Immunoexpression of Trefoil Factor 1 in Non-Neoplastic and Neoplastic Canine Gastric Tissues
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Histological Evaluation
2.4. Immunohistochemistry
2.5. Protein Extraction and Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Case Details
3.2. Specificity of the Monoclonal Antibody
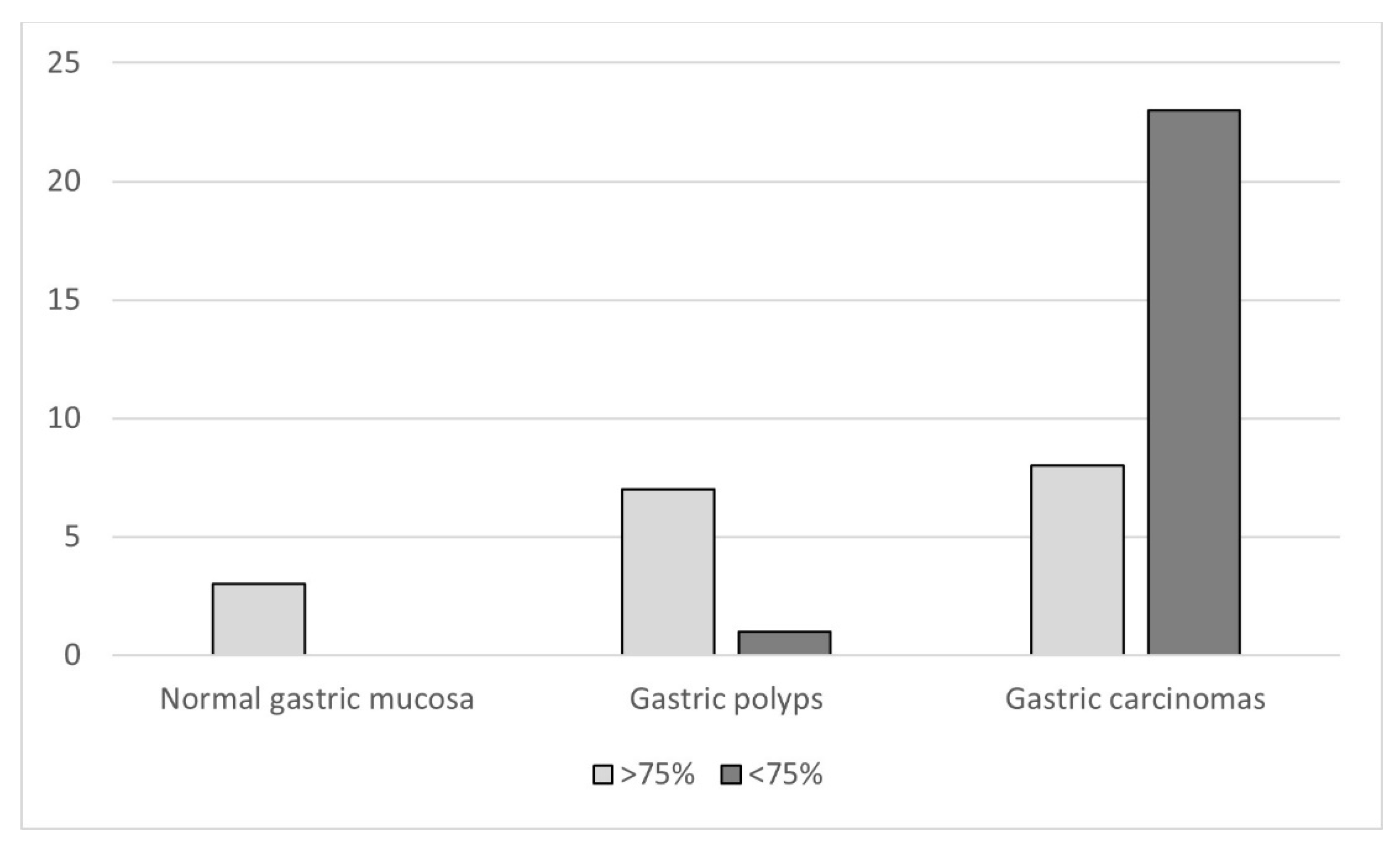
3.3. Immunohistochemistry
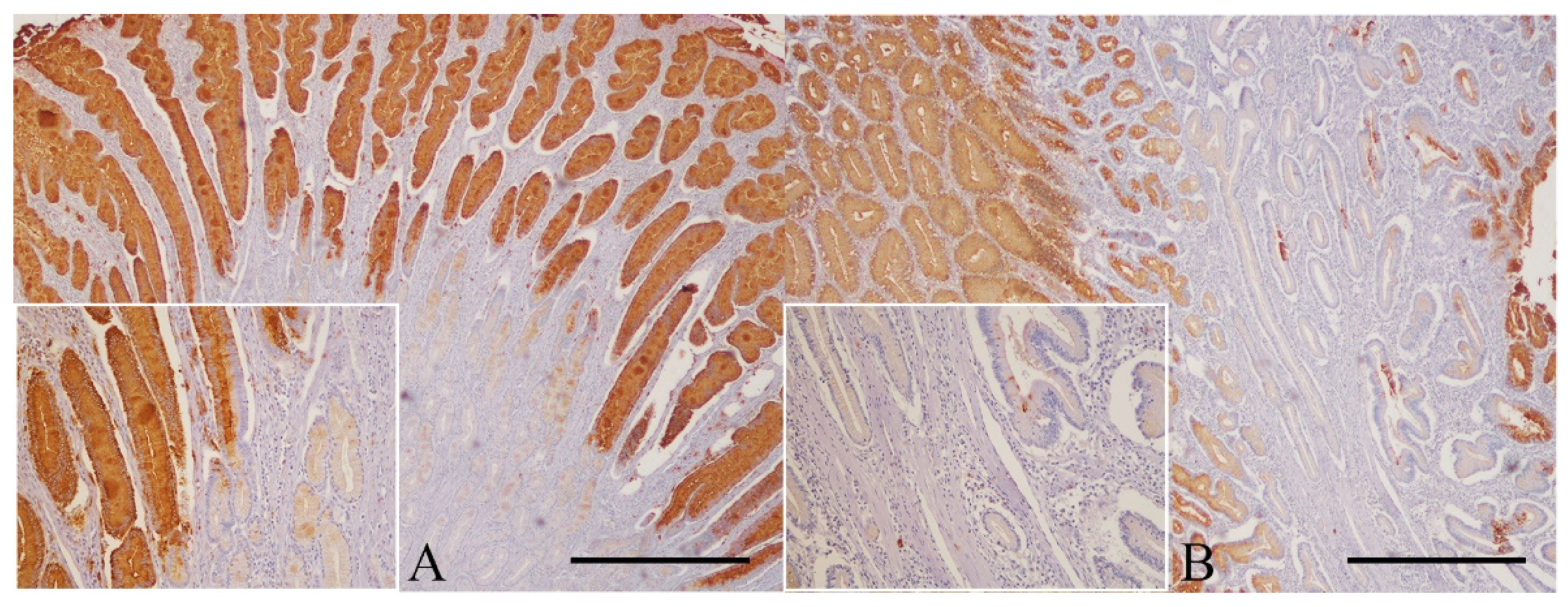
3.3.1. Normal Gastric Mucosa
3.3.2. Gastric Polyps
3.3.3. Non-neoplastic Gastric Mucosa Adjacent to Carcinomas
3.3.4. Gastric Carcinomas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amorim, I.; Taulescu, M.A.; Day, M.J.; Catoi, C.; Reis, C.A.; Carneiro, F.; Gärtner, F. Canine Gastric Pathology: A Review. J. Comp. Pathol. 2016, 154, 9–37. [Google Scholar] [CrossRef]
- Gualtieri, M.; Costa Devoit, C.; Riccardi, E.; Olivero, D. Intestinal Metaplasia and Over-Expression of c-erb2 and p53 in Tissue Adjacent to Dog Gastric Carcinoma. Pak. Vet. J. 2017, 37, 269–274. [Google Scholar]
- Abrams, B.; Wavreille, V.A.; Husbands, B.D.; Matz, B.M.; Massari, F.; Liptak, J.M.; Cray, M.T.; de Mello Souza, C.H.; Wustefeld-Janssens, B.G.; Oblak, M.L.; et al. Perioperative complications and outcome after surgery for treatment of gastric carcinoma in dogs: A Veterinary Society of Surgical Oncology retrospective study of 40 cases (2004–2018). Vet. Surg. 2019, 48, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Head, K.W.; Cullen, J.M.; Dubielzig, R.R.; Else, R.W.; Misdorp, W.; Patnaik, A.K.; Tateyama, S.; van der Gaag, I. Histological Classification of Tumors of the Alimentary System of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 2003; Volume 10, pp. 73–110. [Google Scholar]
- Koterbay, A.M.; Muthupalani, S.; Fox, J.G.; McNiel, E.A. Risk and characteristics of gastric carcinoma in the chow chow dog. Can. Vet. J. 2020, 61, 396–400. [Google Scholar] [PubMed]
- Watanabe, H.; Jass, J.; Sobin, L.; Ota, K.; Jass, J. Histological typing of oesophageal and gastric tumours. In WHO International Histological Classification of Tumours, 2nd ed.; Springer: Berlin, Germany, 1990; pp. 5–9. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Johnson, G.F. Canine gastric adenocarcinoma. Vet. Pathol. 1978, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Fonda, D.; Gualtieri, M.; Scanziani, E. Gastric carcinoma in the dog: A clinicopathological study of 11 cases. J. Small Anim. Pract. 1989, 30, 353–360. [Google Scholar] [CrossRef]
