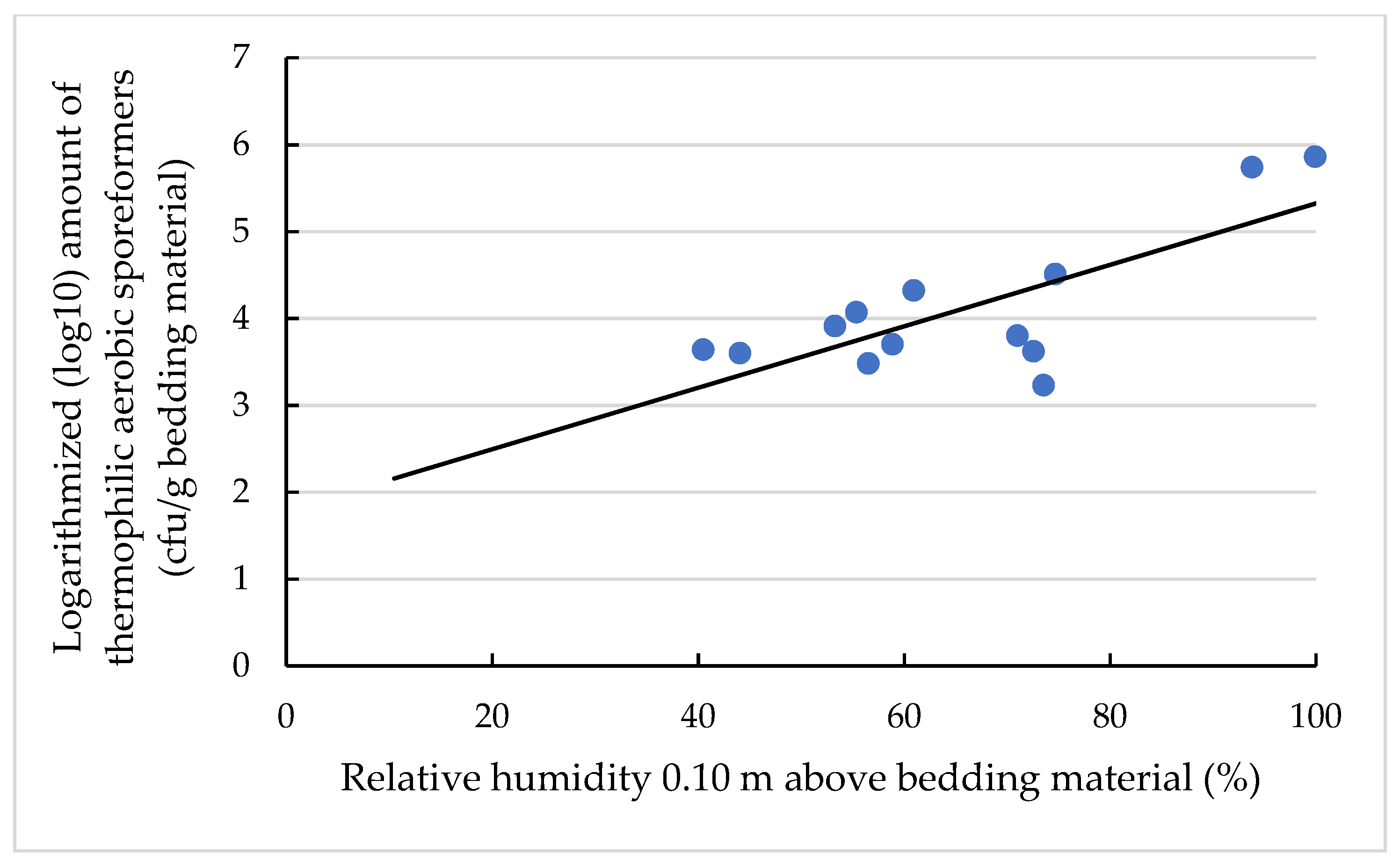
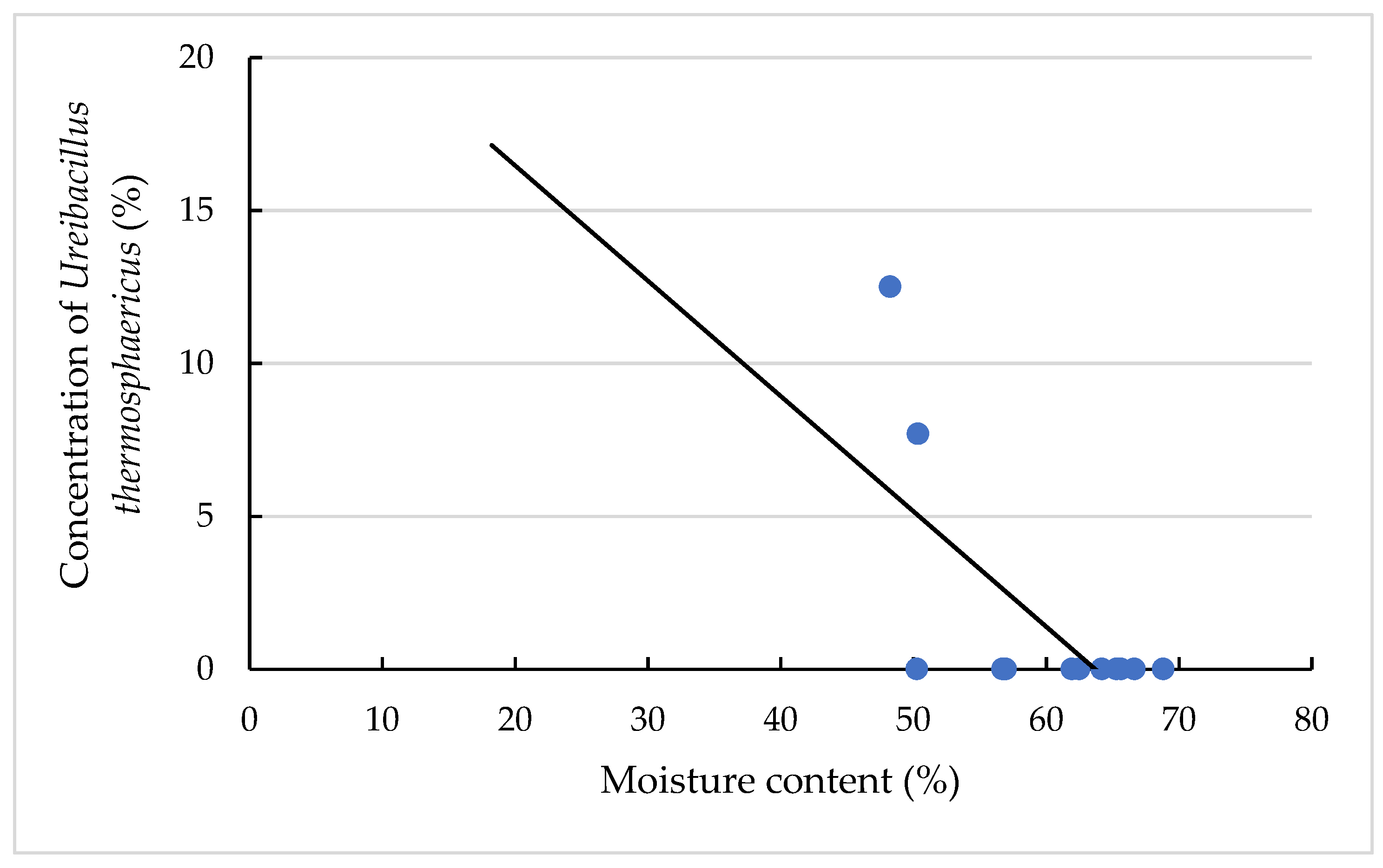
| Sample Number | Sampling Season | CBP Group | Days a | Mc b (%) | Tbed c (°C) | Thigh d (°C) | RHhigh d (%) | Tdown e (°C) | RHdown e (%) | Tamb f (°C) | Bed g (m2) | Picked Colonies (n) | TAS (log10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | winter 2017 | CBP1-lact. h | 8 | 50.24 | 31.13 (±13.80) | 7.92 (±0.54) | 93.78 (±18.37) | 8.16 (±0.86) | 98.70 (±2.94) | 7.40 | 8.90 | 3 | 5.74 |
| 2 | CBP4-dry | 8 | 50.33. | 34.77 (±16.09) | 8.00 (±0.17) | 99.90 (±0.00) | 8.20 (±0.10) | 99.90 (±0.00) | 13.18 | 13 | 5.86 | ||
| 3 | spring 2018 | CBP1-lact. | 113 | 68.80 | 36.08 (±8.46) | 12.10 (±0.81) | 51.50 (±2.76) | 12.22 (±0.66) | 58.87 (±4.72) | 14.30 | 8.42 | 12 | 3.70 |
| 4 | CBP2-lact. | 108 | 66.63 | 33.96 (±7.36) | 12.99 (±0.78) | 49.26 (±3.97) | 13.01 (±0.92) | 53.28 (±4.60) | 7.80 | 7 | 3.91 | ||
| 5 | CBP3-lact. | 80 | 65.28 | 34.96 (±7.18) | 12.08 (±0.27) | 52.38 (±2.40) | 12.13 (±0.27) | 56.53 (±4.26) | 8.31 | 1 | 3.48 | ||
| 6 | CBP4-dry | 113 | 61.93 | 32.83 (±16.80) | 11.60 (±0.20) | 52.37 (±1.23) | 11.70 (±0.20) | 55.37 (±1.36) | 12.99 | 22 | 4.07 | ||
| 7 | summer 2018 | CBP1-lact. | 248 | 64.19 | 42.12 (±6.84) | 31.94 (±0.32) | 40.87 (±1.21) | 31.88 (±0.42) | 44.06 (±0.42) | 34.20 | 8.82 | 2 | 3.60 |
| 8 | CBP2-lact. | 248 | 62.46 | 40.17 (±7.85) | 32.52 (±0.35) | 38.79 (±1.80) | 32.64 (±0.20) | 40.48 (±3.56) | 8.01 | 16 | 3.64 | ||