- Janke, L.; Carlson, C.S.; St Hill, C.A. The novel carbohydrate tumor antigen C2-O-sLe x is upregulated in canine gastric carcinomas. Vet. Pathol. 2010, 47, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.R.; Lemos, I.; Rema, A.; Taulescu, M.; Seixas, F.; Reis, C.A.; Gärtner, F.; Amorim, I. Tn and Sialyl-Tn antigens in canine gastric tissues. Veter-Comp. Oncol. 2020, 18, 615–625. [Google Scholar] [CrossRef]
- Carneiro, F.; David, L.; Seruca, R.; Castedo, S.; Nesland, J.M.; Sobrinho-Simões, M. Hyperplastic polyposis and diffuse carcinoma of the stomach. A study of a family. Cancer 1993, 72, 323–329. [Google Scholar] [CrossRef]
- Machado, J.C.; Carneiro, F.; Blin, N.; Sobrinho-Simões, M. Pattern of pS2 protein expression in premalignant and malignant lesions of gastric mucosa. Eur. J. Cancer Prev. 1996, 5, 169–179. [Google Scholar] [CrossRef]
- Lecoindre, P.; Bystricka, M.; Chevallier, M.; Peyron, C. Gastric carcinoma associated with Menetrier’s-like disease in a West Highland white terrier. J. Small Anim. Pract. 2012, 53, 714–718. [Google Scholar] [CrossRef]
- Qvigstad, G.; Kolbjørnsen, Ø.; Skancke, E.; Waldum, H.L. Gastric neuroendocrine carcinoma associated with atrophic gastritis in the norwegian lundehund. J. Comp. Pathol. 2008, 139, 194–201. [Google Scholar] [CrossRef]
- Munday, J.S.; Aberdein, D.; Cullen, G.D.; French, A.F. Ménétrier disease and gastric adenocarcinoma in 3 Cairn terrier littermates. Vet. Pathol. 2012, 49, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Amorim, I.; Taulescu, M.A.; Ferreira, A.; Rêma, A.; Reis, C.A.; Faustino, A.M.; Cătoi, C.; Gärtner, F. An immunohistochemical study of canine spontaneous gastric polyps. Diagn. Pathol. 2014, 9, 166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Dünker, N. Trefoil factor family peptides—Friends or foes? Biomol. Concepts 2015, 6, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Masiakowski, P.; Breathnach, R.; Bloch, J.; Gannon, F.; Krust, A.; Chambon, P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982, 10, 7895–7903. [Google Scholar] [CrossRef]
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. CMLS 1997, 53, 888–903. [Google Scholar] [CrossRef]
- Rio, M.C.; Bellocq, J.P.; Daniel, J.Y.; Tomasetto, C.; Lathe, R.; Chenard, M.P.; Batzenschlager, A.; Chambon, P. Breast cancer-associated pS2 protein: Synthesis and secretion by normal stomach mucosa. Science 1988, 241, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Wolf, C.; Kédinger, M.; Chenard, M.P.; Tomasetto, C.; Chambon, P.; Rio, M.C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J. Cell Biol. 1993, 122, 191–198. [Google Scholar] [CrossRef] [PubMed]
- May, F.E.; Westley, B.R. Trefoil proteins: Their role in normal and malignant cells. J. Pathol. 1997, 183, 4–7. [Google Scholar] [CrossRef]
- Lefebvre, O.; Chenard, M.P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.C.; Carneiro, F.; Ribeiro, P.; Blin, N.; Sobrinho-Simões, M. pS2 protein expression in gastric carcinoma. An immunohistochemical and immunoradiometric study. Eur. J. Cancer 1996, 32, 1585–1590. [Google Scholar] [CrossRef]
- Müller, W.; Borchard, F. pS2 protein in gastric carcinoma and normal gastric mucosa: Association with clincopathological parameters and patient survival. J. Pathol. 1993, 171, 263–269. [Google Scholar] [CrossRef]
- Luqmani, Y.; Bennett, C.; Paterson, I.; Corbishley, C.M.; Rio, M.C.; Chambon, P.; Ryall, G. Expression of the pS2 gene in normal, benign and neoplastic human stomach. Int. J. Cancer 1989, 44, 806–812. [Google Scholar] [CrossRef]
- Mao, W.; Chen, J.; Peng, T.-L.; Yin, X.-F.; Chen, L.-Z.; Chen, M.-H. Role of trefoil factor 1 in gastric cancer and relationship between trefoil factor 1 and gastrokine 1. Oncol. Rep. 2012, 28, 1257–1262. [Google Scholar] [CrossRef]
- Campbell, B.G.; Jabbes, M. Canine and feline trefoil factor family peptides: Highly conserved molecules with some unique characteristics. Res. Vet. Sci. 2008, 85, 68–73. [Google Scholar] [CrossRef]
- Schmitz, S.; Hill, S.; Werling, D.; Allenspach, K. Expression of trefoil factor genes in the duodenum and colon of dogs with inflammatory bowel disease and healthy dogs. Veter-Immunol. Immunopathol. 2013, 151, 168–172. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumors of the Digestive System; IARC Press: Lyon, France, 2010; Volume 3. [Google Scholar]