| 9 | autumn 2018 | CBP1-lact. | 44 | 48.23 | 55.97 (±17.27) | 6.66 (±0.42) | 70.16 (±2.42) | 6.72 (±0.43) | 74.67 (±3.38) | 6.20 | 8.66 | 8 | 4.51 |
| 10 | CBP2-lact. | 44 | 56.71 | 46.31 (±14.15) | 6.52 (±2.25) | 68.27 (±2.16) | 7.29 (±0.63) | 72.57 (±3.26) | 9.44 | 4 | 3.62 | ||
| 11 | CBP3-lact. | 44 | 56.79 | 56.33 (±5.99) | 6.12 (±0.43) | 68.03 (±1.23) | 6.24 (±0.57) | 70.99 (±1.91) | 9.14 | 6 | 3.80 | ||
| 12 | CBP4-dry | 44 | 56.94 | 56.57 (±0.64) | 5.83 (±0.51) | 72.10 (±3.11) | 6.07 (±0.76) | 73.53 (±4.97) | 11.11 | 1 | 3.23 | ||
| 13 | winter 2019 | CBP4-dry | 121 | 65.62 | 17.93 (± =3.36) | 5.63 (±0.06) | 60.73 (±0.97) | 5.67 (±0.06) | 60.93 (±0.49) | 7.90 | 21.96 | 4 | 4.32 |
| PCR-No. | Primers | Sequences (Position in GenBank Acc. No. X60623 a) | Amplified Hypervariable Region | Reference |
|---|---|---|---|---|
| 1 | 27F | 5′-GAGTTTGATCCTGGCTCA-3′ (3–20) | V1, V2 and V3 | [27,39] |
| V3R | 5′-CGTATTACCGCGGCTGCTG-3′ (539–521) | |||
| 2 | V6F | 5′-TCGAtGCAACGCGAAGAA-3′ (963–980) | V6 | [39] |
| V6R | 5′-ACATtTCACaACACGAGCTGACGA-3′ (1084–1061) |
| TAS Species | |||||||
|---|---|---|---|---|---|---|---|
| Sample Number | Sampling Season | A. thermoaeroph.a | B. lichenif.b | G. thermodenit.c | L. sacch.d | T. vulg.e | U. thermosph.f |
| 1 | winter 2017 | - | 0.67 | - | 0.33 | - | - |
| 2 | 0.08 | 0.46 | 0.15 | 0.23 | - | 0.08 | |