- Lauren, P. The Two Histological Main Types of Gastric Carcinom: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Prachasilpchai, W.; Nuanualsuwan, S.; Chatsuwan, T.; Techangamsuwan, S.; Wangnaitham, S.; Sailasuta, A. Diagnosis of Helicobacter spp. infection in canine stomach. J. Veter-Sci. 2007, 8, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.M.; Machado, J.C.; Carneiro, F.; Reis, C.A.; Gött, P.; Sobrinho-Simões, M. Patterns of expression of trefoil peptides and mucins in gastric polyps with and without malignant transformation. J. Pathol. 1999, 187, 541–548. [Google Scholar] [CrossRef]
- Terragni, R.; Casadei Gardini, A.; Sabattini, S.; Bettini, G.; Amadori, D.; Talamonti, C.; Vignoli, M.; Capelli, L.; Saunders, J.H.; Ricci, M.; et al. EGFR, HER-2 and KRAS in canine gastric epithelial tumors: A potential human model? PLoS ONE 2014, 9, e85388. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, V.; Canfrán, S.; Rodríguez-Franco, F.; Benito, A.; Sáinz, A.; Rodríguez-Bertos, A. Canine gastric carcinoma: Immunohistochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (Hsp27 and Hsp70). Vet. Pathol. 2011, 48, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Swann, H.M.; Holt, D.E. Canine gastric adenocarcinoma and leiomyosarcoma: A retrospective study of 21 cases (1986–1999) and literature review. J. Am. Anim. Hosp. Assoc. 2002, 38, 157–164. [Google Scholar] [CrossRef]
- Karam, S.M.; Tomasetto, C.; Rio, M.C. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 2004, 53, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Luo, J.Y.; Lu, Y.P.; Wang, L.; Shi, H.X. Molecular forms of trefoil factor 1 in normal gastric mucosa and its expression in normal and abnormal gastric tissues. World J. Gastroenterol. 2006, 12, 7361–7364. [Google Scholar] [CrossRef]
- Moss, S.F.; Lee, J.W.; Sabo, E.; Rubin, A.K.; Rommel, J.; Westley, B.R.; May, F.E.; Gao, J.; Meitner, P.A.; Tavares, R.; et al. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: Influence of tumor histology and relationship to prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4161–4167. [Google Scholar] [CrossRef][Green Version]
- Wu, M.S.; Shun, C.T.; Wang, H.P.; Lee, W.J.; Wang, T.H.; Lin, J.T. Loss of pS2 protein expression is an early event of intestinal-type gastric cancer. Jpn. J. Cancer Res. 1998, 89, 278–282. [Google Scholar] [CrossRef]
- Im, S.; Yoo, C.; Jung, J.H.; Choi, H.J.; Yoo, J.; Kang, C.S. Reduced expression of TFF1 and increased expression of TFF3 in gastric cancer: Correlation with clinicopathological parameters and prognosis. Int. J. Med. Sci. 2013, 10, 133–140. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakamura, J.; Kitajima, Y.; Kai, K.; Miyake, S.; Hiraki, M.; Ide, T.; Koga, Y.; Noshiro, H. Loss of trefoil factor 1 is regulated by DNA methylation and is an independent predictive factor for poor survival in advanced gastric cancer. Int. J. Oncol. 2013, 42, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, M.; Yamaguchi, J.; Kokuryo, T.; Ebata, T.; Yokoyama, Y.; Sugawara, G.; Nagino, M. Trefoil factor family 1 expression in the invasion front is a poor prognostic factor associated with lymph node metastasis in pancreatic cancer. Pancreatology 2017, 17, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Iwatsuki, M.; Kurashige, J.; Ishimoto, T.; Baba, Y.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Circulating tumor cells in gastric cancer. J. Cancer Metastasis Treat. 2018, 4, 32. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Y.; Yuan, H.; Bai, R.; Zheng, J.; Zhang, J.; Song, M. DNA methylation of trefoil factor 1 (TFF1) is associated with the tumorigenesis of gastric carcinoma. Mol. Med. Rep. 2014, 9, 109–117. [Google Scholar] [CrossRef]
- Shi, S.Q.; Cai, J.T.; Yang, J.M. Expression of trefoil factors 1 and 2 in precancerous condition and gastric cancer. World J. Gastroenterol. 2006, 12, 3119–3122. [Google Scholar] [CrossRef]
- Suárez, C.; Vizoso, F.; Rodríguez, J.C.; García, I.; Raigoso, P.; Allende, M.T.; García-Muñíz, J.L.; García-Morán, M. Prognostic significance of cytosolic pS2 protein content in gastric cancer. Int. J. Biol. Markers 2001, 16, 37–44. [Google Scholar] [CrossRef]
- Doster, A.R.; Yhee, J.Y.; Kim, J.H.; Im, K.S.; Sur, J.H. CDX-2 and HER-3 expression in canine gastric and colorectal adenocarcinomas. J. Comp. Pathol. 2011, 145, 12–19. [Google Scholar] [CrossRef]



| Case | Breed | Sex/Age (Years) | Location of the Lesion | Histological Diagnosis | |
|---|---|---|---|---|---|
| 1 | Boxer | M/10 | N/A | Normal mucosa | |
| 2 | Basset hound | F/9 | N/A | Normal mucosa | |
| 3 | Yorkshire terrier | M/3 | N/A | Normal mucosa | |
| 4 | Crossbreed | M/16 | Body | Inflammatory polyp | |
| 5 | Poodle | M/12 | Antrum | Hyperplastic polyp | |
| 6 | Boxer | M/10 | Antrum | Hyperplastic polyp | |