| 3 | spring 2018 | - | 0.84 | - | 0.08 | 0.08 | - |
| 4 | 0.29 | 0.71 | - | - | - | - | |
| 5 | - | 1.00 | - | - | - | - | |
| 6 | - | 0.68 | - | 0.14 | 0.18 | - | |
| 7 | summer 2018 | - | 1.00 | - | - | - | - |
| 8 | - | 0.31 | 0.25 | - | 0.43 | - | |
| 9 | autumn 2018 | - | 0.50 | - | - | 0.37 | 0.13 |
| 10 | - | 0.75 | - | - | 0.25 | - | |
| 11 | - | 0.67 | - | 0.33 | - | - | |
| 12 | - | 1.00 | - | - | - | - | |
| 13 | winter 2019 | 0.25 | 0.75 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giambra, I.J.; Jahan, Y.; Yin, T.; Engel, P.; Weimann, C.; Brügemann, K.; König, S. Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods. Animals 2021, 11, 2890. https://doi.org/10.3390/ani11102890
Giambra IJ, Jahan Y, Yin T, Engel P, Weimann C, Brügemann K, König S. Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods. Animals. 2021; 11(10):2890. https://doi.org/10.3390/ani11102890
Chicago/Turabian StyleGiambra, Isabella J., Yeasmin Jahan, Tong Yin, Petra Engel, Christina Weimann, Kerstin Brügemann, and Sven König. 2021. "Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods" Animals 11, no. 10: 2890. https://doi.org/10.3390/ani11102890
APA StyleGiambra, I. J., Jahan, Y., Yin, T., Engel, P., Weimann, C., Brügemann, K., & König, S. (2021). Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods. Animals, 11(10), 2890. https://doi.org/10.3390/ani11102890