| 7 | German shepherd | M/9 | Antrum | Inflammatory polyp | |
| 8 | Irish setter | F/12 | Antrum | Hyperplastic polyp | |
| 9 | Argentine mastiff | F/9 | Antrum | Hyperplastic polyp | |
| 10 | Poodle | M/13 | Antrum | Inflammatory polyp | |
| 11 | Crossbreed | F/16 | Antrum | Hyperplastic polyp | |
| WHO [31] | Lauren [32] | ||||
| 12 | Basset hound | F/12 | Antrum | Tubular | Intestinal |
| 13 | Cocker spaniel | M/13 | Antrum | Signet ring cell | Diffuse |
| 14 | Chow-chow | F/11 | Antrum | Mixed | Indeterminate |
| 15 | Shih Tzu | F/10 | Antrum | Poorly cohesive | Diffuse |
| 16 | Chow-chow | M/10 | Antrum | Signet ring cell | Diffuse |
| 17 | English bulldog | M/6 | Body | Signet ring cell | Diffuse |
| 18 | Labrador retriever | F/14 | Body | Tubular | Intestinal |
| 19 | Shar-pei | M/5 | Body | Signet ring cell | Diffuse |
| 20 | Belgian shepherd | F/11 | Body | Mixed | Indeterminate |
| 21 | Golden retriever | M/14 | Body | Signet ring cell | Diffuse |
| 22 | Labrador retriever | M/8 | Antrum | Mixed | Indeterminate |
| 23 | Siberian husky | F/12 | Antrum | Tubular | Intestinal |
| 24 | Crossbreed | M/10 | Body | Mucinous | Diffuse |
| 25 | Crossbreed | F/8 | Body | Poorly cohesive | Diffuse |
| 26 | Siberian husky | M/13 | Antrum | Tubular | Intestinal |
| 27 | Crossbreed (X German shepherd) | F/13 | Body | Poorly cohesive | Diffuse |
| 28 | Akita | M/9 | Body | Poorly cohesive | Diffuse |
| 29 | Collie | M/11 | Body | Mixed | Indeterminate |
| 30 | Alaska malamute | M/6 | ID | Signet ring cell | Diffuse |
| 31 | Golden retriever | M/10 | Antrum | Signet ring cell | Diffuse |
| 32 | Chow-chow | M/9 | Antrum | Poorly cohesive | Diffuse |
| 33 | Pointer | M/11 | Body | Signet ring cell | Diffuse |
| 34 | Collie | M/11 | Body and antrum | Poorly cohesive | Diffuse |
| 35 | Boxer | M/7 | Antrum | Signet ring cell | Diffuse |
| 36 | Crossbreed | F/7 | Antrum | Poorly cohesive | Diffuse |
| 37 | Chow-chow | M/6 | Body | Mucinous | Diffuse |
| 38 | West highland white terrier | F/13 | Antrum | Signet ring cell | Diffuse |
| 39 | Standard Poodle | M/8 | Antrum | Mixed | Indeterminate |
| 40 | Crossbreed (X Poodle) | F/9 | Antrum | Papillary | Intestinal |
| 41 | Miniature Poodle | F/14 | Antrum | Tubular | Intestinal |
| 42 | German shepherd | M/12 | Body and antrum | Poorly cohesive | Diffuse |
| Parameter | No. of Cases | TFF1 Immunoreactivity | ||
|---|---|---|---|---|
| Preserved N = 8 (25.8%) | Reduced N = 23 (74.2%) | p-Value | ||
| Sex | ||||
| Male | 19 | 4 (21.1) | 15 (78.9) | 0.447 |
| Female | 12 | 4 (33.3) | 8 (66.7) | |
| Age, years | ||||
| <10 | 12 | 2 (16.7) | 10 (83.3) | 0.355 |
| ≥10 | 19 | 6 (31.6) | 13 (68.4) | |
| Tumor location 1 | ||||
| Antrum | 16 | 6 (37.5) | 10 (62.5) | 0.317 |
| Body | 12 | 2 (16.7) | 10 (83.3) | |
| Body + antrum | 2 | 0 (0) | 2 (100) | |
| Histological diagnosis | ||||
| WHO classification [31] | ||||
| Well-differentiated | 6 | 3 (50) | 3 (50) | 0.132 |
| Poorly/undifferentiated | 25 | 5 (20) | 20 (80) | |
| Lauren [32] | ||||
| Intestinal | 6 | 3 (50) | 3 (50) | 0.229 |
| Diffuse | 20 | 4 (20) | 16 (80) | |
| Indeterminate | 5 | 1 (20) | 4 (80) | |
| Depth of tumor invasion 2 | ||||
| Muscular | 10 | 3 (30) | 7 (70) | 0.457 |
| Serosa | 12 | 2 (16.7) | 10 (83.3) | |
| Neoplastic emboli | ||||
| Present | 18 | 3 (16.7) | 15 (83.3) | 0.171 |
| Absent | 13 | 5 (38.5) | 8 (61.5) | |
| Metastatic lesions 3 | ||||
| Present | 9 | 1 (11.1) | 8 (88.9) | 0.186 |
| Absent | 17 | 6 (35.3) | 11 (64.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, A.R.; Castro, M.; Rêma, A.; Mesquita, J.R.; Taulescu, M.; Gärtner, F.; Seixas, F.; Amorim, I. Immunoexpression of Trefoil Factor 1 in Non-Neoplastic and Neoplastic Canine Gastric Tissues. Animals 2021, 11, 2855. https://doi.org/10.3390/ani11102855
Flores AR, Castro M, Rêma A, Mesquita JR, Taulescu M, Gärtner F, Seixas F, Amorim I. Immunoexpression of Trefoil Factor 1 in Non-Neoplastic and Neoplastic Canine Gastric Tissues. Animals. 2021; 11(10):2855. https://doi.org/10.3390/ani11102855
Chicago/Turabian StyleFlores, Ana R., Marisa Castro, Alexandra Rêma, João R. Mesquita, Marian Taulescu, Fátima Gärtner, Fernanda Seixas, and Irina Amorim. 2021. "Immunoexpression of Trefoil Factor 1 in Non-Neoplastic and Neoplastic Canine Gastric Tissues" Animals 11, no. 10: 2855. https://doi.org/10.3390/ani11102855
APA StyleFlores, A. R., Castro, M., Rêma, A., Mesquita, J. R., Taulescu, M., Gärtner, F., Seixas, F., & Amorim, I. (2021). Immunoexpression of Trefoil Factor 1 in Non-Neoplastic and Neoplastic Canine Gastric Tissues. Animals, 11(10), 2855. https://doi.org/10.3390/ani11102855






